Abstract
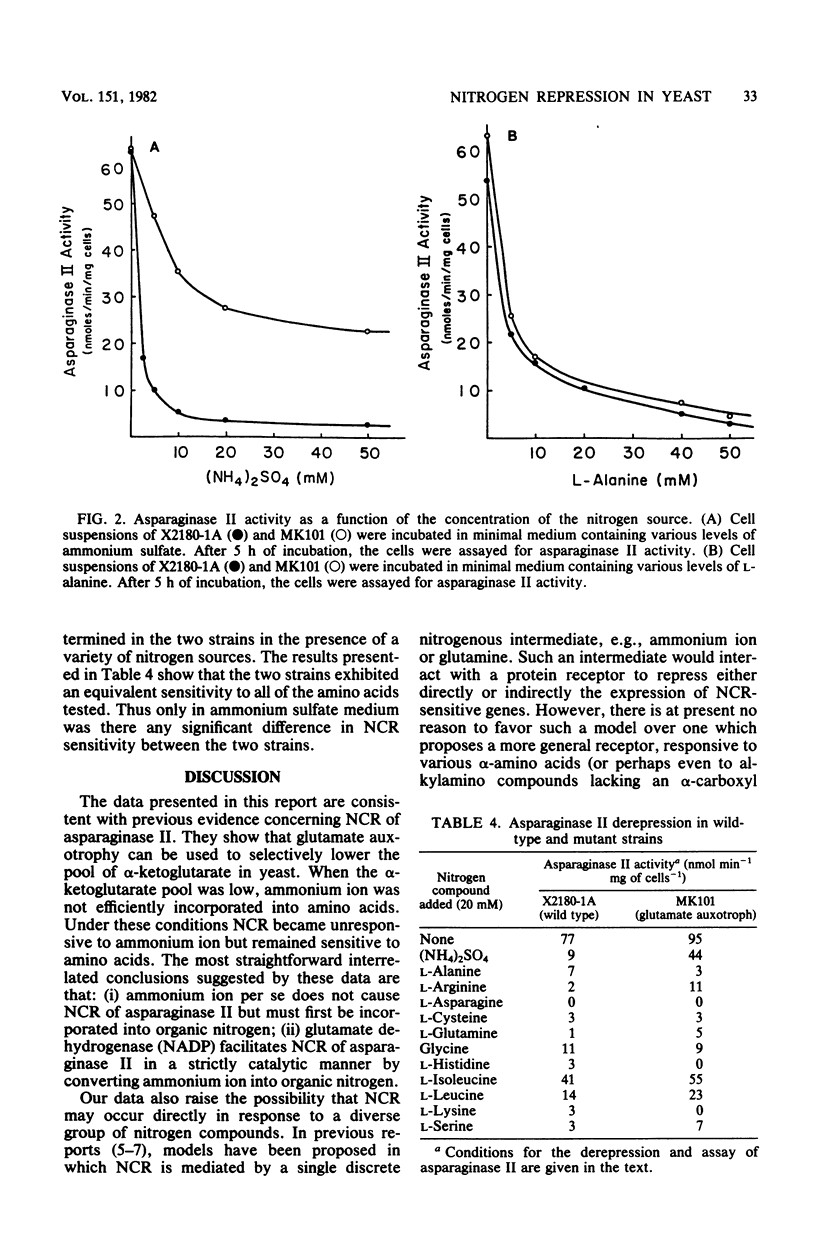
The biosynthesis of asparaginase II in Saccharomyces cerevisiae is subject to nitrogen catabolite repression. In the present study we examined the physiological effects of glutamate auxotrophy on cellular metabolism and on the nitrogen catabolite repression of asparaginase II. Glutamate auxotrophic cells, incubated without a glutamate supplement, had a diminished internal pool of alpha-ketoglutarate and a concomitant inability to equilibrate ammonium ion with alpha-amino nitrogen. In the glutamate auxotroph, asparaginase II biosynthesis exhibited a decreased sensitivity to nitrogen catabolite repression by ammonium ion but normal sensitivity to nitrogen catabolite repression by all amino acids tested.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bossinger J., Cooper T. Possible failure of NADP-glutamate dehydrogenase to participate directly in nitrogen repression of the allantoin degradative enzymes in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1975 Oct 6;66(3):889–892. doi: 10.1016/0006-291x(75)90723-8. [DOI] [PubMed] [Google Scholar]
- Cooper T. G. Mutants of Saccharomyces cerevisiae possessing fully induced levels of urea amido-lyase in the absence of added inducer. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1258–1263. doi: 10.1016/0006-291x(78)90323-6. [DOI] [PubMed] [Google Scholar]
- Drillien R., Aigle M., Lacroute F. Yeast mutants pleiotropically impaired in the regulation of the two glutamate dehydrogenases. Biochem Biophys Res Commun. 1973 Jul 17;53(2):367–372. doi: 10.1016/0006-291x(73)90671-2. [DOI] [PubMed] [Google Scholar]
- Dubois E. L., Grenson M. Absence of involvement of glutamine synthetase and of NAD-linked glutamate dehydrogenase in the nitrogen catabolite repression of arginase and other enzymes in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1974 Sep 9;60(1):150–157. doi: 10.1016/0006-291x(74)90185-5. [DOI] [PubMed] [Google Scholar]
- Dubois E., Grenson M., Wiame J. M. Release of the "ammonia effect" on three catabolic enzymes by NADP-specific glutamate dehydrogenaseless mutations in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1973 Feb 20;50(4):967–972. doi: 10.1016/0006-291x(73)91500-3. [DOI] [PubMed] [Google Scholar]
- Dubois E., Grenson M., Wiame J. M. The participation of the anabolic glutamate dehydrogenase in the nitrogen catabolite repression of arginase in Saccharomyces cerevisiae. Eur J Biochem. 1974 Oct 2;48(2):603–616. doi: 10.1111/j.1432-1033.1974.tb03803.x. [DOI] [PubMed] [Google Scholar]
- Dubois E., Vissers S., Grenson M., Wiame J. M. Glutamine and ammonia in nitrogen catabolite repression of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1977 Mar 21;75(2):233–239. doi: 10.1016/0006-291x(77)91033-6. [DOI] [PubMed] [Google Scholar]
- Dunlop P. C., Meyer G. M., Ban D., Roon R. J. Characterization of two forms of asparaginase in Saccharomyces cerevisiae. J Biol Chem. 1978 Feb 25;253(4):1297–1304. [PubMed] [Google Scholar]
- Dunlop P. C., Meyer G. M., Roon R. J. Nitrogen catabolite repression of asparaginase II in Saccharomyces cerevisiae. J Bacteriol. 1980 Jul;143(1):422–426. doi: 10.1128/jb.143.1.422-426.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop P. C., Meyer G. M., Roon R. J. Reactions of asparaginase II of Saccharomyces cerevisiae. A mechanistic analysis of hydrolysis and hydroxylaminolysis. J Biol Chem. 1980 Feb 25;255(4):1542–1546. [PubMed] [Google Scholar]
- Dunlop P. C., Roon R. J., Even H. L. Utilization of D-asparagine by Saccharomyces cerevisiae. J Bacteriol. 1976 Mar;125(3):999–1004. doi: 10.1128/jb.125.3.999-1004.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop P. C., Roon R. J. L-Asparaginase of Saccharomyces cerevisiae: an extracellular Enzyme. J Bacteriol. 1975 Jun;122(3):1017–1024. doi: 10.1128/jb.122.3.1017-1024.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Dubois E., Piotrowska M., Drillien R., Aigle M. Ammonia assimilation in Saccharomyces cerevisiae as mediated by the two glutamate dehydrogenases. Evidence for the gdhA locus being a structural gene for the NADP-dependent glutamate dehydrogenase. Mol Gen Genet. 1974;128(1):73–85. doi: 10.1007/BF00267295. [DOI] [PubMed] [Google Scholar]
- Grenson M., Hou C. Ammonia inhibition of the general amino acid permease and its suppression in NADPH-specific glutamate dehydrogenaseless mutants of saccharomyces cerevisiae. Biochem Biophys Res Commun. 1972 Aug 21;48(4):749–756. doi: 10.1016/0006-291x(72)90670-5. [DOI] [PubMed] [Google Scholar]
- Lacroute F. Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast. J Bacteriol. 1971 May;106(2):519–522. doi: 10.1128/jb.106.2.519-522.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogur M., Coker L., Ogur S. Glutamate auxotrophs in Saccharomyces 1. I. The biochemical lesion in the glt-1 mutants-2. Biochem Biophys Res Commun. 1964;14:193–197. doi: 10.1016/0006-291x(64)90254-2. [DOI] [PubMed] [Google Scholar]
- Roon R. J., Larimore F., Levy J. S. Inhibition of amino acid transport by ammonium ion in Saccharomyces cerevisiae. J Bacteriol. 1975 Oct;124(1):325–331. doi: 10.1128/jb.124.1.325-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roon R. J., Levy J. S., Larimore F. Negative interactions between amino acid and methylamine/ammonia transport systems of Saccharomyces cerevisiae. J Biol Chem. 1977 Jun 10;252(11):3599–3604. [PubMed] [Google Scholar]
- van de Poll K. W. Ammonium repression in a mutant of Saccharomyces carlsbergensis lacking NADP dependent glutamate dehydrogenase activity. FEBS Lett. 1973 Jun 1;32(2):265–266. doi: 10.1016/0014-5793(73)80848-8. [DOI] [PubMed] [Google Scholar]


