Abstract
Chemotherapeutic regimes are typically limited by nonspecific toxicity. To address this problem we have developed a broadly applicable drug-masking chemistry that operates in conjunction with a unique broad-scope catalytic antibody. This masking chemistry is applicable to a wide range of drugs because it is compatible with virtually any heteroatom. We demonstrate that generic drug-masking groups may be selectively removed by sequential retro-aldol–retro-Michael reactions catalyzed by antibody 38C2. This reaction cascade is not catalyzed by any known natural enzyme. Application of this masking chemistry to the anticancer drugs doxorubicin and camptothecin produced prodrugs with substantially reduced toxicity. These prodrugs are selectively unmasked by the catalytic antibody when it is applied at therapeutically relevant concentrations. We have demonstrated the efficacy of this approach by using human colon and prostate cancer cell lines. The antibody demonstrated a long in vivo half-life after administration to mice. Based on these findings, we believe that the system described here has the potential to become a key tool in selective chemotherapeutic strategies.
The specific elimination of virus-infected or cancer cells with potent chemotherapeutic regimes is limited by nonspecific toxicity that results from an inability to direct these agents to their appropriate targets. The development of strategies that provide for selective chemotherapy presents significant multidisciplinary challenges. Selective chemotherapy might, in the case of cancer, be based on the enzymatic activation of a prodrug at the tumor site. Unless the tumor displays a specific enzymatic activity that can be used for prodrug activation (1), the enzymatic activity must be directed to the site with a targeting molecule, usually an antibody, that recognizes a cell-surface molecule selectively expressed at the tumor site. Because a single molecule of enzyme catalyzes the activation of many molecules of prodrug, a localized and high concentration of drug may be maintained at the tumor site. This concept of antibody-directed enzyme prodrug therapy (ADEPT) holds promise as a general and selective chemotherapeutic strategy if several specific criteria can be met (for a recent review, cf. ref. 2).
A number of antigens that are expressed on the surface of tumor cells or in their supporting vasculature have been shown to be effective targets for antibody-mediated cancer therapy. Thus, for the most part, the targeting antibody component of this strategy is not limiting. By contrast, the requirements of the enzyme component and complementary prodrug chemistries for ADEPT are difficult to achieve. First, selective prodrug activation requires the catalysis of a reaction that must not be accomplished by endogenous enzymes in the blood or normal tissue of the patient. Enzymes of nonhuman origin that meet these needs are, however, likely to be highly immunogenic, a fact that makes repeated administration impossible. Also, the chemistry used to convert a drug into a prodrug should be versatile enough to allow for the modification of many drug classes while not interfering with the operation of the enzyme so that a single enzyme could be used for the activation of a multiplicity of prodrugs. To overcome these limitations, it has been suggested that the enzyme component for ADEPT might be a catalytic antibody (3–5). The potential of catalytic antibodies (6) for ADEPT are indeed compelling; both catalysis of reactions not catalyzed by human enzymes and minimal immunogenicity through antibody humanization are feasible. In this concept, the ADEPT conjugate translates into a bispecific antibody (7) consisting of targeting and catalytic arms.
To satisfy the requirements of ADEPT, we sought to exploit the unusual chemistry of catalytic antibody 38C2. Catalytic antibody 38C2 was generated by using the process of reactive immunization by which the enamine mechanism of natural aldolases was imprinted within the antibody-binding site (8). Through a reactive lysine buried in a hydrophobic pocket at the base of the substrate binding site, antibody 38C2 catalyzes aldol and retro-aldol reactions at rates approaching those of natural aldolases (9). However, unlike natural aldolases or other catalytic antibodies, antibody 38C2 accepts a wide variety of substrates making it a versatile tool for asymmetric synthesis (9, 10). We recognized that the broad scope of antibody 38C2 might be used for the activation of a number of structurally distinct prodrugs, a characteristic that would be advantageous not only for the treatment of a wide range of tumors but also for the treatment of tumors that develop resistance to a particular drug.
Here we develop a prodrug chemistry designed to take advantage of the broad scope and mechanism of catalytic antibody 38C2 and evaluate the potential of this system for ADEPT. Our drug masking/activation concept is based on a sequential retro-aldol–retro-Michael reaction catalyzed by antibody 38C2 (11). This reaction is not known to be catalyzed by any other enzyme and has a very low background, i.e., the reaction is very slow in the absence of the catalyst. We have applied this chemistry to the anticancer drugs doxorubicin and camptothecin. We demonstrate that weakly toxic or nontoxic concentrations of their corresponding prodrugs can be activated by therapeutically relevant concentrations of antibody 38C2 to kill colon and prostate cancer cell lines. To further test the therapeutic relevance of our model system, we show that antibody 38C2 remains catalytically active over weeks after i.v. injection into mice. Based on these findings, we believe that the system described here has the potential to become a key tool in selective chemotherapy.
MATERIALS AND METHODS
Synthesis of Linkers and Prodrugs.
Compound 1. 2-Methylallylmagnesium chloride (0.5 M solution in THF, 22.7 ml, 11.4 mmol) was added dropwise to a stirred solution of 4-hydroxy-2-butanone (500 mg, 5.7 mmol) in THF at −78°C. The mixture was stirred for 10 min, allowed to warm to room temperature, poured over ice, and extracted with ether. The product was purified by column chromatography on silica gel (ethyl acetate/hexane 50:50) to give compound 1 (680 mg, 81%).
1H NMR (400 MHz, CDCl3) δ 4.94 (s, 1H), 4.74 (s, 1H), 3.90 (m, 1H), 3.86 (m, 1H), 2.84 (br, 1H), 2.49 (br, 1H), 2.30 (d, J = 10.4 Hz, 1H), 2.18 (d, J = 10.4 Hz, 1H), 1.83 (s, 3H), 1.81 (m, 1H), 1.69 (m, 1H).
Compound 2.
p-Nitrophenyl chloroformate (432 mg, 3 mmol) was dissolved in 10 ml of methylene chloride and added dropwise to a stirred solution of compound 1 (605 mg, 3.0 mmol) in 20 ml of methylene chloride and 2 ml of triethylamine. The mixture was stirred for 60 min and the solvent was removed under reduced pressure. The product was purified by column chromatography on silica gel (ethyl acetate/hexane 75:25) to give compound 2 (583 mg, 63%).
1H NMR (400 MHz, CDCl3) δ 8.27 (d, J = 7.2 Hz, 1H), 7.38 (d, J = 7.2 Hz, 1H), 4.98 (s, 1H), 4.79 (s, 1H), 4.49 (m, 2H), 2.38 (d, J = 10.8 Hz, 1H), 2.19 (d, J = 10.8 Hz, 1H), 1.97 (m, 2H), 1.86 (s, 3H), 1.27 (s, 3H).
Linker 3.
Compound 2 (309 mg, 1.0 mmol) was dissolved in methylene chloride and osmium tetraoxide (2.5% solution in t-butanol, 1.25 ml, 0.1 mmol), and 4-methylmorpholine N-oxide (50% solution in water, 228 μl, 1.1 mmol) was added. The mixture was stirred for about 1 hr (TLC confirmed the disappearance of all starting material). Lead tetraacetate (490 mg, 1.1 mmol) was added, the mixture was stirred for 5 min, and the solvent was separated from the solids and removed under reduced pressure. The remaining residue was purified by column chromatography over silica gel (ethyl acetate/hexane 70:20) to give linker 3 (305 mg, 98%).
1H NMR (400 MHz, CDCl3) δ 8.27 (d, J = 7.2 Hz, 2H), 7.37 (d, J = 7.2 Hz, 2H), 4.44 (m, 2H), 4.00 (s, 1H), 2.82 (d, J = 14.0 Hz, 1H), 2.58 (d, J = 14.0 Hz, 1H), 2.20 (s, 3H), 1.96 (m, 2H), 1.24 (s, 3H).
Linker 4.
4-Methylaminobutyric acid hydrochloride salt (153.5 mg, 1.0 mmol) was dissolved in 1 ml of methanol and triethylamine (210 μl, 1.5 mmol). Linker 3 (311 mg, 1.0 mmol), dissolved in 1 ml of methanol, was added to the reaction mixture. The mixture was stirred for 16 hr. After removal of the solvent, the product was purified by column chromatography over silica gel (ethyl acetate/acetic acid 97:3) to give linker 4 (285 mg, 98%).
1H NMR (400 MHz, CDCl3) δ 4.21 (t, J = 5.2 Hz, 3H), 3.33 (m, 2H), 2.88 (br, 3H), 2.64 (t, J = 14.0 Hz, 2H), 2.35 (m, 2H), 2.18 (s, 3H), 1.89 (m, 4H), 1.25 (s, 3H).
Prodrug 5.
Doxorubicin hydrochloride (Fluka; 10 mg, 0.017 mmol) was dissolved in 1 ml of dimethylformamide and triethylamine (3.5 μl, 0.025 mmol). Linker 3 (7.8 mg, 0.025 mmol) was dissolved in minimal amount of dimethylformamide and added to the reaction vessel. The mixture was stirred for 16 hr, after which the solvent was removed under reduced pressure. The remaining residue was dissolved in ethylacetate, filtered, and purified by column chromatography over silica gel (ethyl acetate/methanol 90:10) to give prodrug 5 (9.5 mg, 78%). NMR indicates that prodrug 5 exists as mixture of two diastereoisomers. High-resolution mass spectrometry (MH+) calculated for C35H41NO15, 716.2554; observed, 716.2528.
Prodrug 8.
Camptothecin (Aldrich; 35 mg, 0.1 mmol) was suspended in 5 ml of acetonitrile; linker 4 (29 mg, 0.1 mmol) was added followed by diisapropyl carbodiimide (DIPC) (24 μl, 0.15 mmol) and dimethylaminopyridine (DMAP) (24 mg, 0.2 mmol). The mixture was stirred for 16 hr, the solvent was removed, and the product purified by column chromatography over silica gel (ethyl acetate/methanol 95:5) to give prodrug 8 (42 mg, 67%).
NMR indicated that prodrug 8 exists as mixture of two diastereoisomers. HRMS (MH+) calculated for C33H37N3O9, 619.2502; observed, 619.2511.
Antibody Preparation.
The generation and purification of mouse mAb 38C2 was described (8). A stock solution of 15 mg/ml (100 μM) 38C2 IgG in PBS (pH 7.4), stored at 4°C, was used. Antibody 38C2 is commercially available from Aldrich.
Kinetic Analysis.
All antibody reactions were carried out in PBS (pH 7.4) at 37°C in microcentrifuge tubes. Reactions were typically carried out at concentrations between 20 and 200 μM substrate and 5 μM antibody. Kinetic data were derived from Lineweaver–Burk plots. Antibody-catalyzed reactions were monitored at 254 nm by reverse-phase HPLC (Hitachi L-6200A equipped with an AS-2000 autosampler) with a C-18 column (25 cm × 4.6 mm, 5 μm) (micrometer) by using various proportions of acetonitrile/water at 1 ml/min.
Cell Lines.
Human colon carcinoma cell lines HT29 and LIM1215 were kindly provided by Lloyd J. Old (Ludwig Institute for Cancer Research, New York). Human prostate cancer cell line LNCap was purchased from American Type Culture Collection. All cell lines were maintained in RPMI 1640 medium (HyClone) supplemented with 10% fetal bovine serum (Life Technologies, Gaithersburg, MD) and antibiotic-antimycotic reagents (Life Technologies) containing penicillin, streptomycin, and amphotericin B. The cell lines were cultivated in culture flasks at 37°C in a humidifying incubator in an atmosphere of 5% CO2.
Cell Growth Inhibition Assays.
Stock solutions of 5 mM doxorubicin and prodoxorubicin in dimethylformamide, respectively, were stored at 4°C. For cell-growth inhibition assays, 250-μm solutions of doxorubicin and prodoxorubicin in PBS (pH 7.4) were freshly prepared from the 5-mM stock solutions and further diluted in cell culture medium to yield 10 nM–25 μM solutions. Methyl vinyl ketone was purchased from Aldrich. Stock solutions of 1 mM camptothecin and procamptothecin in dimethylformamide, respectively, were stored at 4°C. For cell-growth inhibition assays, 100-μM solutions of camptothecin and procamptothecin in PBS (pH 7.4) were freshly prepared from the 1-mM stock solutions and further diluted in cell culture medium to yield 10 nM–1-μM solutions. Cells grown in culture flasks were trypsinized, washed with PBS, resuspended in cell culture medium, and reduced to a single-cell suspension by passing through an 18 gauge needle. After counting, the cells were plated at a density of 5 × 103 (HT29) or 1 × 104 (LIM1215, LNCap) cells per well in 96-well tissue culture plates and maintained in culture as described above. Drugs were diluted to a final concentration of 10 nM–25 μM in 100 μl of 10% fetal bovine serum in RPMI 1640 and added to the cells 24 hr after plating. For the antibody experiments, prodrug and 38C2 IgG were mixed just before adding to the cells. After drug addition, the cells were maintained at 37°C in 5% CO2 for 3–5 days (72–120 hr). Before the quantitative cell-growth inhibition assay, the cell density was qualitatively analyzed by microscopy. This visual evaluation always matched the result of the quantitative analysis. The cells were then washed with 150 μl of PBS (pH 7.5) and incubated with 100 μl of 9% (vol/vol) Triton X-100 (Sigma) for 45 min at 37°C. The supernatant was then transferred to a 96-well V-shaped plate and centrifuged to remove cell debris. In a 96-well plate, 50 μl of the supernatants were combined with 50 μl of freshly reconstituted substrate reaction mixtures containing 2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyltetrazolium chloride that is converted into a red formazan product in the presence of lactate dehydrogenase released from the cells (12). The substrate reaction mixture was purchased from Promega. The color reaction was incubated for 5–15 minutes at room temperature and then stopped by adding 50 μl of 1 M acetic acid. For quantification, 50-μl aliquots were transferred to another 96-well plate, and the color was read at 490 nm in an ELISA plate reader. The absorbance data were converted to percent cell density, with the untreated controls being 100% and the background of the color reaction being 0%.
In Vivo Activity Assay.
Mice were injected i.v. (tail vein) with 100 μl of 15 mg/ml 38C2 IgG in PBS or the same amount of a control antibody. Blood samples were obtained every 24 hr, and sera were prepared by centrifuging. To a final volume of 100 μl, 5 μl of serum (final concentration 1:20) were mixed with 93 μl of PBS and 2 μl of 5 mM methodol (final concentration 100 μM). Product formation was followed with a fluorescence plate-reader by monitoring at λabs = 330 nm and λem = 452 nm (11). By using softmax pro software (Molecular Devices), specific activities were determined from 2000–5000 s after reaction start. For a standard, specific activities were also derived from defined concentrations of 38C2 IgG. Concentrations in the range of 50–500 nM were in linear proportion with the initial rates and were used to derive the serum concentration of 38C2 IgG from specific activities by linear regression analysis.
RESULTS AND DISCUSSION
Design and Synthesis of a Generic Drug-Masking Trigger.
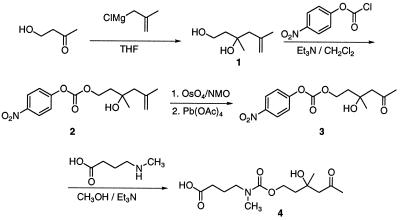
The products of aldol reactions are ketones and aldehydes. Thus, in isolation, aldol chemistry is of little utility for drug masking/activation strategies. To solve this problem, we have based our prodrug strategy (Fig. 1) on a tandem retro-aldol–retro-Michael reaction catalyzed by antibody 38C2. Our finding that antibody 38C2 catalyzes the retro-Michael reaction of β-heterosubstituted ketones and aldehydes to generate free amine, hydroxyl, and thiol groups supports our concept of prodrug activation catalyzed by antibody 38C2. Many drugs contain free amine, hydroxyl, or thiol groups, whose masking often results in a significant decrease of the activity. Because of the high background rate of the retro-Michael reaction, however, retro-Michael substrates themselves are generally not suitable as prodrugs. We reasoned that this problem could be solved with a tandem reaction. In this reaction scheme, the carbon—carbon bond-cleaving retro-aldol reaction is used to reveal a hidden retro-Michael substrate. This allows us to couple the low background reaction inherent in the aldol with the versatile carbon-heteroatom bond cleavage chemistry of the retro-Michael reaction.
Figure 1.
Prodrug activation via a tandem retro-aldol–retro-Michael reaction. X stands for heteroatoms N, O, or S.
By linking the retro-Michael reaction to an upstream retro-aldol reaction in a fluorogenic substrate, where both reactions are catalyzed by antibody 38C2, we have demonstrated significant enhancements in the kcat/kuncat ratio (11). Based on these results, we designed generic heteroatom-masking linkers that are recognized and cleaved by antibody 38C2, leading to free drug release (Fig. 1). The synthesis of linkers 3 and 4 for the masking of amine and hydroxyl groups, respectively, is shown in Fig. 2. Three examples of antibody-mediated prodrug activation have been reported in the literature (3–5). All of these reports use tetrahedral phosphonates as stable transition state analogs for antibody generation. Prodrug activation, i.e., release of the free drug, was achieved by hydrolysis of an ester or a carbamate. Many endogenous enzymes catalyze these types of reactions. Furthermore, immunization with transition-state analogs generates antibodies that are generally highly specific for their designed substrates. Therefore, this approach is usually applicable to the activation of a single prodrug. By contrast, our approach based on an antibody prepared by using reactive immunization is designed to allow for the generic masking/activation of potentially any drug that contains a functionally active heteroatom.
Figure 2.
Synthesis of generic linkers 3 and 4, which can be used to mask functionally active amine and hydroxyl groups of drugs, respectively.
Design and Synthesis of a Doxorubicin Prodrug that Is a Substrate for Antibody 38C2.
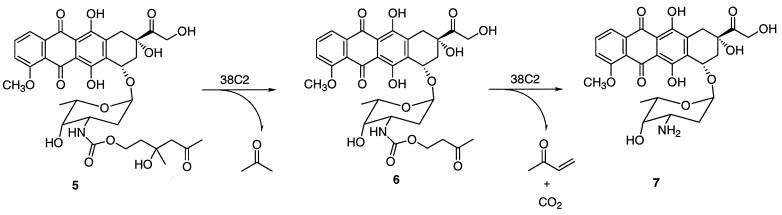
We chose the topoisomerase I and II inhibitor doxorubicin as a model compound for our prodrug activation concept because its structure activity relationship is well characterized and it has been used previously in ADEPT systems (2). Doxorubicin is a drug approved for cancer therapy that suffers from misdirected toxicity. As a candidate prodrug of doxorubicin, subsequently referred to as prodoxorubicin, the carbamate derivative 5 was synthesized in one step from commercially available doxorubicin 7 and linker 3. Antibody 38C2 was found to catalyze both reactions of the retro-aldol–retro-Michael cascade cleavage of prodoxorubicin 5, leading to the release of free doxorubicin 7, acetone, methyl vinyl ketone, and carbon dioxide as byproducts (Fig. 3). We chose a tertiary aldol as the first reaction because no known natural enzyme catalyzes this particular type of retro-aldol reaction. In accordance with its mechanism, catalysis was completely inhibited by 2,4-pentanedione and followed Michaelis–Menten kinetics with kcat = 0.00174 min−1, kcat/kuncat > 105, and Km = 43 μM. Typically, 38C2 is highly enantioselective, however, we observed that under the conditions studied, the reaction proceeded to completion, indicative of loss of enantioselectivity and complete activation of the prodrug. According to a pharmacokinetic analysis of ADEPT systems, low kcat values combined with high Km values are expected to increase the tumor/blood ratio of the free drug, leading to a reduction in peripheral toxicity (13). However, despite our high kcat/kuncat ratio, the relatively low kcat value for the prodoxorubicin activation could be improved by optimization of both the masking linker and the catalytic antibody.
Figure 3.
Doxorubicin prodrug activation via a tandem retro-aldol–retro-Michael reaction catalyzed by antibody 38C2.
Cell Growth Inhibition by Doxorubicin and Prodoxorubicin.
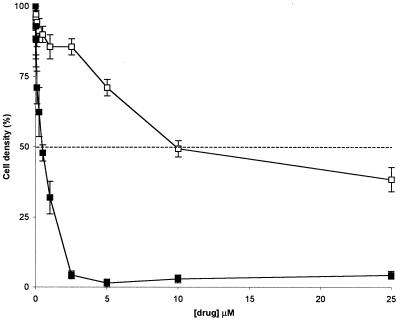
The antiproliferative effects of drug and prodrug were monitored by quantifying the cell growth in the presence of a range of concentrations of doxorubicin and prodoxorubicin, respectively. Three different human cancer cell lines were evaluated, human colon carcinoma cell lines HT29 and LIM1215 and human prostate cancer cell line LNCap. The cells were lysed 72 hr or 120 hr after drug addition, and the activity of the cytoplasmic enzyme lactate dehydrogenase released from the cells was assayed by using a color reaction. The lactate dehydrogenase activity correlated with the cell growth as revealed by microscopic analysis and gave consistent readings as revealed by standard deviations <10% in quadruplicate assays. The inhibition of cell growth of LIM1215 cells 120 hr after addition of doxorubicin and prodoxorubicin is shown (Fig. 4). The results of studies of three cancer cell lines at the two different time points are summarized in Table 1. As expected, prodoxorubicin was significantly less effective at inhibiting cell growth. The ratio between antiproliferative effects of prodoxorubicin and doxorubicin varied from about 10 in the case of HT29 cells to 40 for LNCap cells (Table 1). These ratios are in agreement with comparable anthracycline prodrugs with carbamate linkage (2).
Figure 4.
Growth inhibition of LIM1215 human colon carcinoma cells in vitro by doxorubicin (■) and prodoxorubicin (□)(Bars indicate SD; n = 4). The data were determined by using the assay described in Fig. 5 and are summarized in Table 1. The dashed line indicates 50% decrease in the cell density as compared with the untreated control. Note the reduced capacity of prodoxorubicin for cell growth inhibition.
Table 1.
Growth inhibition of human cancer cells in vitro by doxorubicin and prodoxorubicin
| Time, hr | Treatment | IC50, μM
|
||
|---|---|---|---|---|
| HT29 | LIM1215 | LNCaP | ||
| 72 | doxorubicin | 3.0 | 0.4 | 0.4 |
| prodoxorubicin | >25 | 18 | 18 | |
| 120 | doxorubicin | 2.5 | 0.4 | 0.2 |
| prodoxorubicin | 22 | 10 | 8 | |
Functional Activation of Prodoxorubicin by Antibody 38C2.
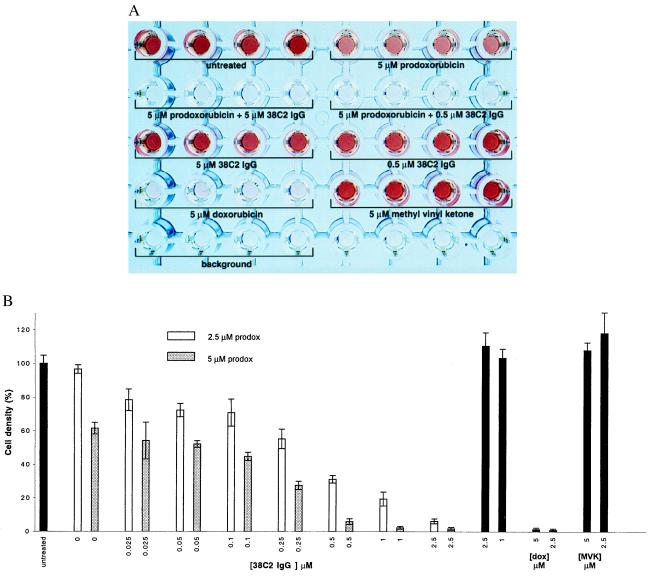
The inhibition of cell growth by a combination of prodoxorubicin and antibody 38C2 was analyzed by using the assay described above (Fig. 5A). This analysis revealed that the combination of prodoxorubicin and antibody 38C2 strongly inhibits cell growth, whereas the same concentration of prodoxorubicin alone is far less potent. Antibody 38C2 alone does not inhibit cell growth. The results of studies of LIM1215 cells 120 hr after drug addition are shown (Fig. 5). Microscopic analysis revealed that no cells survived the combined treatment. The fact that cell growth inhibition is complete for both molar ratios of substrate/antibody of 1:1 and 10:1 indicates the catalytic activation of prodoxorubicin by antibody 38C2 (Fig. 5A). At even higher ratios, i.e., lower antibody concentrations, we found that cell growth inhibition clearly correlates with the antibody concentration (Fig. 5B). A byproduct of the conversion of prodoxorubicin to doxorubicin is methyl vinyl ketone, which is potentially toxic. However, we did not detect inhibition of cell growth by methyl vinyl ketone when it was applied alone at similar concentrations (Fig. 5B). These data demonstrate that antibody 38C2 functionally activates prodoxorubicin, resulting in inhibition of cell growth. It is important to stress that despite the relatively low kcat value for prodoxorubicin activation, submicromolar concentrations of antibody 38C2 were found to be sufficient for effective cell growth inhibition (Fig. 5). This result compares favorably to earlier reports of prodrug activation by catalytic antibodies (3–5).
Figure 5.
Growth inhibition of LIM1215 human colon carcinoma cells by prodoxorubicin in the presence of antibody 38C2. (A) Growth inhibition assay. Cells in quadruplicate wells in a 96-well plate were lysed 120 hr after drug addition, and the activity of the cytoplasmic enzyme lactate dehydrogenase released from the cells was detected by using a colorimetric assay. The intensity of the red color correlates with the number of cells in the well. (B) Growth inhibition in the presence of two fixed concentrations of prodoxorubicin and increasing concentrations of antibody 38C2. Controls are shown in black columns. dox, doxorubicin; prodox, prodoxorubicin; MVK, methyl vinyl ketone. Bars indicate SD; n = 4.
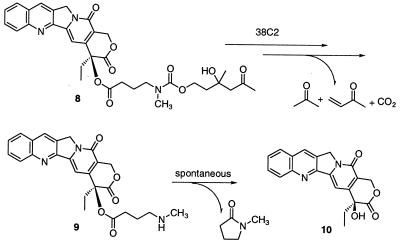
Design and Synthesis of a Camptothecin Prodrug that Is a Substrate for Antibody 38C2.
To demonstrate the general applicability of our prodrug activation concept by masking a different drug and a different heteroatom, we chose the powerful topoisomerase I inhibitor camptothecin for modification (14). In contrast to doxorubicin, camptothecin has not been used in ADEPT studies before. From analysis of the structure–activity relationship of camptothecin, it was found that two functional groups of camptothecin (camptothecin 10, Fig. 6) are required for its activity: the six-membered lactone ring E and the 20-hydroxyl group in α position to the lactone (14, 15). It was also found that the lactone ring of camptothecin is not stable in vivo and is rapidly hydrolyzed. Maintenance of the lactone ring is essential to the activity of the drug. Esterification of the tertiary alcohol at position 20 was shown to increase the stability of the lactone ring. A few examples of camptothecin prodrugs that use the 20-hydroxyl group to generate transport forms of the drug with a stabilized lactone ring, reduced toxicity, and higher solubility in water have been reported (15, 16). Based on these reports, we chose the 20-hydroxyl group for masking camptothecin with our retro-aldol–retro-Michael linker. As a candidate prodrug of camptothecin, subsequently referred to as procamptothecin, the ester derivative of camptothecin 8 was synthesized in one step from commercially available camptothecin 10 and linker 4. Antibody 38C2 was found to catalyze the retro-aldol and retro-Michael reactions to give self-immolative amine 9 (Fig. 6). After spontaneous lactamization, the free drug was released. As expected, the addition of the polar linker to campothecin increased its solubility in water. In the absence of antibody 38C2, the ester linkage between camptothecin and the linker was found to be much more stable than reported for other 20-hydroxyl ester derivatives of camptothecin (data not shown).
Figure 6.
Camptothecin prodrug activation via a tandem retro-aldol–retro-Michael reaction catalyzed by antibody 38C2 followed by a spontaneous lactamization.
Biological Evaluation of Procamptothecin.
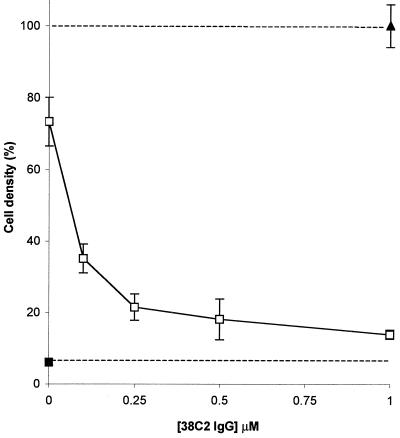
The antiproliferative effects of camptothecin and procamptothecin were monitored by quantifying the growth of HT29, LIM1215, and LNCap cells in the presence of a range of concentrations of drug and prodrug. All camptothecin assays were analyzed 72 hr after drug addition. The IC50 values of camptothecin were in the range of 0.1–0.25 μM for HT29 and LIM1215 cells and 0.01–0.025 μM for LNCap cells, revealing that camptothecin inhibits cell growth at substantially lower concentrations than doxorubicin (Table 1). Significantly, procamptothecin demonstrated a toxicity that was reduced by more than a factor of ten as compared with camptothecin, suggesting that the ester linkage of the retro-aldol–retro-Michael mask to camptothecin is relatively stable even in cell culture medium containing serum (data not shown). This result encouraged us to examine the functional activation of procamptothecin by antibody 38C2. The growth inhibition assay with HT29 cells is shown (Fig. 7). Antibody 38C2 activated procamptothecin in a concentration-dependent manner, revealing the full toxic effect of the drug. This activation was also seen in assays with LIM1215 and LNCap cells. Intriguingly, a concentration as low as 0.1 μM 38C2 IgG was highly effective in inhibiting cell growth (Fig. 7). This result is relevant to the potential in vivo applications of our prodrug activation system. The local concentration of antibody 38C2 that can be achieved at the tumor site through a tumor-targeting moiety conjugated to antibody 38C2 is likely to be in the submicromolar range depending on the accessibility of the target antigen, its expression level, and its affinity for the tumor-targeting moiety. An optimized combination of prodrugs that can be activated by antibody 38C2 may increase the sensitivity further.
Figure 7.
Growth inhibition of HT29 human colon carcinoma cells by procamptothecin in the presence of increasing concentrations of antibody 38C2. (▴) untreated control; (□), 1 μM procamptothecin; (■), 1 μM camptothecin. Bars indicate SD; n = 4.
In Vivo Activity of Antibody 38C2.
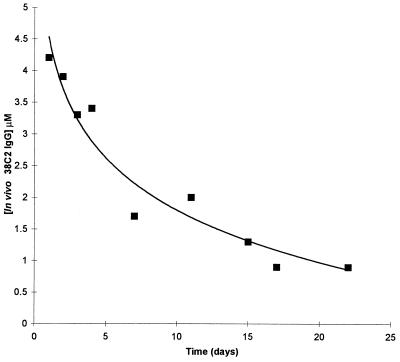
As an evaluation of the in vivo relevance of our prodrug activation concept, 38C2 IgG was injected i.v. into mice, and sera was prepared after defined time points and analyzed for catalytic activity. By using the highly sensitive conversion of the fluorogenic aldol sensor methodol into fluorescent 6-methoxy-2-napthaldehyde (11), catalytic activity was detectable only in mice that had been treated with antibody 38C2. No catalytic activity was detectable in sera from mice treated with the same amount of a control antibody. This result again confirms the stability of retro-aldol substrates of antibody 38C2 in the absence of the antibody, a prerequisite for our prodrug activation concept. By using defined concentrations of 38C2 IgG as a standard, the in vivo concentration of 38C2 IgG was calculated from its catalytic activity. As shown in Fig. 8, 38C2 IgG was detectable for more than 3 weeks with a clearance rate typical of an IgG in vivo. This result demonstrates that antibody 38C2 retains its catalytic activity over therapeutically relevant time periods in vivo. Potential in vivo problems like rapid clearance or inhibition of the catalytic activity of 38C2 by covalently binding diketones or other potential inhibitors were not observed. Moreover, mice treated with antibody 38C2 did not show any apparent abnormalities, suggesting that the antibody is not toxic.
Figure 8.
In vivo activity of antibody 38C2. Mice were injected with 100 μl of 15 mg/ml 38C2 IgG in PBS on day 0. The concentration of 38C2 IgG in mouse sera was studied as a function of time after the injection. Activity was calculated based on the antibody 38C2-catalyzed conversion of the fluorogenic aldol sensor methodol into fluorescent 6-methoxy-2-naphtaldehyde (11). Typical data derived from one mouse are shown. The initial 38C2 IgG concentration on day 0 can be estimated to be about 6 μM based on a blood volume of 1.5 ml and 1.5 mg of injected antibody. Catalysis was not detectable in sera from mice injected with 100 μl of 15 mg/ml control antibody in PBS.
CONCLUSION
We have developed a system that includes a drug-masking strategy and a complementary catalytic antibody for prodrug activation that demonstrates characteristics of the ideal ADEPT system. These characteristics include a versatile masking chemistry that may be applied to a wide range of drugs. Application of this masking strategy to two drugs suitable for human cancer therapy produced prodrugs with favorable toxicity ratios. No known enzyme possesses the catalytic activity required for the activation of these prodrugs, however, activation of diverse prodrug structures may be achieved with the broad-scope catalytic antibody 38C2. These unique features will allow treatment regimes involving cocktails of prodrugs to be studied. Antibody 38C2 demonstrated long-lived catalytic activity in vivo and was shown to selectively activate prodrugs and potentiate killing of colon and prostate cancer cell lines when applied at therapeutically relevant concentrations in culture. Humanization of 38C2 should endow the final characteristic of immunosilence to the antibody, allowing us to explore the potential of this system for the selective targeting of tumors and their supporting vasculature. A gene encoding a humanized 38C2 should also find application in gene therapy strategies, allowing for the selective ablation of cells expressing it.
Acknowledgments
We thank Diane Kubitz for technical assistance and Dr. Lloyd J. Old for providing the human carcinoma cell lines HT29 and LIM1215. This study was supported by Grant CA27489 from the National Institutes of Health (R.A.L. and C.F.B) and by a Clinical Investigation Grant from the Cancer Research Institute (C.R. and C.F.B.).
ABBREVIATION
- ADEPT
antibody-directed enzyme prodrug therapy
References
- 1.Denmeade S R, Nagy A, Gao J, Lilja H, Schally A V, Isaacs J T. Cancer Res. 1998;58:2537–2540. [PubMed] [Google Scholar]
- 2.Niculescu-Duvaz I, Springer C J. Adv Drug Delivery Rev. 1997;26:151–172. doi: 10.1016/s0169-409x(97)00032-x. [DOI] [PubMed] [Google Scholar]
- 3.Miyashita H, Karaki Y, Kikuchi M, Fujii I. Proc Natl Acad Sci USA. 1993;90:5337–5340. doi: 10.1073/pnas.90.11.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell D A, Gong B, Kochersperger L M, Yonkovich S, Gallop M A, Schultz P G. J Am Chem Soc. 1994;116:2165–2166. [Google Scholar]
- 5.Wentworth P, Datta A, Blakey D, Boyle T, Partridge L J, Blackburn G M. Proc Natl Acad Sci USA. 1996;93:799–803. doi: 10.1073/pnas.93.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz P G, Lerner R A. Science. 1995;269:1835–1842. doi: 10.1126/science.7569920. [DOI] [PubMed] [Google Scholar]
- 7.Cao Y, Suresh M R. Bioconjugate Chem. 1998;9:635–644. doi: 10.1021/bc980044l. [DOI] [PubMed] [Google Scholar]
- 8.Wagner J, Lerner R A, Barbas C F., III Science. 1995;270:1797–1800. doi: 10.1126/science.270.5243.1797. [DOI] [PubMed] [Google Scholar]
- 9.Barbas C F, III, Heine A, Zhong G, Hoffmann T, Gramatikova S, Björnstedt R, List B, Anderson J, Stura E A, Wilson I A, Lerner R A. Science. 1997;278:2085–2092. doi: 10.1126/science.278.5346.2085. [DOI] [PubMed] [Google Scholar]
- 10.Sinha S C, Barbas C F, III, Lerner R A. Proc Natl Acad Sci USA. 1998;95:14603–14608. doi: 10.1073/pnas.95.25.14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.List B, Barbas C F, III, Lerner R A. Proc Natl Acad Sci USA. 1998;95:15351–15355. doi: 10.1073/pnas.95.26.15351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korzeniewski C, Callewaert D M. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 13.Yuan F, Baxter L T, Jain R K. Cancer Res. 1991;51:3119–3130. [PubMed] [Google Scholar]
- 14.Potmesil M. Cancer Res. 1994;54:1431–1439. [PubMed] [Google Scholar]
- 15.Greenwald R B, Pendri A, Conover C, Gilbert C, Yang R, Xia J. J Med Chem. 1996;39:1938–1940. doi: 10.1021/jm9600555. [DOI] [PubMed] [Google Scholar]
- 16.Wall M E, Wani M C, Nicholas A W, Manikumar G, Tele C, Moore L, Truesdale A, Leitner P, Besterman J M. J Med Chem. 1993;36:2689–2700. doi: 10.1021/jm00070a013. [DOI] [PubMed] [Google Scholar]