Abstract
The role of various adhesion molecules in lymphocyte homing to the brain and in inflammatory autoimmune disease of the central nervous system (CNS) was examined in mice. Activated T cell lines and clones expressed CD44 and integrin α4, but not L-selectin, and entered the CNS independent of their antigen specificity. mAbs directed against CD44 and integrin α4 prevented the transfer of experimental autoimmune encephalomyelitis (EAE) by myelin basic protein-specific T cells. T cells preincubated with anti-CD44 or antiintegrin α4 were blocked only partially from entering the brain parenchyma. However, both antibodies efficiently prevented CNS inflammation and clinical expression of EAE when injected in vivo. This effect lasted as long as antibodies were administered. Antibodies specific for L-selectin had no effect on homing of encephalitogenic T cells to the brain or development of EAE. Antiintegrin α4 and anti-CD44 did not impair the activation and function of encephalitogenic T cells in vitro and did not deplete integrin α4- or CD44-positive cells in vivo. These data suggest that, in the absence of leukocyte recruitment, the entry of a reduced number of activated myelin basic protein-reactive T cells in the CNS is not sufficient for the development and expression of EAE. We propose that antibodies to integrin α4 and CD44 prevent clinical disease by partially targeting the primary influx of encephalitogenic T cells and by preventing the secondary influx of leukocytes to lesions initiated by the transferred T cells.
The immune system is capable of recognizing and responding with specificity to various antigens while maintaining overall tolerance to self-components. This process involves the generation and selection of a diverse population of immunocompetent but naive lymphocytes from a large number of precursor cells in the primary lymphoid organs and the efficient initiation of an immune response by immunocompetent cells on capture of antigen in the secondary lymphoid organs (1). These highly specialized immune microenvironments are linked with each other and with the extralymphoid sites of the body through lymphocyte homing and recirculation (2).
Unstimulated lymphocytes recirculate continuously, going from blood to lymphoid tissue and back to blood again, having a cycle time that is measured in hours (3). Lymphocyte recirculation occurs in secondary lymphoid organs such as lymph nodes and Peyer’s patches. Antigen-activated lymphocytes rapidly lose lymphoid-organ homing receptors and exit from these organs to enter the bloodstream, where they migrate to inflammatory sites as well as various organs throughout the body (4, 5). These properties of activated leukocytes permit an efficient surveillance of tissues for injury and infection.
In experimental autoimmunity, inflammation can be induced in naive animals by the transfer of activated autoreactive T cells (6). Experimental autoimmune encephalomyelitis (EAE) is an inflammatory disease of the central nervous system (CNS) with similarities to multiple sclerosis, including episodes of relapsing and remitting paralysis (6–10). EAE is mediated by major histocompatibility complex class II-restricted, myelin basic protein (MBP) or other CNS antigen-specific CD4 positive T lymphocytes. On adoptive transfer, activated T cells penetrate the blood–brain barrier and enter the CNS. The expression of clinical disease in EAE, i.e., neurological deficits such as relapsing paralysis, is a multistep event. An early event is the extravasation and entry of activated MBP-specific encephalitogenic T-lymphocytes into the brain parenchyma across the blood–brain barrier (11–14). Consecutively, other types of leukocytes are recruited. An inflammatory lesion consisting of macrophages, granulocytes, and lymphocytes is formed before the expression of clinical disease (6, 7, 9, 15).
The exact mechanism by which activated lymphocytes cross the blood–brain barrier remains unclear. It has been shown that mAbs specific for integrin α4 can inhibit inflammation, including EAE (16–18). Baron et al. (18) demonstrated a direct role for integrin α4 in the entry of activated T cells into normal CNS tissue. However, transendothelial migration of leukocytes is thought to involve several independent, specialized adhesion pathways that are mediated by various classes of homing receptors and their vascular addressins (2, 19). Selectins have been shown to be involved in the primary step of leukocyte migration whereas integrins can be involved in primary as well as secondary (firm) adhesion (2, 19, 20). Recently, a novel rolling interaction between lymphoid cells and endothelial cells has been described (21). This interaction is not mediated by selectin or integrin α4 but has as its basis the interaction between CD44 and its principal ligand, the glycosaminoglycan hyaluronate (HA).
CD44 is a family of cell surface glycoproteins thought to function as cell adhesion molecules playing a role in leukocyte extravasation, lymphocyte homing, myelo- and leukopoesis, and binding to the extracellular matrix (21–24). Here, we analyzed the role of CD44, L-selectin, and integrin α4 in the various stages during the pathogenesis of EAE, namely primary lymphocyte homing to the brain and secondary recruitment of inflammatory leukocytes.
MATERIALS AND METHODS
Animals.
Female (PL × SJL)F1 and C57BL/6 mice used in this study were purchased from The Jackson Laboratory. Mice were kept under specific pathogen-free conditions in the Department of Laboratory Animal Medicine at Stanford University according to approved protocols and were used when between 5 and 10 weeks of age.
Establishment of Antigen-Specific T Cell Lines and Clones.
T cell lines and clones specific for MBP peptide Ac1-11 were established from (PL × SJL)F1 mice as described (6, 25). T cells specific for Listeria monocytogenes were established from C57BL/6 mice as described (26). T cell lines and clones specific for Mycobacterium tuberculosis (H37Ra, Difco) were established following the same protocols.
Induction of Adoptively Transferred EAE and in Vivo Antibody Treatment.
For adoptive transfer of EAE, 1.25–5 × 106 activated cells of the T cell lines were injected i.v. in 500 μl of PBS into naive (PL × SJL)F1 mice 6–8 weeks of age. Purified mAbs (250 μg) were injected i.p. on the indicated days. We evaluated all animals daily for disease signs and scored them according to a scale, with disease scores ranging from 1 to 5 as described (25).
mAbs.
The following anti-mouse mAbs were used in the experiments: IM7.8.1 (rat IgG2b anti-mouse CD44, anti-PGP-1), R1-2 [rat IgG2b anti-mouse CD49d, anti-α4-chain of integrin α4β1 (VLA-4) or integrin α4β7 (LPAM-1)], PS/2 [rat IgG2b anti-mouse CD49d, anti-α4-chain of integrin α4β1 (VLA-4) or integrin α4β7 (LPAM-1)], MEL-14 (rat IgG2a anti-mouse CD62L, anti-L-selectin), M1/70 [rat IgG2b anti-mouse CD11b, anti-αM-chain of integrin αMβ2 (Mac-1)], 5C6 [rat IgG2b anti-mouse CD11b, anti-αM-chain of integrin αMβ2 (Mac-1)], 53.7.3 (rat IgG2a anti-mouse CD5, anti-Ly-1), 53.2.1 (rat IgG2a anti-mouse Thy1.2), F23.1 (anti-T cell antigen receptor Vβ8, IgG2a), and GK1.5 (rat IgG2b anti-mouse CD4, anti-L3T4). Hybridomas were grown in a Cell-Pharm hollow fiber cell culture system (Unisyn Tech., Hopkinton, MA). mAb 5C6 was kindly provided as ascites by Hugh Rosen (University of Oxford). Protein G affinity columns (Amersham Pharmacia) were used for mAb purification. Purity and concentration of mAbs was determined by gel electrophoresis and photometric assay at an optical density of 280 nm (OD280). Antibodies were dialyzed and stored at −70°C until use. Directly fluorescein- or phycoerythrin-conjugated mAbs for staining and flow cytometry to analyze T cell surface marker expression were purchased from PharMingen and were used according to standard procedures.
In Vivo T Cell Trafficking Assay.
Ten million antigen-activated T cells were suspended in RPMI medium 1640 supplemented with 10% FCS and were labeled in vitro with 10 μg/ml of fluorescent cell linker PKH2 (green; Sigma) or 5 μg/ml PKH26 (red; Sigma) according to the manufacturer’s instructions. In some experiments, 10 μg/ml DiO C18 (green; Molecular Probes) and 5 μg/ml DiI C18 (red; Molecular Probes) were used in fluorescent labeling. Cells were incubated for 30 minutes at 37°C. After washing, cells labeled with the red dye were incubated for 30 minutes with 10 μg/ml of one of the mAbs R1-2, PS/2, or IM7.8.1, and the cells labeled with the green dye were incubated with 10 μg/ml of one of the mAbs GK1.5, 53.7.3, 53.2.1, or IM7.8.1. Then, these cells were washed free of unbound antibody, were mixed in 1:1 ratio, and immediately were injected i.v. in PBS into naive (PL × SJL)F1 mice. Each animal received simultaneously two sets of 107 fluorescently labeled, antibody-treated cells. The exact ratio of green and red cells in the initial cell mix was determined by fluorescence microscopy. Brain, spleen, and liver were harvested 5–24 h later and were snap-frozen. Labeled cells in the brain parenchyma, spleen white pulp, and liver were counted by using a fluorescence microscope, and the ratio of red versus green cells was calculated. The results are given relative to the ratio of labeled cells in the cell mix before injection.
Effect of mAb Treatment on Peripheral Blood Leukocyte Subsets.
To determine the absolute cell counts of peripheral blood leukocyte subsets in mAb-treated animals, naive (PL × SJL)F1 mice were administered 250 μg of purified mAbs GK1.5, R1-2, IM7.8.1, or PBS i.p. (n = 5 per treatment group). Peripheral blood leukocytes were isolated by red cell hemolysis 24 h later for counting (Coulter Counter) and fluorescence-activated cell sorter analysis with directly conjugated reagents for the percentage of B cells (B220+; mAb 6B2), T cells (T200+; mAb 53.7.1), and CD4+ T cells (mAb GK1.5). Specific staining of cells was analyzed on a FACScan (Becton Dickinson) according to standard procedures.
Histological Staining.
Preparation of tissue specimens and histological staining were performed as described (25).
In Vitro T Cell Proliferation Assay.
Seven to ten days after antigenic stimulation, cells of the encephalitogenic T cell line TLMSB-9 were tested for their specific proliferative responses to MBP peptide Ac1-11 in the presence of various antibodies. Procedures have been described elsewhere (25, 26).
RESULTS
Expression of Adhesion Molecules by T Cells with Different in Vivo Activities.
To detect possible patterns of adhesion molecules uniquely expressed on encephalitogenic T cells, we stained T cell clones and lines specific for M. tuberculosis, L. monocytogenes, and the MBP epitopes Ac1-11 and 87-99 with several antibodies directed against adhesion molecules (Table 1). All clones tested were negative for mAb MEL-14, recognizing CD62L (L-selectin), the murine peripheral lymph node-specific homing receptor (27, 28). All T cells tested were positively stained by integrin α4-chain-specific mAb R1-2 (29). All cells were highly positive for PGP-1 (CD44) detected by mAb IM7.8.1. T cells were also positive for LFA-1 αL-chain-specific mAb 2D7 (between ++ and ++++, according to grading in Table 1) and integrin β2-chain-specific mAb C71/16 (between +++ and ++++, according to grading in Table 1; ref. 30). In addition, MBP-specific T cell line L9 stained positively with mAb M301 directed against integrin β7 (data not shown). Most T cells had a defined in vivo activity, such as induction of EAE (31) or protection against facultative intracellular bacteria (26). No specific pattern of expression of adhesion molecules could be clearly associated with encephalitogenicity of T cells specific for MBP peptides (Table 1).
Table 1.
Summary of the properties of mouse T cell lines and clones used in this study
| T cell lines/clones | Antibody staining
|
In vivo function | ||
|---|---|---|---|---|
| MEL-14, anti-L-selectin | R1-2, antiintegrin α4 | IM7.8.1, anti-PGP-1 | ||
| T cell lines and clones specific for Mycobacterium tuberculosis (H37 Ra) | ||||
| MBTF1 | Not tested | ++* | ++++ | Not tested |
| C4 BL/6 | Not tested | ++ | ++++ | Not tested |
| T cell clones specific for L. monocytogenes† | ||||
| THKL-C6 | − | ++ | ++++ | Protection and chemotaxis |
| THKL-C7 | − | +++ | ++++ | Protection and chemotaxis |
| THKL-C13 | − | +++ | ++++ | Protection and chemotaxis |
| TSAG-C65 | − | ++ | ++++ | Chemotaxis |
| T cell lines and clones specific for MBP epitopes‡ | ||||
| MBP Ac1-11 | ||||
| L4 | +++ | ++++ | EAE | |
| L5 | +++ | ++++ | EAE | |
| L7 | ++ | ++++ | EAE | |
| L9 | − | +++ | ++++ | EAE |
| L9C48 | − | ++/+++ | ++++ | EAE |
| C1 | − | ++ | ++++ | No EAE |
| PJR25 | − | ++ | ++++ | EAE |
| PJB20 | − | +++ | ++++ | EAE |
| PJB18 | − | ++ | ++++ | No EAE |
| MBP 87-99 | ||||
| L10 | − | ++ | ++++ | EAE |
| L10 C1 | − | ++ | +++ | EAE |
| L10 C9 | − | ++ | ++++ | No EAE |
| L10 C23 | − | + | ++++ | No EAE |
| L10 C32 | − | ++ | ++++ | No EAE |
| L10 C14 | − | + | ++++ | No EAE |
The Entry of T Cells to the Brain Is Independent of Antigen Specificity and in Vivo Function.
To follow the migration of activated T lymphocytes after adoptive transfer, an in vivo homing assay with fluorescently stained T cells had been used. Labeled T cells could be visualized and counted in the various organs 5 h after adoptive transfer (data not shown). The majority of transferred T cells was found in the liver and the mantle zone of the spleen. Only a few cells had entered the brain parenchyma at this time, but these were presumably responsible for inducing inflammation in the CNS and EAE. Both MBP-specific and L. monocytogenes-specific T cells entered the brain parenchyma after activation in vitro by the relevant antigen (data not shown). These data indicate that entry of T cells into the brain is apparently independent of their antigen specificity.
Antibodies to CD44 and Integrin α4 Reduce the Entry of Autoreactive T Cells to the Brain.
Labeling of the T cells had no effect on their ability to mediate EAE (data not shown). Pre-coating of the encephalitogenic T cells with mAbs R1-2 and IM7.8.1 before adoptive transfer reduced the number of cells entering the brain parenchyma to 82 ± 6% for R1-2 and to 44 ± 7% for IM7.8.1 when the entry of noncoated or control antibody coated cells was determined as the norm (100%) (Table 2).
Table 2.
Ratios of red vs. green T cells in different organs of (PL × SJL)F1 mice 18-24 h after adoptive transfer
| Red:green cells coated with mAbs* | Cell mix at time of injection | Brain | Spleen | Liver |
|---|---|---|---|---|
| Antiintegrin α4 mAb vs. control mAb | ||||
| R1–2 (α4):53.7.3 (control), n = 2 | 1.0 | 0.78 | 0.89 | 0.97 |
| R1–2 (α4):GK 1.5(control), n = 2 | 1.0 | 0.79 | 0.96 | 1.2 |
| R1–2 (α4):53.2.1 (control), n = 2 | 1.0 | 0.89 | 1.2 | 1.1 |
| PS/2 (α4):53.7.3 (control), n = 2 | 1.0 | 0.50 | 0.82 | 1.0 |
| Anti-CD44 mAb vs. control mAb | ||||
| IM7.8.1 (CD44):53.7.3 (control), n = 2 | 1.0 | 0.49 | 1.0 | 1.3 |
| IM7.8.1 (CD44):53.2.1 (control), n = 2 | 1.0 | 0.39 | 1.0 | Not determined |
| Anti-CD44 mAb vs. antiintegrin α4 mAb | ||||
| R1–2 (α4):IM7.8.1 (CD44), n = 2 | 1.0 | 1.3 | 1.1 | 0.90 |
| Control | ||||
| No Ab:No Ab | 1.0 | 1.0 | 1.1 | 1.1 |
Each mouse received simultaneously two sets of 107 fluorescently labeled, antibody-treated T cells. The exact ratio of green and red cells in the initial cell mix was determined by fluorescence microscopy. Brain, spleen, and liver were harvested 5–24 h later and were snap-frozen. The results are given relative to the ratio of red vs. green T cells in various organs of sacrificed mice.
Antibodies to CD44 and Integrin α4 Prevent the Establishment of Inflammatory Lesions in the Brain.
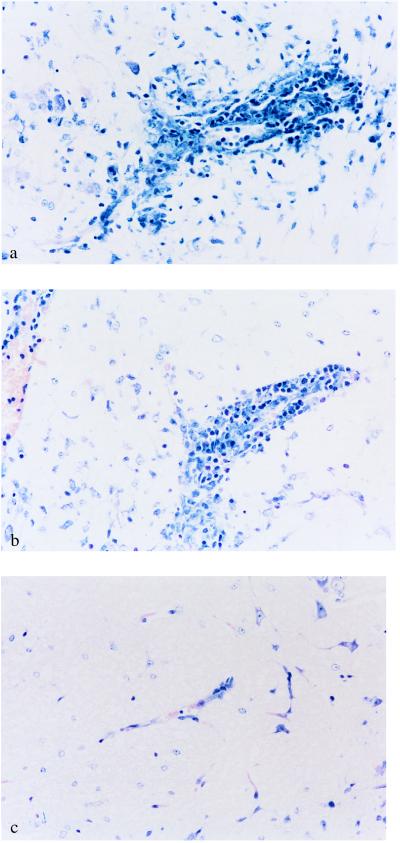
Although a substantial number of potentially encephalitogenic T cells were still capable of entering the brain when pretreated with mAbs IM7.8.1, R1-2 or PS/2, no inflammatory lesions developed under continuous in vivo treatment with those antibodies (Fig. 1). Eight days after transfer of MBP Ac1-11 specific T cell line L9, PBS-treated mice or mice treated with mAb MEL-14 showed perivascular infiltrates composed of lymphocytes, granulocytes, and macrophages (Fig. 1 a and b). The infiltrates developed preferentially in the spinal cord, the brainstem, the cerebellum, and, to a lesser extent, in other areas of the white or parts of the gray matter in the brain. At this time, mice started to develop clinical signs of EAE. In contrast, treatment of mice with mAbs IM7.8.1 or R1-2 prevented appearance of inflammatory lesions in the brain after transfer of T cell line L-9 (Fig. 1c; data not shown).
Figure 1.
Prevention of T cell-induced inflammation in the white matter of the CNS by antibodies directed against CD44 and integrin α4 but not L-selectin. Mice were treated continuously with different antibodies for 8 days after adoptive transfer of 5 × 106 MBP peptide Ac1-11-specific T cells. Thereafter, brains of mice treated i.p. with the indicated antibodies or PBS were removed, fixed, sectioned, and stained with hematoxylin and eosin. Typical regions were photographed. The original magnification was 200×. (a) Histopathology of mice treated with PBS. Shown is perivascular inflammatory infiltrate in the brainstem of a mouse with adoptive EAE. Clinical disease score at the time of death was grade 3. (b) Histopathology of mice treated with mAb MEL-14 (anti-L-selectin). Shown is perivascular inflammatory infiltrate in the brainstem of a mouse with adoptive EAE. Clinical disease score at the time of death was grade 2. (c) Histopathology of mice treated with mAb IM7.8.1 (anti-CD44). Shown is the absence of perivascular infiltrates in the brainstem of a mouse after adoptive transfer of encephalitogenic T cells. Clinical disease score was grade 0.
Antibodies to CD44 and Integrin α4, but not L-Selectin, Block EAE in a Dose-Dependent Manner.
Administered in vivo, mAbs IM7.8.1 and R1-2 prevented adoptive transfer of EAE (Fig. 2). The therapeutic effect of antibodies depended on the total amount of antibody injected and on the mean disease score in a particular experiment. The protection of animals from EAE lasted as long as antibodies were administered (Fig. 2 b and c). Three to eight days after the last antibody injection, all mice developed EAE with paralysis, although the mean disease severity was always lower than in the control animals at the same time point (Fig. 2 b and c). In severe disease, mAb R1-2 delayed significantly the lethal outcome of EAE (data not shown). In contrast, when disease course was mild, animals could be protected from EAE for a longer period of time after the last antibody injection (Fig. 2d). One or two injections of mAb IM7.8.1 at the time of transfer of T cell line L9 had no effect on the expression of EAE (Fig. 2a). Continuous injections of mAb MEL-14, specific for L-selectin, also had no effect on the course of EAE (Fig. 2).
Figure 2.
Prevention of adoptively transferred EAE by treatment with anti-CD44 and antiintegrin α4 mAbs and amelioration of clinical signs by treatment with antiintegrin αM mAbs. MBP peptide Ac1-11-specific T cells (5 × 106/mouse, n = 5–15 mice/group) were transferred into naive (PL × SJL)F1 mice after in vitro activation with antigen for 3 days. Mice in each group were treated twice (a) or over a longer time period (b) with mAbs IM7.8.1 (open circles), MEL-14 (closed circles), or PBS as control (open squares). Each mAb (250 μg) was given on the indicated days (marked by i). Mice were scored daily, and the mean EAE scores (± SEM) at each day after transfer are shown. The figure shows representative results from at least three experiments. In a second series of experiments (c and d), mice were treated with 250 μg of mAbs R1-2 (open circles), MEL-14 (closed circles), or PBS as control (open squares) on days as indicated by i. Mice were scored daily, and the mean EAE scores (± SEM) at each day after transfer are shown. The figures shows representative results from at least three experiments. (c) Continuous injections of antiintegrin α4 mAb R1-2 prevent clinical signs of EAE as long as antibodies are administered. (d) During a mild course of EAE, continuous injections of R1-2 fully prevent clinical signs of EAE for at least 24 days. In the last series of experiments (e and f), mice were treated with antiintegrin αM mAb M1/70 (e, full circles), 5C6 (f, full circles), or PBS as control (open squares). Clinical effects of treatment with these mAbs were moderate.
Antibodies to Integrin αM Ameliorate the Clinical Course of EAE.
mAbs M1/70 (Fig. 2e) and 5C6 (Fig. 2f), specific for the αM chain of integrin αMβ2 (Mac-1), ameliorated the clinical severity of EAE without reducing the number of sick animals (Fig. 2). This effect was not correlated with changes in the histology of EAE lesions in the CNS when compared with PBS-treated or control antibody (MEL 14)-treated mice (data not shown).
mAbs IM7.8.1 and R1-2 Do Not Deplete CD4+ T Lymphocytes in Vivo.
Depletion of effector cells could be responsible for the therapeutic effect of a mAb in vivo. For this reason, we tested a panel of mAbs for their effect on leukocyte subsets after in vivo treatment of mice. We found that mAbs directed against CD4 depleted CD4+ lymphocytes in vivo (ref. 32; Fig. 3 whereas treatment of mice with mAb R1-2 or IM7.8.1 did not significantly influence the overall number of T cells circulating in the bloodstream (Fig. 3). Of note, the total white blood cell count, B cell, and non-B/non-T compartments were slightly reduced after treatment with IM7.8.1 in vivo (Fig. 3).
Figure 3.
Effect of mAbs GK1.5 and IM7.8.1 on leukocyte subsets in vivo. Purified mAbs (250 μg) GK1.5, R1-2, IM7.8.1, or PBS were administered i.p. into naive (PL × SJL)F1 mice (n = 5 per treatment group). Peripheral blood leukocytes were isolated 24 h later for counting and fluorescence-activated cell sorter analysis with directly conjugated reagents for the percentage of B cells (B220+), T cells (T200+), and CD4+ T cells (mAb GK1.5+). Results are expressed as mean ± SD of absolute cell counts (×1,000/μl of blood) adjusted to the total white blood cell counts as determined by the Coulter Counter.
The Role of Adhesion Molecules in the Activation of Encephalitogenic T Cells.
The proliferative response of encephalitogenic T cell line L-9 to MBP Ac1-11 was tested in the presence of mAbs (data not shown). As expected, anti-mouse CD4 mAb GK1.5 inhibits the proliferative response by >90%. This antibody in vivo also prevents and reverses EAE (32). Neither mAb IM7.8.1 nor R1-2 reduced the antigen-specific proliferative response of T cell line L9. Similarly, isotype-matched control antibody M1/70 had no blocking effect on the proliferative response of T cell line L9 in vitro (data not shown).
DISCUSSION
In EAE, T cells are induced and expanded in the peripheral immune system after immunization with myelin proteins and glycoproteins (25, 33). Some T cells are able to generate an inflammatory disease of the CNS after entering meninges, the perivascular space, and, finally, brain or spinal cord parenchyma. Requirements for the induction of a T cell-mediated autoimmune inflammation in the CNS include appropriate antigen-specificity, functional (effector) phenotype of T cells, and the ability of autoreactive T cells to gain access to the target organ by crossing the blood–brain barrier. It has been shown previously that T cells can enter the brain parenchyma of naive mice depending on their activation state (11, 13). Although initial homing of T cells to the CNS is independent of their antigen-specificity, only cells capable of recognizing CNS antigens remain in the tissue for longer periods (7, 11, 13, 34–40).
Many models of leukocyte-endothelial cell recognition preceding transendothelial migration of leukocytes have been characterized as a multistep process involving reversible adhesion, leukocyte activation, and activation-dependent binding (2, 19, 20, 41–45). In this study, we show that integrin α4-positive and CD44-positive activated T cells with different in vivo functions can enter the brain of naive mice. It is noteworthy that the MBP-specific encephalitogenic T cell line L9 is positive for integrin β7 (T.V., unpublished data) because both integrin α4β1 and integrin α4β7 can act as adhesion receptors for vascular cell adhesion molecule 1 and fibronectin c-peptide (46). We found that the ability of the lines and clones tested to enter the CNS of mice was independent of T cell antigen receptor usage, antigen specificity, and genetic background of mice. After adoptive transfer, a small number of T cells specific for myelin antigens apparently induced inflammation with massive cellular infiltrates in the CNS and clinical signs of EAE. The difference between encephalitogenic and nonencephalitogenic T cells seems to rest in their ability to induce an inflammatory reaction once they have entered the brain parenchyma. The inflammatory lesion in the CNS in EAE is composed of macrophages, lymphocytes, and granulocytes (15, 25, 47) and includes a heterogeneous T cell population (9, 38).
Antibodies directed against CD44 and integrin α4 could interfere in several ways with the establishment of inflammatory lesions in the CNS: (i) At the vascular lumen, antibodies directed at adhesion molecules could directly block the entry of autoreactive T cells to the brain; (ii) antibodies to CD44 or integrin α4 could interfere with the successful reactivation of encephalitogenic T cells by myelin antigens presented by glial cells, and possibly other antigen presenting cells, within CNS lesions; CD44 has been shown to be expressed on glial cells in EAE lesions and plays a role in the interaction between a T cell hybridoma and astrocytes in vitro (48); (iii) as in i, antibodies could block the secondary influx of leukocytes into the CNS at a later stage in the inflammatory process; and (iv), the antibodies might deplete the peripheral leukocyte pool substantially, preventing the organism from mounting an effective inflammatory response.
Our results indicate that the major effect of mAbs R1-2 and IM7.8.1 could be directed against events that lead to the secondary influx of inflammatory cells that are crucial for reversible clinical disease and demyelination. In that view, the entry of encephalitogenic cells into the brain parenchyma was not sufficient for the induction of histolopathological or clinical disease as long as leukocyte recruitment was blocked by antiadhesion molecule antibodies. The notion that the secondary influx of leukocytes was targeted by antiintegrin α4 mAbs with clinical relevance is supported by the study of Yednock et al. (17), who demonstrated that adoptive EAE in the rat can be suppressed by antiintegrin α4 antibodies that were given after adoptive transfer of encephalitogenic T cells. In the mouse, when mAb treatment is stopped after 16 days, encephalitogenic T cells still resided within or migrated from the periphery into the CNS parenchyma, inducing inflammation and EAE. Thus, the antibodies did not seem to deplete the encephalitogenic cells in situ. The secondary recruitment of cells to the CNS inflammatory foci could result from any step in a multistep process. For example, in situ, T cells might require adhesive events to meet, adhere to, and be recruited by particular brain antigen-presenting cells. These antigen-presenting cells and the environment surrounding them could express adhesive elements such as vascular cell adhesion molecule 1, fibronectin c-peptide, HA, and Mac-1 (integrin αMβ2). Successful reactivation of T cells in situ could result in expression of new or quantitatively augmented addressins on CNS vessels resulting in an augmented, secondary influx. Thus, each of these antiadhesins that affect the generation of EAE pathology could operate within the CNS proper and/or on the vessels that serve it.
Several lines of evidence point to an important function of CD44 as an adhesion molecule, with the observed roles for CD44 ranging from extracellular matrix binding, cell migration, lymphocyte development, metastasis, and lymphocyte homing (22–24). CD44 is a proteoglycan with a wide tissue distribution whose polypeptide chain exists in various isoforms as a result of alternative exon splicing (22). The sequence of CD44 displays a limited homology with the HA-binding domains of proteoglycan core and link proteins, indicating a role for CD44 as a receptor for HA (49, 50). Of note, proteoglycans (51, 52), including CD44 (52, 53), have been shown to present cyto- and chemokines to leukocytes, resulting in the promotion of T cell adhesion. Several recent studies indicate an in vivo role for the interaction between CD44 expressed on leukocytes and HA or other extracellular matrix ligands. First, CD44 has been shown to be required for leukocyte extravasation into an inflammatory site involving nonlymphoid tissue in a model of cutaneous DTH reaction (23) and for short-term homing of activated T cells to inflamed peritoneum induced by local administration of superantigen (24). Moreover, anti-CD44 treatment abrogates tissue edema and leukocyte infiltration in murine arthritis (54). During a T cell response in vivo, binding of CD44 to hyaluronate is transiently up-regulated (55). It is noteworthy that both integrin α4 and CD44 have been shown to mediate primary events in lymphocyte adhesion, namely, tethering and rolling under physiologic flow (21, 24, 56, 57), whereas additional, as-yet uncharacterized molecules might play a role in primary adhesion of human T cells (58). DeGrendele (21) demonstrated that lymphocytes with an increased potential of CD44-HA binding and subsequent rolling are generated during an inflammatory immune response in vivo. These functions of integrin α4 and CD44 might explain the modest reduction in the number of T cells arriving in the CNS parenchyma after in vitro-pretreatment of cells with blocking antiintegrin α4 and anti-CD44 mAbs.
Strikingly, anti-L-selectin directed mAb MEL-14 had no effect on the generation of inflammatory lesions and clinical disease in EAE. Although L-selectin is expressed on many classes of leukocytes (27, 59) and plays an important role in leukocyte adhesion, rolling, and migration (20, 27, 60–62), it is not expressed on antigen-activated T or B cells. It has become clear recently that selectins are not always required for leukocyte migration (43). Conversely, antibodies directed at the C3b adhesion receptor (Mac-1) expressed on monocytes and macrophages ameliorated EAE significantly, as has been shown (63). Possibly, the relevant Mac1-positive cells are in the brain and mediate an important antigen presenting cell–T cell interaction.
We propose that CD44, possibly through interaction with HA expressed on inflamed endothelium (64, 65), and integrin α4, but not L-selectin, play an important role in the events that lead to or are directly responsible for secondary recruitment of inflammatory leukocytes by T cells reactive with CNS autoantigens in EAE (66–68) or by T cells in mediating other autoimmune disorders (69, 70).
Acknowledgments
The authors are grateful to Dr. Hugh Rosen for providing mAb 5C6 and to Drs. Henry McFarland and William Biddison for critical comments. This work was supported by grants from Systemix and Sandoz Corporations (I.L.W. and T.V.), the National Institutes of Health (S.B., T.V., and L.S.), and the Israel Science Foundation administered by the Israel Academy of Sciences (S.B.).
ABBREVIATIONS
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- MBP
myelin basic protein
- HA
hyaluronate
Footnotes
Present address: Biotie Therapies, Biocity, FIN-20520 Turku, Finland.
References
- 1.Weissman I L. Cell. 1994;76:207–218. doi: 10.1016/0092-8674(94)90329-8. [DOI] [PubMed] [Google Scholar]
- 2.Butcher E C, Picker L J. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 3.Ford W L, Gowans J L. Semin Hematol. 1969;6:67–83. [PubMed] [Google Scholar]
- 4.Mackay C. Curr Opin Immunol. 1993;5:423–427. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- 5.Dailey M O, Fathman C G, Butcher E C, Pillemer E, Weissman I L. J Immunol. 1982;128:2134–2136. [PubMed] [Google Scholar]
- 6.Zamvil S S, Steinman L. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 7.Raine C S. Ann Neurol. 1994;36:S61–S72. doi: 10.1002/ana.410360716. [DOI] [PubMed] [Google Scholar]
- 8.Hafler D A, Weiner H L. Immunol Rev. 1995;144:75–107. doi: 10.1111/j.1600-065x.1995.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 9.Dal Canto M C, Melvold R W, Kim B S, Miller S D. Microsc Res Tech. 1995;15:215–229. doi: 10.1002/jemt.1070320305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinman L. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 11.Wekerle H, Linington C, Lassmann H, Meyermann R. Trends Neurosci. 1986;6:271–277. [Google Scholar]
- 12.Cross A H, Canella B, Brosnan C F, Raine C S. Lab Invest. 1990;63:162–170. [PubMed] [Google Scholar]
- 13.Hickey W F, Hsu B L, Kimura H. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 14.Wekerle H. Int Rev Immunol. 1992;9:231–241. doi: 10.3109/08830189209061793. [DOI] [PubMed] [Google Scholar]
- 15.Lassmann H, Zimprich F, Rossler K, Vass K. Rev Neurol (Paris) 1991;147:763–781. [PubMed] [Google Scholar]
- 16.Issekutz T B, Issekutz A C. Clin Immunol Immunopathol. 1991;6:436–447. doi: 10.1016/s0090-1229(05)80014-5. [DOI] [PubMed] [Google Scholar]
- 17.Yednock T A, Cannon C, Fritz L C, Sanchez-Madrid F, Steinman L, Karin N. Nature (London) 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 18.Baron J L, Madri J A, Ruddle N H, Hashim G, Janeway C A., Jr J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Springer T A. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 20.Springer T A. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 21.DeGrendele H C, Estess P, Picker L J, Siegelman M H. J Exp Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesley J, Hyman R, Kincade P W. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- 23.Camp R L, Scheynius A, Johansson C, Pure E. J Exp Med. 1993;178:497–507. doi: 10.1084/jem.178.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeGrendele H C, Estess P, Siegelman M H. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 25.Brocke S, Gijbels K, Steinman L. Autoimmune Disease Models: A Guidebook. San Diego: Academic; 1994. [Google Scholar]
- 26.Brocke S, Hahn H. Infect Immun. 1991;59:4531–4539. doi: 10.1128/iai.59.12.4531-4539.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallatin W M, Weissman I L, Butcher E C. Nature (London) 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 28.Siegelman M H, van de Rijn M, Weissman I L. Science. 1989;243:1165–1172. doi: 10.1126/science.2646713. [DOI] [PubMed] [Google Scholar]
- 29.Holzman B, McIntyre B W, Weissman I L. Cell. 1989;56:37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- 30.Springer T, Davignon D, Ho M, Kurzinger E, Martz E, Sanchez-Madrid F. Immunol Rev. 1982;68:171–195. doi: 10.1111/j.1600-065x.1982.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 31.Brocke S, Gaur A, Piercy C, Gijbels K, Fathman C G, Steinman L. Nature (London) 1993;365:642–644. doi: 10.1038/365642a0. [DOI] [PubMed] [Google Scholar]
- 32.Waldor M K, Sriram S, Hardy R, Herzenberg L A, Herzenberg L A, Lanier L, Lim M, Steinman L. Science. 1985;227:415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- 33.Quarles R H. J Mol Neurosci. 1997;8:1–12. doi: 10.1007/BF02736858. [DOI] [PubMed] [Google Scholar]
- 34.Trotter J, Steinman L. J Immunol. 1984;132:2919–2923. [PubMed] [Google Scholar]
- 35.Hafler D A, Weiner H L. Ann Neurol. 1987;22:89–93. doi: 10.1002/ana.410220121. [DOI] [PubMed] [Google Scholar]
- 36.Raine C S, Cannella B, Duijvestijn A M, Cross A H. Lab Invest. 1990;63:476–489. [PubMed] [Google Scholar]
- 37.Raine C S. Neuropathol Appl Neurobiol. 1991;17:265–274. doi: 10.1111/j.1365-2990.1991.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 38.Bell B B, Lindsey J W, Sobel R A, Hodgkinson S, Steinman L. J Immunol. 1993;150:4085–4092. [PubMed] [Google Scholar]
- 39.Mor F, Cohen I R. J Clin Invest. 1992;90:2447–2455. doi: 10.1172/JCI116136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindsey J, Steinman L. J Neuroimmunol. 1993;48:227–234. doi: 10.1016/0165-5728(93)90196-6. [DOI] [PubMed] [Google Scholar]
- 41.Springer T A. Nature (London) 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 42.Butcher E C. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 43.Mackay C. Curr Biol. 1995;5:733–736. doi: 10.1016/s0960-9822(95)00147-3. [DOI] [PubMed] [Google Scholar]
- 44.Carr M W, Alon R, Springer T A. Immunity. 1996;4:179–187. doi: 10.1016/s1074-7613(00)80682-2. [DOI] [PubMed] [Google Scholar]
- 45.Gallatin W M, St. John T P, Siegelman M, Reichert R, Butcher E C, Weissman I L. Cell. 1986;14:673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- 46.Crowe D T, Chiu H, Fong S, Weissman I L. J Biol Chem. 1994;269:14411–14418. [PubMed] [Google Scholar]
- 47.Lassmann H. Schriftenr Neurol. 1983;25:1–135. [PubMed] [Google Scholar]
- 48.Haegel H, Toelg C, Hoffmann M, Ceredig R. J Cell Biol. 1993;122:1067–1077. doi: 10.1083/jcb.122.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyake K, Underhill C B, Lesley J, Kincade P W. J Exp Med. 1990;172:69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyake K, Kincade P W. Curr Top Microbiol Immunol. 1990;166:87–90. doi: 10.1007/978-3-642-75889-8_12. [DOI] [PubMed] [Google Scholar]
- 51.Ebnet K, Kaldjian E P, Anderson A O, Shaw S. Annu Rev Immunol. 1996;14:155–177. doi: 10.1146/annurev.immunol.14.1.155. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka Y, Adams D H, Hubscher S, Hirano H, Siebenlist U, Shaw S. Nature (London) 1993;361:79–81. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka Y, Adams D H, Shaw S. Immunol Today. 1994;14:111–114. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- 54.Mikecz K, Brennan F R, Kim J H, Glant T T. Nat Med. 1995;1:558–563. doi: 10.1038/nm0695-558. [DOI] [PubMed] [Google Scholar]
- 55.Lesley J, Howes N, Perschl A, Hyman R. J Exp Med. 1994;180:383–387. doi: 10.1084/jem.180.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alon R, Kassner P D, Carr M W, Finger E B, Hemler M E, Springer T A. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berlin C, Bargatze R F, von Andrian U H, Szabo M C, Hasslen S R, Nelson R D, Berg E L, Erlandsen S L, Butcher E C. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 58.Jones D A, McIntire L V, Smith C W, Picker L J. J Clin Invest. 1994;94:2443–2450. doi: 10.1172/JCI117612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tedder T F, Penta A C, Levine H B, Freedman A S. J Immunol. 1990;144:532–540. [PubMed] [Google Scholar]
- 60.Spertini O, Luscinskas F W, Kansas G S, Munro J M, Griffin J D, Gimbrone M A, Tedder T F. J Immunol. 1991;147:2565–2573. [PubMed] [Google Scholar]
- 61.Arbones M L, Ord D C, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon D J, Tedder T F. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 62.Picker L J, Butcher E C. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 63.Gordon E J, Myers K J, Dougherty J P, Rosen H, Ron Y. J Neuroimmunol. 1995;62:153–160. doi: 10.1016/0165-5728(95)00120-2. [DOI] [PubMed] [Google Scholar]
- 64.Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. J Clin Invest. 1998;101:97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeGrendele H, Kosfiszer M, Estess P, Siegelman M. J Immunol. 1997;159:2549–2553. [PubMed] [Google Scholar]
- 66.Wekerle H, Kojima K, Lannes-Vieira J, Lassmann H, Linington C. Ann Neurol. 1994;36:S47–53. doi: 10.1002/ana.410360714. [DOI] [PubMed] [Google Scholar]
- 67.Hohlfeld R. Brain. 1997;120:865–916. doi: 10.1093/brain/120.5.865. [DOI] [PubMed] [Google Scholar]
- 68.Miller S D, Karpus W J. Immunol Today. 1994;15:356–361. doi: 10.1016/0167-5699(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 69.Wittig B, Schwarzler C, Fohr N, Gunthert U, Zoller M. J Immunol. 1998;161:1069–1073. [PubMed] [Google Scholar]
- 70.Estess P, DeGrendele H C, Pascual V, Siegelman M H. J Clin Invest. 1998;102:1173–1182. doi: 10.1172/JCI4235. [DOI] [PMC free article] [PubMed] [Google Scholar]