Abstract
Obesity is associated with a cluster of abnormalities, including hypertension, insulin resistance, hyperinsulinemia, and elevated levels of both plasminogen activator inhibitor 1 (PAI-1) and transforming growth factor β (TGF-β). Although these changes may increase the risk for accelerated atherosclerosis and fatal myocardial infarction, the underlying molecular mechanisms remain to be defined. Although tumor necrosis factor α (TNF-α) has been implicated in the insulin resistance associated with obesity, its role in other disorders of obesity is largely unknown. In this report, we show that in obese (ob/ob) mice, neutralization of TNF-α or deletion of both TNF receptors (TNFRs) results in significantly reduced levels of plasma PAI-1 antigen, plasma insulin, and adipose tissue PAI-1 and TGF-β mRNAs. Studies in which exogenous TNF-α was infused into lean mice lacking individual TNFRs indicate that TNF-α signaling of PAI-1 in adipose tissue can be mediated by either the p55 or the p75 TNFR. However, TNF-α signaling of TGF-β mRNA expression in adipose tissue is mediated exclusively via the p55 TNFR. Our results suggest that TNF-α is a common link between the insulin resistance and elevated PAI-1 and TGF-β in obesity. The chronic elevation of TNF-α in obesity thus may directly promote the development of the complex cardiovascular risk profile associated with this condition.
Obesity is rapidly increasing in Western societies and with it there is a steadily increasing risk for obesity-related disorders (syndrome X; refs. 1 and 2), including cardiovascular disease (3, 4). In spite of the magnitude and cost of this problem, the molecular changes in obesity that promote these conditions remain to be defined. Tumor necrosis factor α (TNF-α) is chronically elevated in adipose tissues of obese rodents and humans (5–8) and may represent an important link between obesity and insulin resistance (5, 9, 10). However, it is not yet clear whether this cytokine also contributes to other obesity-related pathologies. Experimental support for this possibility would help to clarify the role of TNF-α in the various metabolic and cardiovascular disorders associated with obesity.
The increased incidence of cardiovascular disease (3, 4) may be related to the elevated levels of hemostatic factors like fibrinogen, factor VII, and plasminogen activator inhibitor 1 (PAI-1) observed in the plasma of obese patients (11–14). PAI-1 is the primary inhibitor of plasminogen activation in vivo, and increased plasma PAI-1 compromises normal fibrin clearance mechanisms and promotes thrombosis (15, 16), including myocardial infarction (16, 17). Although PAI-1 is dramatically up-regulated in human obesity (11–14), little is known about the origin of this plasma inhibitor in obesity or about the signals that control its biosynthesis. Recent evidence suggests that the adipose tissue itself may contribute to the elevated expression of PAI-1 in obesity. For example, relatively high levels of PAI-1 mRNA were detected in murine adipose tissue (18), and clinical studies of obese patients demonstrate that plasma PAI-1 levels decrease after weight loss because of surgery or dietary intervention (2, 3, 19–21). Moreover, in genetically obese (ob/ob) mice, adipocyte PAI-1 expression and plasma PAI-1 levels are significantly elevated (22). Significant expression of PAI-1 mRNA also has been demonstrated in cultured 3T3-L1 adipocytes (23), the visceral and s.c. fat of obese rats (24), and adipose tissues from human subjects (25).
Several observations implicate TNF-α in the increased expression of PAI-1 in obese adipose tissue. TNF-α expression is elevated in adipose tissue of both rodents and humans (5–8). Additionally, TNF-α stimulates PAI-1 biosynthesis by a variety of cells, including 3T3-L1 adipocytes (16, 18, 23). Intraperitoneal administration of TNF-α into lean mice increases PAI-1 mRNA in the adipose tissue and does so in a pattern of cellular expression that is similar to that observed in obese mice (22, 23). Recent studies show that human adipose tissue explants respond to exogenous TNF-α with increased PAI-1 expression, and that the addition of pentoxifylline (a methylxanthine known to inhibit TNF-α) decreases basal and TNF-α-induced PAI-1 levels (26). Interestingly, insulin (22) and transforming growth factor β (TGF-β) (27) also have been implicated in the increased expression of PAI-1 in obesity, and TNF-α contributes to the increase in both of these mediators (9, 10, 27). These observations support the hypothesis that the chronic elevation of TNF-α in obesity (7, 8) may stimulate PAI-1 and TGF-β biosynthesis in the adipose tissue and contribute to the elevated levels of plasma PAI-1 observed in obese humans and rodents. In this study, we directly address this hypothesis. We show that inhibition of TNF-α in vivo by infusion of neutralizing antibodies, or deletion of both the p55 and p75 TNF-α receptors (TNFRs), leads to a 50% decrease in plasma PAI-1 antigen and an 80–85% reduction of adipose tissue PAI-1 and TGF-β mRNAs in ob/ob mice. These results directly implicate TNF-α in the elevated PAI-1 and TGF-β gene expression previously observed in the adipose tissue of obese mice. Although TNF-α also appears to play a significant role in the elevated plasma PAI-1 associated with murine obesity, the failure to decrease plasma PAI-1 by more than 50% in these experiments suggests that other mechanisms are involved as well.
MATERIALS AND METHODS
Animals.
Adult male obese mice (C57BL/6J ob/ob) and their lean counterparts (C57BL/6J +/?) were obtained from The Jackson Laboratory. ob/ob mice deficient in both TNFRs (p55−/− p75−/−) were generated by crossing and back-crossing lean mice deficient in these receptors to ob/ob mice as described (28) and genotyped by using PCR-based assays (28). In some experiments, obese mice were injected i.p. with either 25 μg of the IgG fraction of a neutralizing hamster mAb to mouse TNFα (Genzyme) or with equivalent amounts of normal hamster IgG as a control. Six hours later, the mice were anesthetized by inhalation of metofane (Pitman–Moore, Mundelein, IL), their blood was collected into 20 mM (final concentration) EDTA, pH 8.0, and their adipose tissues were rapidly removed and immediately frozen in liquid nitrogen for preparation of total RNA (23). In other experiments, wild-type lean mice (C57BL/6J), as well as lean mice lacking p55 TNFR, p75 TNFR, or both, were injected i.p. with recombinant murine TNF-α, 4 μg per mouse in 100 μl of sterile saline (kind gift of Richard Ulevitch, The Scripps Research Institute). Control animals were injected with 100 μl of saline alone. Three hours later, blood was collected and adipose tissues were removed and processed for in situ hybridization (below) or the preparation of total RNA.
Determination of PAI-1, Insulin, and TGF-β Levels in Plasma.
Active PAI-1 antigen in plasma was determined by using the t-PA binding assay as described (29). The results were compared with a standard curve constructed by using recombinant mouse PAI-1. Plasma insulin levels were determined by using a rat insulin ELISA assay from Crystal Chem (Chicago) together with a mouse insulin standard according to the manufacturer’s instructions. Total plasma TGF-β levels were measured by using the PREDICTA TGF-β1 kit from Genzyme, according to the manufacturer’s instructions.
RNA Analysis.
The concentration of PAI-1 and TGF-β mRNAs in tissues was determined by quantitative reverse transcription–PCR by using a competitor cRNA containing upstream and downstream primers for PAI-1, TGF-β, and β-actin (internal control) as described (22, 23). After reverse transcription and PCR amplification in the presence of 32P end-labeled 5′ primers, PCR products were electrophoresed on 1.8% agarose gels. The appropriate bands corresponding to the internal standard cRNA product and the target mRNA product were excised from the gel, and the incorporated radioactivity was quantified by using a scintillation counter. A standard curve for the internal control cRNA was constructed and used to determine the specific activity of the target mRNA as described (22, 23, 27). Variations in sample loading were assessed by direct comparison to β-actin mRNA. In situ hybridization on paraffin-embedded tissue sections (3–5 μ) was performed as described by using 35S-labeled antisense or sense riboprobes (30). Slides were exposed in the dark at 4°C for 4–8 weeks. After developing the slides, they were counterstained with hematoxylin and eosin.
Statistical Analysis.
Statistical comparison of results was performed by using the unpaired Student’s t test.
RESULTS
Changes in PAI-1 Expression in ob/ob Mice After Injection of a Neutralizing Antibody to Mouse TNF-α.
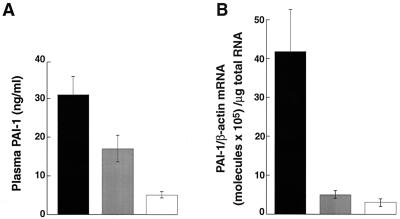
We previously demonstrated that pretreatment of mice with neutralizing antibodies to TNF-α blocked the subsequent induction of PAI-1 (31) and CD14 (32) by endotoxin. Experiments therefore were performed to determine the effects of these neutralizing antibodies on the levels of PAI-1 in the plasma and adipose tissues of obese mice. Fig. 1 shows that obese mice treated with the neutralizing antibody for 6 hr had approximately 45% lower plasma PAI-1 antigen levels than the obese controls injected with normal hamster IgG (Fig. 1A; P < 0.02). The antiserum reduced PAI-1 mRNA in adipose tissues by approximately 90% (P < 0.0001), to levels that approached those in adipose tissues from lean mice (Fig. 1B). These results demonstrate the direct involvement of TNF-α in the elevated plasma and adipose tissue PAI-1 observed in this model of murine obesity.
Figure 1.
Changes in PAI-1 expression in ob/ob mice after injection of a neutralizing antibody to TNF-α. Obese (ob/ob) male mice (four each, 14 weeks old) were injected i.p. with a control hamster IgG (black bars) or with a neutralizing hamster mAb against murine TNF-α (gray bars). Six hours later, the plasma (A) or adipose tissue (B) were collected and analyzed for PAI-1 antigen (A) or mRNA (B) as described in Materials and Methods. The level of PAI-1 antigen in plasma (A) or PAI-1 mRNA (B) in age-matched lean mice (n = 4) also is shown (empty bars). Error bars represent mean ± SD.
Effect of Deletion of TNFRs on PAI-1 Expression in ob/ob Mice.
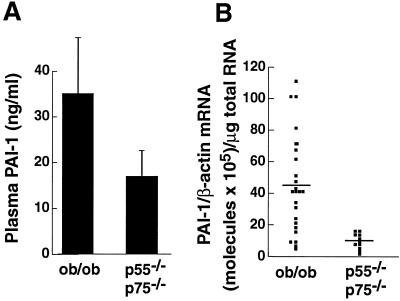
The potential role of TNF-α in the elevated PAI-1 levels in obesity was further examined in mice that lacked both TNFRs (p55 and p75) and thus could not respond to endogenous TNF-α. When compared with normal ob/ob mice, the receptor-deficient obese mice had significantly reduced levels of PAI-1 antigen in the plasma (52% reduction; P < 0.01; Fig. 2A) and PAI-1 mRNA in the adipose tissues (78% reduction; P < 0.0009; Fig. 2B). These results are comparable to the results obtained in the antibody experiments described above and thus prove the hypothesis that TNF-α plays a key role in the elevated expression of plasma and adipose tissue PAI-1 observed in this model.
Figure 2.
Effect of deletion of both TNFRs on PAI-1 expression in ob/ob mice. Plasma and adipose tissue were collected from 20- to 24-week-old male and female ob/ob mice and ob/ob mice lacking both TNFRs (p55−/−, p75−/−). Plasma PAI-1 antigen (A) or adipose tissue PAI-1 mRNA (B) were analyzed as described in Materials and Methods. (A) n = 6 for each group; bars represent mean ± SD. Comparison of ob/ob vs. ob/ob p55−/−, p75−/−: P < 0.01. (B) Error bars represent the mean for each group. Comparison of ob/ob vs. ob/ob p55−/−, p75−/−: P < 0.0009.
Effect of Deletion of TNFRs on Plasma Insulin and Adipose Tissue TGF-β Levels in ob/ob Mice.
TNF-α has been implicated in the compensatory hyperinsulinemia (9, 10) and elevated TGF-β (27) that accompany obesity. To directly investigate the role of TNF-α in the regulation of these mediators in vivo, we measured plasma insulin and adipose tissue TGF-β mRNA levels in ob/ob mice lacking TNFRs. Plasma insulin levels were reduced significantly (P < 0.02) in ob/ob mice lacking both TNFRs when compared with control ob/ob mice (Table 1), in agreement with previous reports (28, 33). TGF-β gene expression in the adipose tissue also was significantly decreased (i.e., by over 80%; P < 0.01) in male mice lacking both TNFRs. There also was a trend toward decreased total TGF-β antigen levels in the plasma of ob/ob mice lacking both receptors (4.6 ± 2.4 ng/ml) compared with wild-type ob/ob mice (13.7 ± 6.9 ng/ml). However, this difference was not significant (P < 0.08) probably because of the small number of animals used (n = 6). Thus, elevated endogenous TNF-α contributes to the observed elevation in plasma insulin and adipose tissue TGF-β mRNA in obese mice.
Table 1.
Mice lacking both TNFRs have reduced levels of plasma insulin antigen and adipose tissue TGF-β mRNA
| Determination | ob/ob mice | TNFR-deficient ob/ob mice, p55−/−, p75−/− | Lean mice |
|---|---|---|---|
| Insulin antigen | 47 ± 12* | 20 ± 12 | 5 ± 1.4 |
| (P < 0.02) | |||
| TGF-β mRNA | 3.4 × 105 ± 2 × 105† | 0.45 × 105 ± 0.3 × 105 | 1.5 × 105 ± 0.8 × 105 |
| (P < 0.01) |
n = 6 ± SD.
ng/ml as determined by using a commercially available ELISA assay.
Molecules of TGF-β mRNA/μg total RNA.
Effect of Exogenous TNF-α Treatment on PAI-1 Expression in Wild-Type and TNFR-Deficient Lean Mice.
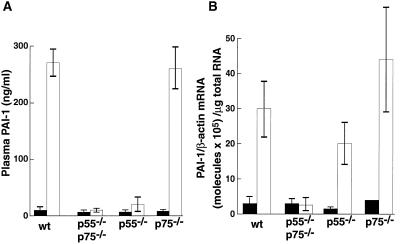
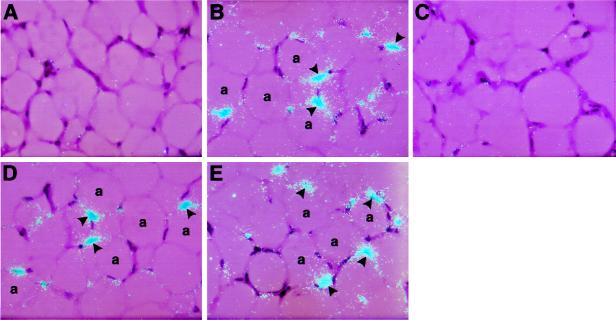
To begin to investigate the role of p55 TNFR and p75 TNFR in TNF-α-induced elevation of PAI-1, we determined plasma PAI-1 antigen and adipose tissue PAI-1 mRNA expression after injection of TNF-α (4 μg/mouse) into wild-type and TNFR-deficient lean mice. PAI-1 antigen in the plasma of the wild-type mice was induced approximately 30-fold (P < 0.000001) by TNF-α treatment, and this response was not observed in mice lacking both receptors (Fig. 3A). This lack of response in the receptor-deficient animals seemed to be primarily caused by the absence of p55 TNFR because mice lacking p75 TNFR appeared to have a normal response. Moreover, induction in the p55 TNFR-deficient mice, while significant (P < 0.04), was only about 10% of that of the wild-type mice. In the adipose tissue, PAI-1 mRNA levels were induced approximately 10-fold in the wild-type lean mice (Fig. 3B; P < 0.009), and this response again was abolished in the mice lacking both receptors. In contrast to the plasma results (Fig. 3A), the induction of PAI-1 mRNA in the adipose tissue of mice lacking either the p75 or p55 TNFR appeared to be relatively normal (P < 0.004 and P < 0.004, respectively). This observation suggests that TNF-α signaling of PAI-1 mRNA in the adipose tissue can be mediated by both receptors. These results were confirmed by in situ hybridization (Fig. 4). For example, no signal for PAI-1 mRNA was detected in adipose tissues from untreated mice (Fig. 4A). However, after TNF-α treatment, a strong hybridization signal was apparent in adipocytes in the wild-type lean mice (Fig. 4B), but not in the TNF-α-treated mice lacking both receptors (Fig. 4C). A positive signal also was observed in lean mice lacking either p55 (Fig. 4D) or p75 (Fig. 4E) TNFR. Taken together, these results suggest that in the adipose tissue signaling in response to exogenously administered TNF-α can be mediated by either the p55 or p75 TNFR.
Figure 3.
Effect of exogenous TNF-α on PAI-1 expression in lean wild-type and TNFR-deficient lean mice. Lean mice or TNFR-deficient lean mice (four each, 20–24 weeks old) were injected i.p. with 4 μg of murine recombinant TNF-α (open bars) or saline (filled bars). Three hours later, the plasma (A) or adipose tissue (B) were collected and analyzed for PAI-1 antigen (A) or mRNA (B) as described in Materials and Methods. Error bars represent mean ± SD.
Figure 4.
Effect of TNF-α on the cellular localization of PAI-1 mRNA in wild-type and TNFR-deficient mice. In situ hybridization was performed on paraffin sections of adipose tissues from wild-type untreated mice (A) and TNF-α-treated wild-type (B), p55,p75 TNF-deficient (C), p55 TNFR-deficient (D), and p75 TNFR-deficient (E) mice. Slides were exposed for 4 weeks at 4°C and then stained with hematoxylin and eosin. a, adipocytes. Arrowheads indicate positive signals for PAI-1 mRNA in the fat (B, D, and E). Magnification: ×400 for all sections.
Effect of Exogenous TNF-α Treatment on TGF-β Expression in Wild-Type and TNFR-Deficient Lean Mice.
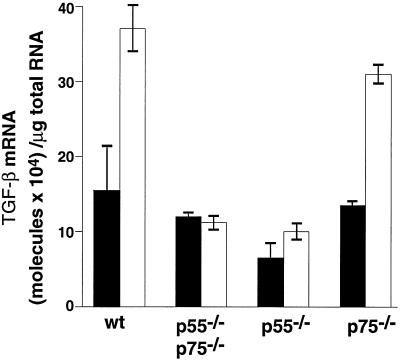
We previously demonstrated that exogenous i.p. injection of TNF-α (4 μg/mouse) increased TGF-β mRNA expression in adipose tissue of normal CB6 mice (27). Here we determined the role of the p55 and p75 TNFRs in this induction. TGF-β mRNA levels were significantly induced by TNF-α treatment in adipose tissues from wild-type lean mice (P < 0.03, Fig. 5), and this response was abolished in mice lacking both receptors. The response to TNF-α in the p75 TNFR-deficient mice appeared to be relatively normal (P < 0.004), whereas the p55 TNFR-deficient animals showed no response at all. These results suggest that the predominant mode of signaling by TNF-α for TGF-β expression in the adipose tissue is through the p55 TNFR.
Figure 5.
Effect of exogenous TNF-α treatment on TGF-β mRNA expression in lean wild-type and lean TNFR-deficient mice. Lean wild-type mice or TNFR-deficient lean mice (20–24 weeks old) were injected i.p. with 4 μg of murine recombinant TNF-α (open bars) or saline (filled bars). Three hours later, adipose tissues were collected and analyzed for TGF-β mRNA levels as described in Materials and Methods. n = 3, error bars represent ± SD.
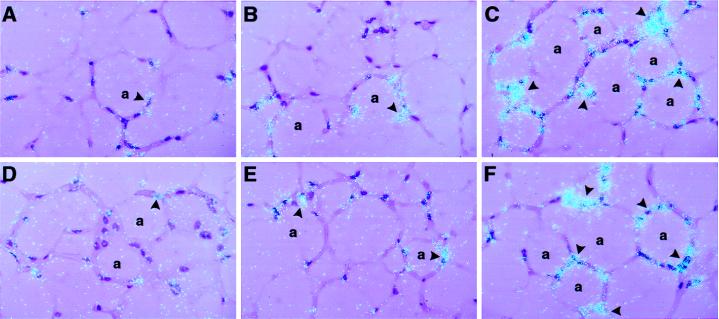
These results also were confirmed by in situ hybridization analysis (Fig. 6). A weak, but consistent, signal for TGF-β mRNA was detected in multiple cell types in the fat from untreated mice (Fig. 6 A and B). This signal was increased after TNF-α treatment of the wild-type mice (Fig. 6C) and mice lacking only the p75 TNFR (Fig. 6F). However, the signal for TGF-β mRNA was not increased after treatment of mice lacking either both TNFRs (Fig. 6D) or the p55 TNFR (Fig. 6E).
Figure 6.
Effect of TNF-α on the cellular localization of TGF-β mRNA in wild-type and TNFR-deficient mice. In situ hybridization was performed on paraffin sections of adipose tissues from wild-type untreated mice (A and B) and TNF-α-treated wild-type (C), p55,p75 TNFR-deficient (D), p55 TNFR-deficient (E), and p75 TNFR-deficient (E) mice. Slides were exposed for 8 weeks at 4°C and stained with hematoxylin and eosin. a, adipocytes. Arrowheads indicate positive signals for TGF-β mRNA in the fat. Magnification: ×400 for all sections.
In spite of the results shown in Figs. 5 and 6, we were unable to demonstrate significant increases in plasma TGF-β in response to TNF-α (not shown). Thus, the increased TGF-β mRNA level in adipose tissue is not reflected as increased TGF-β antigen in the plasma, suggesting that either additional posttranscriptional mechanisms are involved or that TGF-β in the fat may play a more local autocrine or paracrine function.
DISCUSSION
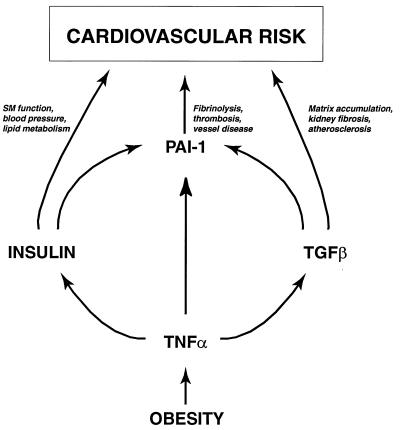
Obesity is a complex metabolic disorder that often is associated with insulin resistance and hyperinsulinemia as well as with accelerated atherosclerosis, hyperlipidemia, hypertension, elevated PAI-1, and elevated TGF-β (2, 3, 11–14, 27). The fact that these changes frequently occur in obesity (syndrome X, ref. 1) suggests a common etiology. Although several observations indicate that TNF-α is elevated in obesity and contributes to the insulin-resistant state (5–8, 10), the role of this cytokine in other pathologies of obesity is largely unknown. In this study, we demonstrate that obese mice treated with neutralizing antibodies to TNF-α, or lacking both the p55 and p75 TNFRs, not only have increased insulin sensitivity as reflected by reduced levels of plasma insulin (Table 1) (28, 33), but also have significantly reduced levels of plasma PAI-1 antigen and adipose tissue PAI-1 and TGF-β mRNAs (Figs. 1 and 2; Table 1). These observations provide direct evidence that TNF-α is a common link between obesity, insulin resistance/hyperinsulinemia, PAI-1, and TGF-β, and thus establish a central role for TNF-α in a number of the metabolic disorders associated with obesity (Fig. 7). The recent demonstration by other investigators (34) that TNF-α also regulates plasma triglyceride levels and glucose homeostasis further supports this conclusion. Taken together, these observations raise the possibility that this cytokine may be a useful target for pharmacological interventions designed to treat the complex cardiovascular risk profile associated with this condition.
Figure 7.
TNF-α and cardiovascular disease in obesity. TNF-α is chronically elevated in adipose tissue in obesity and contributes to the insulin resistance/hyperinsulinemia and elevated TGF-β associated with this condition. TNF-α, insulin, and TGF-β may, in turn, lead to elevated PAI-1, tissue factor, and possibly other hemostatic genes that may promote cardiovascular risk.
The biological responses of TNF-α are mediated through its two receptors, and these receptors are expressed by most nucleated cells, including adipocytes (28, 33, 35–37). The p55 TNFR elicits a variety of proinflammatory cellular responses and appears to mediate many of the biological effects of TNF-α (38–42). Although p75 TNFR also signals in response to TNF-α, it appears to be involved in a more limited number of cellular responses, including proliferation/cell viability and cytokine production (43–45). Experiments therefore were performed to define the role played by each of these receptors in the elevation of plasma PAI-1 antigen and adipose tissue PAI-1 and TGF-β mRNAs. Relatively normal induction of plasma PAI-1 antigen (Fig. 3A), adipose tissue (Fig. 3B), and adipocyte (Fig. 4 D and E) PAI-1 mRNAs were observed when exogenous TNF-α was administered to lean mice lacking p75 TNFR. Unexpectedly, significant induction of plasma PAI-1 antigen (i.e., 2.5-fold) and adipose tissue PAI-1 mRNA (8- to 10-fold) also was observed when TNF-α was administered to the p55-deficient mice (Figs. 3 and 4). These results indicate that in the absence of the p55 receptor, TNF-α can induce PAI-1 through p75 TNFR. Thus, although p55 TNFR and p75 TNFR are unrelated proteins and therefore presumably control different intracellular signaling events (37), they both have the potential to regulate PAI-1 in the adipose tissue. These considerations suggest that the two pathways share common elements for the regulation of PAI-1 by exogenous TNF-α. The involvement of both receptors in the TNF-α-mediated regulation of PAI-1 in the adipose tissue is unusual because single receptors seem to mediate the effects of TNF-α reported for other systems (43–45). In this regard, the induction of TGF-β mRNA by TNF-α in adipose tissue also appears to be mediated by a single receptor, p55 TNFR (Fig. 6). Finally, it should be emphasized that these results with lean mice may not apply to obese mice. Obviously, analysis of PAI-1 expression in ob/ob mice lacking either the p55 TNFR or p75 TNFR is necessary to conclusively determine the role of each receptor in the regulation of PAI-1 in these animals.
The mechanism(s) by which neutralization of TNF-α or deletion of its receptors leads to reduced PAI-1 may be complex. TNF-α appears to directly induce PAI-1 gene expression in a variety of cells and tissues, including the adipose tissue. However, TNF-α also leads to elevated plasma insulin and elevated TGF-β mRNA in the adipose tissue of obese mice, and both insulin and TGF-β have been shown to independently increase PAI-1 expression in the plasma and adipose tissue (22, 23, 27). Thus, it is possible that the observed decrease in PAI-1 in ob/ob mice lacking both receptors or treated with the neutralizing antibodies may reflect not only the absence of TNF-α but also the decreased insulin and TGF-β associated with this condition (Table 1). Additional experiments need to be performed to resolve this issue. Finally, and importantly, it should be noted that whereas adipose tissue expression of PAI-1 and TGF-β mRNAs was reduced by 75–90% in mice lacking TNF-α or a TNF-α signaling system (Figs. 1 and 2; Table 1), the PAI-1 antigen in the plasma was reduced only by 45–50%. We considered the possibility that the apparent discrepancy between the partial decrease in plasma PAI-1 and the quasitotal decrease in adipose tissue PAI-1 mRNA (Fig. 1) in anti-TNF-α-treated ob/ob mice may be a kinetic “artifact.” However, plasma PAI-1 levels were still only decreased by 50% at later times (i.e., 24 hr; data not shown). This observation, together with the very short half-life of plasma PAI-1 (10–15 min), argues that the difference between plasma PAI-1 antigen and adipose tissue PAI-1 mRNA is not a kinetic effect. Rather, this apparent discrepancy probably reflects the fact that the origin of plasma PAI-1 antigen is a complex process. For example, multiple tissues besides adipose tissue probably contribute to the final plasma PAI-1 levels. Moreover, changes in the rate of PAI-1 clearance, and mediators other than TNF-α, may be involved in the regulation of plasma PAI-1 levels. In this regard, the levels of plasma PAI-1 antigen and adipose tissue PAI-1 mRNA in lean mice lacking both TNFRs were not significantly different from those in wild-type lean mice (Fig. 3 A and B). Thus, constitutive synthesis of PAI-1 in lean adipose tissue does not appear to be mediated by TNF-α. Therefore, it is also possible that part of the increase in plasma PAI-1 observed in ob/ob mice may be the result of increased constitutive (i.e., not induced by TNF-α) production of PAI-1 by the adipose tissues, because in obese animals, the size and number of adipocytes, and thus the amount of adipose tissue mass, typically increases several-fold.
In summary (Fig. 7), we have conclusively demonstrated a key role for TNF-α in the elevated expression of PAI-1 in obese mice. We also have shown that TNF-α contributes to the elevated plasma insulin and adipose tissue TGF-β expression in obesity (Table 1). The fact that insulin and TGF-β are mediators with broad biological effects implies that the initial elevation of TNF-α in obesity is rapidly amplified through the activation of these and perhaps other genes. There are a number of potential pathophysiological consequences of these changes. For example, elevations in TGF-β not only stimulate PAI-1, but also induce adipose tissue expression of tissue factor, the primary initiator of the coagulation cascade (46). In addition, TGF-β has been implicated in atherosclerosis (47, 48) and fibrotic kidney disease (49, 50), two common complications of obesity. Insulin itself has been reported to stimulate PAI-1 (reviewed in ref. 51), and clearly also influences smooth muscle function, blood pressure/hypertension, and lipid metabolism, all common features of obesity. These considerations suggest that chronic elevation of TNF-α in obesity initiates a cascade of events that directly contribute to the cardiovascular risk associated with this condition. The identification of the signals responsible for the increased TNF-α will aid in our understanding of the molecular basis of the abnormal expression of PAI-1 and other genes in obesity.
Acknowledgments
We thank Dr. J. Peschon (Immunex Research and Development Corp.) for the TNFR-deficient mice. We also thank T. Thinnes for technical assistance, Laurence Hervio for assistance with the graphics, and M. McRae for her expert secretarial skills. This work was supported in part by grants from the National Institutes of Health (HL 47819) and Novartis to D.J.L. and in part by Grant DK 52539-01A1 from the National Institutes of Health to G.S.H. This is Scripps Research Institute Manuscript No. 11248-VB.
ABBREVIATIONS
- PAI-1
plasminogen activator inhibitor 1
- TGF-β
transforming growth factor β
- TNF-α
tumor necrosis factor α
- TNFR
TNF receptor
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Hjermann I. J Cardiovasc Pharmacol. 1992;8:S5–S10. doi: 10.1097/00005344-199200208-00002. [DOI] [PubMed] [Google Scholar]
- 2.Finer N. Br Med Bull. 1997;53:229–450. doi: 10.1093/oxfordjournals.bmb.a011620. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen S, Jensen M D. Curr Opin Lipidol. 1997;8:200–204. doi: 10.1097/00041433-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Björntorp P. Ann Med. 1992;24:15–18. doi: 10.3109/07853899209164140. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil G S, Shargill N S, Spiegelman B M. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann C, Lorenz K, Braithwaite S S, Colca J R, Palazuk B J, Hotamisligil G S, Spiegelman B M. Endocrinology. 1994;134:264–270. doi: 10.1210/endo.134.1.8275942. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil G S, Arner P, Caro J F, Atkinson R L, Spiegelman B M. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kern P A, Saghizadeh M, Ong J M, Bosch R J, Deem R, Simsolo R B. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi J K, Hotamisligil G S. Semin Cell Dev Biol. 1999;10:19–29. doi: 10.1006/scdb.1998.0273. [DOI] [PubMed] [Google Scholar]
- 10.Peraldi P, Spiegelman B. Mol Cell Biochem. 1998;182:169–175. [PubMed] [Google Scholar]
- 11.McGill J B, Schneider D J, Arfken C L, Lucore C L, Sobel B E. Diabetes. 1994;43:104–109. doi: 10.2337/diab.43.1.104. [DOI] [PubMed] [Google Scholar]
- 12.Potter van Loon B J, Kluft C, Radder J K, Blankenstein M A, Meinders A E. Metabolism. 1993;42:945–949. doi: 10.1016/0026-0495(93)90005-9. [DOI] [PubMed] [Google Scholar]
- 13.Legnani C, Maccaferri M, Tonini P, Cassio A, Cacciari E, Coccheri S. Fibrinolysis. 1988;2:211–214. [Google Scholar]
- 14.Vague P, Juhan-Vague I, Chabert V, Alessi M C, Atlan C. Metabolism. 1989;38:913–915. doi: 10.1016/0026-0495(89)90241-2. [DOI] [PubMed] [Google Scholar]
- 15.Sprengers E D, Kluft C. Blood. 1987;69:381–387. [PubMed] [Google Scholar]
- 16.Fearns C, Samad F, Loskutoff D J. In: Synthesis and Localization of PAI-1 in the Vessel Wall. Van Hinsbergh V W M, editor. Amsterdam, The Netherlands: Harwood; 1995. pp. 207–226. [Google Scholar]
- 17.Carmeliet P, Collen D. Curr Opin Lipidol. 1997;8:118–125. doi: 10.1097/00041433-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Sawdey M S, Loskutoff D J. J Clin Invest. 1991;88:1346–1353. doi: 10.1172/JCI115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Primrose J N, Davies J A, Prentice C R M, Hughes R, Johnston D. Thromb Haemostasis. 1992;68:396–399. [PubMed] [Google Scholar]
- 20.Juhan-Vague I, Alessi M C. Thromb Haemostasis. 1997;78:656–660. [PubMed] [Google Scholar]
- 21.Calles-Escandon J, Ballor D, Harvey-Berino J, Ades P, Tracy R, Sobel B. Am J Clin Nutr. 1996;64:7–11. doi: 10.1093/ajcn/64.1.7. [DOI] [PubMed] [Google Scholar]
- 22.Samad F, Loskutoff D J. Mol Med. 1996;2:568–582. [PMC free article] [PubMed] [Google Scholar]
- 23.Samad F, Yamamoto K, Loskutoff D J. J Clin Invest. 1996;97:37–46. doi: 10.1172/JCI118404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, et al. Nat Med. 1996;2:800–803. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 25.Alessi M C, Peiretti F, Morange P, Henry M, Nalbone G, Juhan-Vague I. Diabetes. 1997;46:860–867. doi: 10.2337/diab.46.5.860. [DOI] [PubMed] [Google Scholar]
- 26.Cigolini M, Tonoli M, Borgato L, Frigotto L, Manzato F, Zeminian S, Cardinale C, Camin M, Chiaramonte E, De Sandre G, Lunardi C. Atherosclerosis. 1999;143:81–90. doi: 10.1016/s0021-9150(98)00281-0. [DOI] [PubMed] [Google Scholar]
- 27.Samad F, Yamamoto K, Pandey M, Loskutoff D. Mol Med. 1997;3:37–48. [PMC free article] [PubMed] [Google Scholar]
- 28.Uysal K T, Wiesbrock S M, Marino M W, Hotamisligil G S. Nature (London) 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 29.Schleef R R, Sinha M, Loskutoff D J. J Lab Clin Med. 1985;106:408–415. [PubMed] [Google Scholar]
- 30.Keeton M, Eguchi Y, Sawdey M, Ahn C, Loskutoff D J. Am J Pathol. 1993;142:59–70. [PMC free article] [PubMed] [Google Scholar]
- 31.Fearns C, Loskutoff D J. Am J Pathol. 1997;150:579–590. [PMC free article] [PubMed] [Google Scholar]
- 32.Fearns C, Loskutoff D J. Infect Immun. 1997;65:4822–4831. doi: 10.1128/iai.65.11.4822-4831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uysal K T, Wiesbrock S M, Hotamisligil G S. Endocrinology. 1998;139:4832–4838. doi: 10.1210/endo.139.12.6337. [DOI] [PubMed] [Google Scholar]
- 34.Ventre J, Doebber T, Wu M, MacNaul K, Stevens K, Pasparakis M, Kollias G, Moller D E. Diabetes. 1997;46:1526–1531. doi: 10.2337/diab.46.9.1526. [DOI] [PubMed] [Google Scholar]
- 35.Vandenabeele P, Declercq W, Beyaert R, Fiers W. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- 36.Bazzoni F, Beutler B. J Inflam. 1995;45:221–238. [PubMed] [Google Scholar]
- 37.Tartaglia L A, Weber R F, Figari I S, Reynolds C, Palladino M A, Jr, Goeddel D V. Proc Natl Acad Sci USA. 1991;88:9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tartaglia L A, Rothe M, Hu Y F, Goeddel D V. Cell. 1993;73:213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- 39.Tartaglia L A, Ayres T M, Wong G H, Goeddel D V. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 40.Tartaglia L A, Goeddel D V. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 41.Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. J Exp Med. 1993;177:1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong G H, Tartaglia L A, Lee M S, Goeddel D V. J Immunol. 1992;149:3350–3353. [PubMed] [Google Scholar]
- 43.Tartaglia L A, Goeddel D V, Reynolds C, Figari I S, Weber R F, Fendly B M, Palladino M A., Jr J Immunol. 1993;151:4637–4641. [PubMed] [Google Scholar]
- 44.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Nature (London) 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 45.Vandenabeele P, Declercq W, Vercammen D, Van de Craen M, Grooten J, Loetscher H, Brockhaus M, Lesslauer W, Fiers W. J Exp Med. 1992;176:1015–1024. doi: 10.1084/jem.176.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samad F, Pandey M, Loskutoff D J. Proc Natl Acad Sci USA. 1998;95:7591–7596. doi: 10.1073/pnas.95.13.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X L, Liu S X, Wilcken D E L. Cardiovasc Res. 1997;34:404–410. doi: 10.1016/s0008-6363(97)00033-3. [DOI] [PubMed] [Google Scholar]
- 48.Nabel E G, Shum L, Pompili V J, Yang Z-Y, San H, Shu H B, Liptay S, Gold L, Gordon D, Derynck R, Nabel G J. Proc Natl Acad Sci USA. 1993;90:10759–10763. doi: 10.1073/pnas.90.22.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeiffer A, Schatz H. Exp Clin Endocrinol Diabetes. 1995;103:7–14. doi: 10.1055/s-0029-1211323. [DOI] [PubMed] [Google Scholar]
- 50.Border W A. Curr Opin Nephrol Hypertens. 1994;3:54–58. doi: 10.1097/00041552-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Loskutoff D J, Samad F. Arterioscler Thromb Vasc Biol. 1998;18:1–6. doi: 10.1161/01.atv.18.1.1. [DOI] [PubMed] [Google Scholar]