Abstract
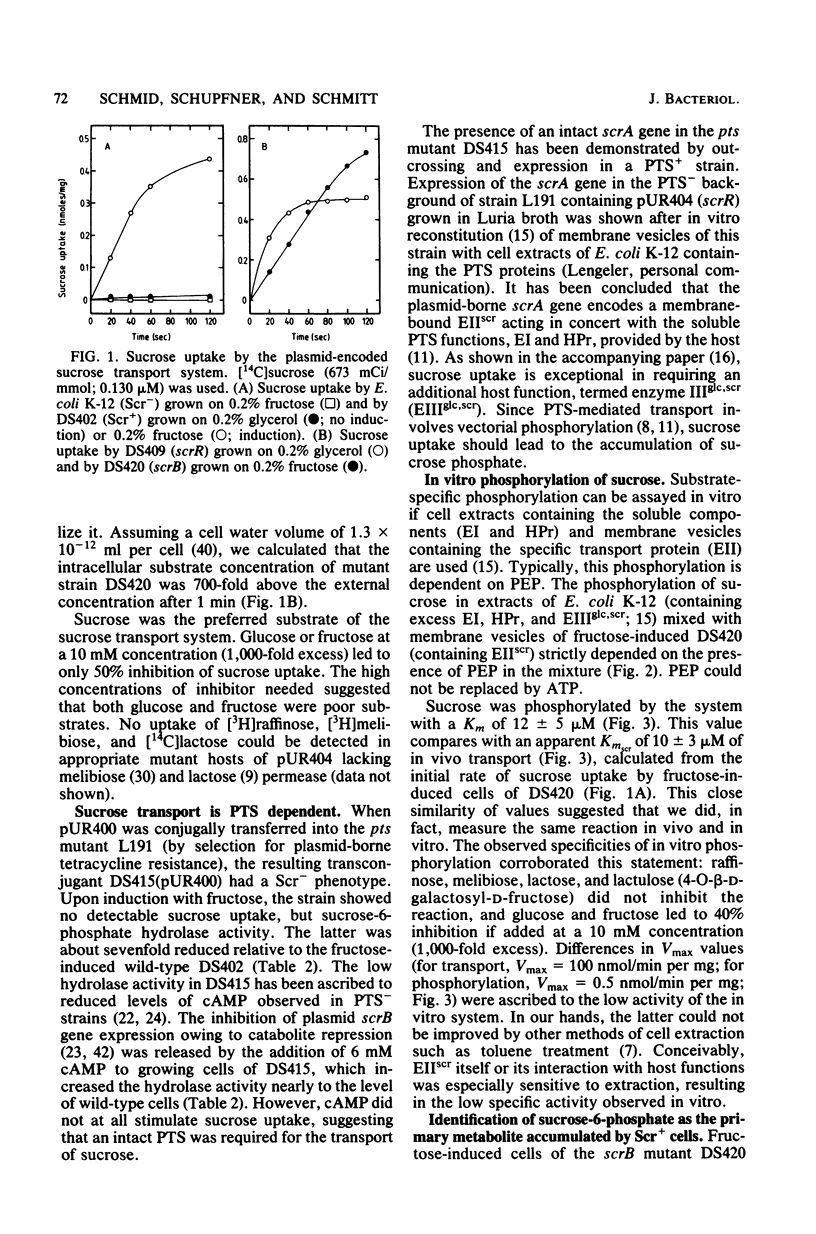
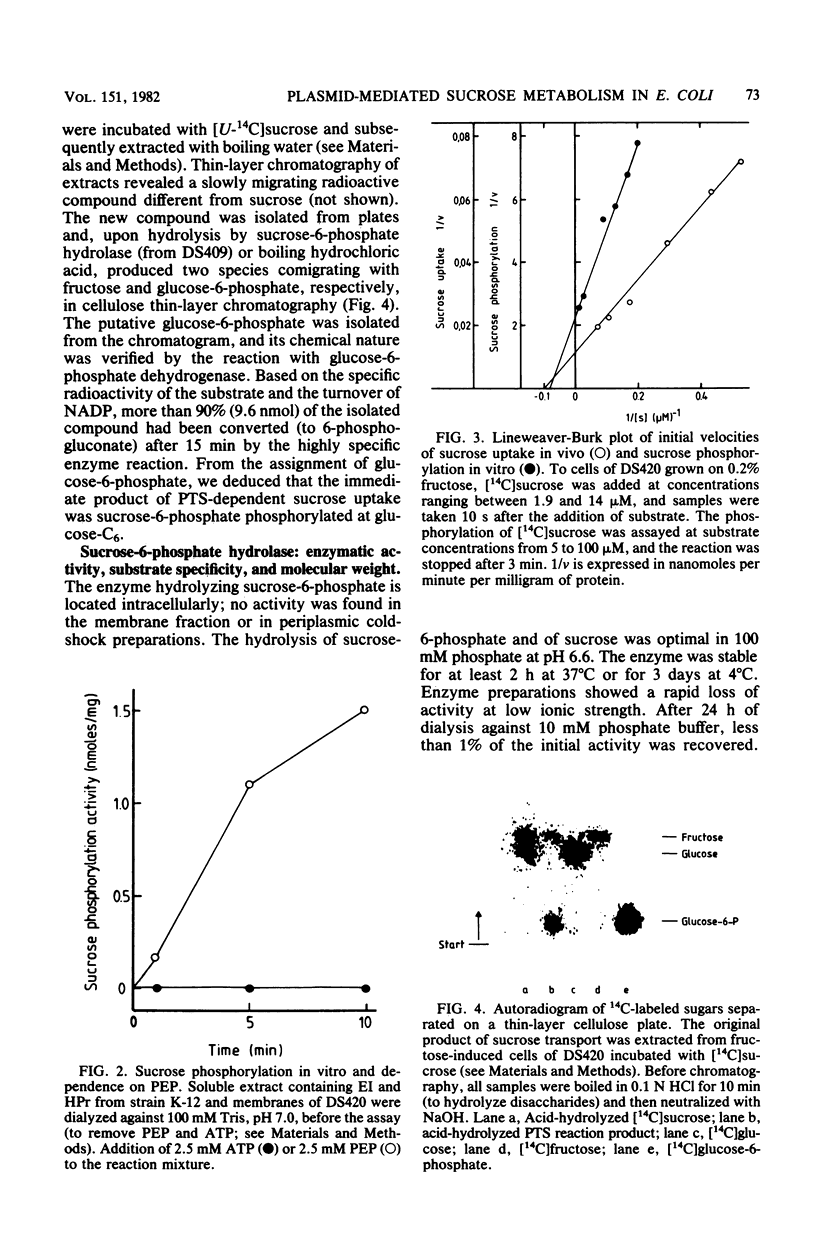
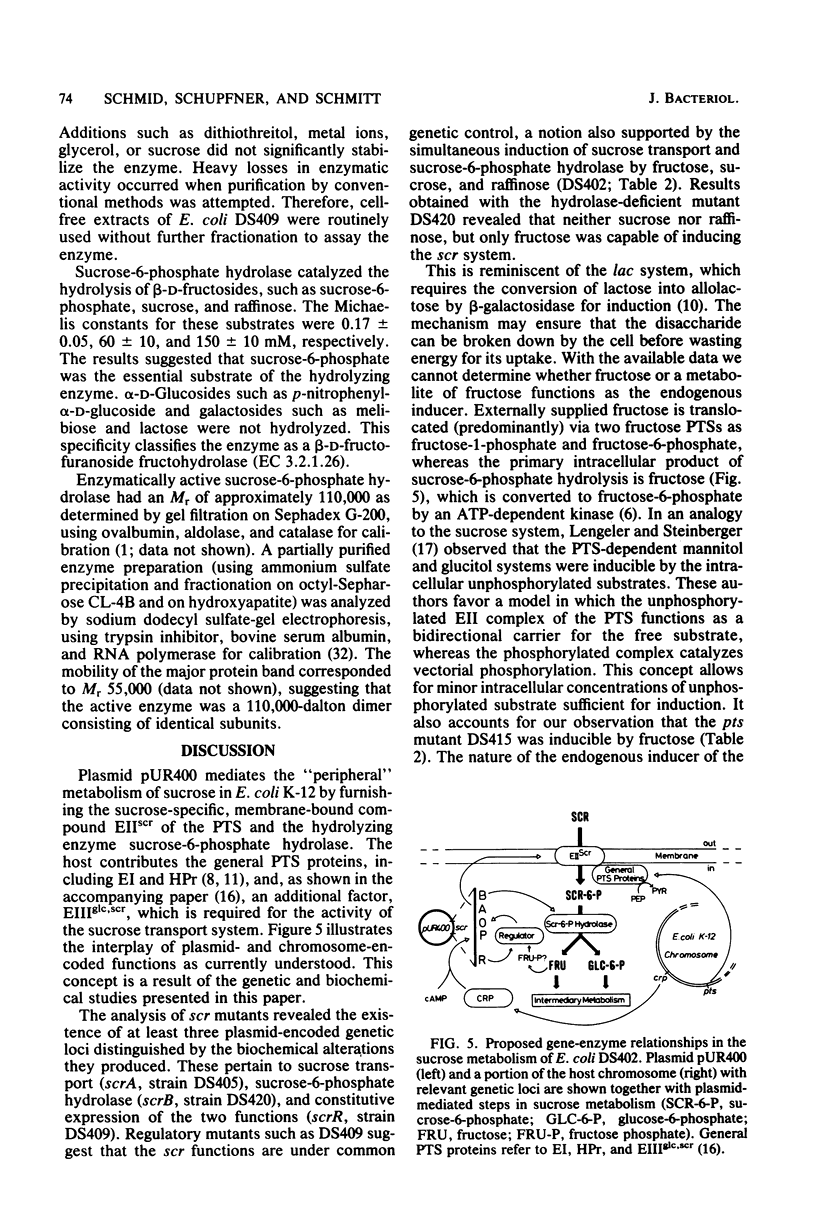
The conjugative plasmid pUR400 determines tetracycline resistance and enables cells of Escherichia coli K-12 to utilize sucrose as the sole carbon source. Three types of mutants affecting sucrose metabolism were derived from pUR400. One type lacked a specific transport system (srcA); another lacked sucrose-6-phosphate hydrolase (scrB); and the third, a regulatory mutant, expressed both of these functions constitutively (scrR). In a strain harboring pUR400, both transport and sucrose-6-phosphate hydrolase were inducible by fructose, sucrose, and raffinose; if a scrB mutant was used, fructose was the only inducer. These data suggested that fructose or a derivative acted as an endogenous inducer. Sucrose transport and sucrose-6-phosphate hydrolase were subject to catabolite repression; these two functions were not expressed in an E. coli host (of pUR400) deficient in the adenosine 3-,5'-phosphate receptor protein. Sucrose uptake (apparent Km = 10 microM) was dependent on the scrA gene product and on the phosphoenolpyruvate-dependent sugar:phosphotransferase system (PTS) of the host. The product of sucrose uptake (via group translocation) was identified as sucrose-6-phosphate, phosphorylated at C6 of the glucose moiety. Intracellular sucrose-6-phosphate hydrolase catalyzed the hydrolysis of sucrose-6-phosphate (Km = 0.17 mM), sucrose (Km = 60 mM), and raffinose (Km = 150 mM). The active enzyme was shown to be a dimer of Mr 110,000.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Porter E. V. Initial characterization of sucrose-6-phosphate hydrolase from Streptococcus mutans and its apparent identity with intracellular invertase. Biochem Biophys Res Commun. 1979 Jul 12;89(1):307–314. doi: 10.1016/0006-291x(79)90979-3. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Sommer H., Saedler H. Transposon Tn951 (TnLac) is defective and related to Tn3. Mol Gen Genet. 1981;184(2):241–248. doi: 10.1007/BF00272911. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Gachelin G. Studies on the alpha-methylglucoside permease of Escherichia coli. A two-step mechanism for the accumulation of alpha-methylglucoside 6-phosphate. Eur J Biochem. 1970 Oct;16(2):342–357. doi: 10.1111/j.1432-1033.1970.tb01088.x. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jobe A., Bourgeois S. lac Repressor-operator interaction. VI. The natural inducer of the lac operon. J Mol Biol. 1972 Aug 28;69(3):397–408. doi: 10.1016/0022-2836(72)90253-7. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Minor L., Coynault C., Rohde R., Rowe B., Aleksic S. Localisation plasmidique du déterminant génétique du caractète atypique "saccharose plus" des Salmonella. Ann Microbiol (Paris) 1973 Oct;124(3):295–306. [PubMed] [Google Scholar]
- Lengeler J. W., Mayer R. J., Schmid K. Phosphoenolpyruvate-dependent phosphotransferase system enzyme III and plasmid-encoded sucrose transport in Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):468–471. doi: 10.1128/jb.151.1.468-471.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J., Lin E. C. Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol. 1972 Nov;112(2):840–848. doi: 10.1128/jb.112.2.840-848.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J., Steinberger H. Analysis of the regulatory mechanisms controlling the synthesis of the hexitol transport systems in Escherichia coli K12. Mol Gen Genet. 1978 Aug 17;164(2):163–169. doi: 10.1007/BF00267381. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Repression of beta-galactosidase synthesis by glucose in phosphotransferase mutants of Escherichia coli. Repression in the absence of glucose phosphorylation. J Biol Chem. 1969 Nov 10;244(21):5836–5842. [PubMed] [Google Scholar]
- Perlman R., Pastan I. Cyclic 3'5-AMP: stimulation of beta-galactosidase and tryptophanase induction in E. coli. Biochem Biophys Res Commun. 1968 Mar 27;30(6):656–664. doi: 10.1016/0006-291x(68)90563-9. [DOI] [PubMed] [Google Scholar]
- Prasad I., Schaefler S. Regulation of the beta-glucoside system in Escherchia coli K-12. J Bacteriol. 1974 Nov;120(2):638–650. doi: 10.1128/jb.120.2.638-650.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. L. Transduction studies on the relation between prophage and host chromosome. J Mol Biol. 1965 Jul;12(3):892–912. doi: 10.1016/s0022-2836(65)80336-9. [DOI] [PubMed] [Google Scholar]
- Sabourin D., Beckwith J. Deletion of the Escherichia coli crp gene. J Bacteriol. 1975 Apr;122(1):338–340. doi: 10.1128/jb.122.1.338-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Ritschewald S., Schmitt R. Relationships among raffinose plasmids determined by the immunochemical cross-reaction of their alpha-galactosidases. J Gen Microbiol. 1979 Oct;114(2):477–481. doi: 10.1099/00221287-114-2-477. [DOI] [PubMed] [Google Scholar]
- Schmid K., Schmitt R. Raffinose metabolism in Escherichia coli K12. Purification and properties of a new alpha-galactosidase specified by a transmissible plasmid. Eur J Biochem. 1976 Aug 1;67(1):95–104. doi: 10.1111/j.1432-1033.1976.tb10637.x. [DOI] [PubMed] [Google Scholar]
- Schmitt R. Analysis of melibiose mutants deficient in alpha-galactosidase and thiomethylgalactoside permease II in Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):462–471. doi: 10.1128/jb.96.2.462-471.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Sheppard D. E., Englesberg E. Further evidence for positive control of the L-arabinose system by gene araC. J Mol Biol. 1967 May 14;25(3):443–454. doi: 10.1016/0022-2836(67)90197-0. [DOI] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979 Jun;24(3):821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Parsell Z. Transmissible substrate-utilizing ability in enterobacteria. J Gen Microbiol. 1975 Mar;87(1):129–140. doi: 10.1099/00221287-87-1-129. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Regulation and function of sucrose 6-phosphate hydrolase in Streptococcus mutans. Infect Immun. 1979 Nov;26(2):487–491. doi: 10.1128/iai.26.2.487-491.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Uptake and metabolism of sucrose by Streptococcus lactis. J Bacteriol. 1981 Aug;147(2):543–551. doi: 10.1128/jb.147.2.543-551.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebauer K., Schraml S., Shales S. W., Schmitt R. Tetracycline resistance transposon Tn1721: recA-dependent gene amplification and expression of tetracycline resistance. J Bacteriol. 1981 Sep;147(3):851–859. doi: 10.1128/jb.147.3.851-859.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]
- Wohlhieter J. A., Lazere J. R., Snellings N. J., Johnson E. M., Synenki R. M., Baron L. S. Characterization of transmissible genetic elements from sucrose-fermenting Salmonella strains. J Bacteriol. 1975 May;122(2):401–406. doi: 10.1128/jb.122.2.401-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]



