Abstract
The action of nuclear hormone receptors is tripartite, involving the receptor, its ligands, and its coregulator proteins. The estrogen receptor (ER), a member of this superfamily, is a hormone-activated transcription factor that mediates the stimulatory effects of estrogens and the inhibitory effects of antiestrogens such as tamoxifen in breast cancer and other estrogen target cells. To understand how antiestrogens and dominant negative ERs suppress ER activity, we used a dominant negative ER as bait in two-hybrid screening assays from which we isolated a clone from breast cancer cells that potentiates the inhibitory activities of dominant negative ERs and antiestrogen-liganded ER. At higher concentrations, it also represses the transcriptional activity of the estradiol-liganded ER, while having no effect on other nuclear hormone receptors. This clone, denoted REA for “repressor of estrogen receptor activity,” encodes a 37-kDa protein that is an ER-selective coregulator. Its competitive reversal of steroid receptor coactivator 1 enhancement of ER activity and its direct interaction with liganded ER suggest that it may play an important role in determining the sensitivity of estrogen target cells, including breast cancer cells, to antiestrogens and estrogens.
Keywords: tamoxifen, dominant negative receptors, breast cancer
The action of nuclear hormone receptors is tripartite, involving the receptor, its ligands, and its coregulator proteins (1–4). The estrogen receptor (ER), a member of this receptor superfamily, is a hormone-activated transcription factor that mediates the biological effects of estrogens in breast cancers and in a variety of other target tissues. In these tissues, the ER stimulates the expression of specific estrogen-regulated genes (for reviews, see refs. 5–9). Because of the role of estrogens in promoting the growth and progression of breast cancers (5, 6), there currently is great interest in exploring ways to functionally inactivate the ER, so as to suppress ER-mediated gene expression and cell proliferation. These approaches have involved the use of antiestrogens such as tamoxifen, as well as dominant negative ERs. Antiestrogens compete with estrogens for binding to the ER but fail to activate the ER, and they are used widely in breast cancer treatment (5, 7, 8). Dominant negative ERs are slightly altered forms of the ER protein that lack transcriptional activity and that suppress the activity of the wild-type ER when they are coexpressed in the same cells (9–12).
We previously identified dominant negative ERs altered in the C-terminal, helix 12 region of the ligand-binding domain of the receptor through structure–function mutagenesis analysis (9). Further studies on the mechanism of action of these dominant negative ERs indicated that their inhibitory effectiveness depended on their heterodimerization with the wild-type ER and implied an active mechanism to support their repression of wild-type ER activity (10, 11). Thus, we hypothesized that the dominant negative ER might recruit into the dominant negative ER complex a repressive protein. We imagined, as well, that a similar mechanism might be involved in mediating the inhibitory effects of antiestrogens operating through wild-type ER.
Because the activation function 2 (AF-2) region of the ER has been shown to be important in its interaction with other protein factors (2, 13–18), and our dominant negative ER was altered by a point mutation in this AF-2 region (L540Q ER), we thought it likely that the dominant negative ER might be suppressing wild-type ER action by recruiting repressor factors to this region of the receptor protein. Intriguingly, it is also the AF-2 region of ER that undergoes a marked shift in conformation in an ER-antiestrogen complex (19).
To investigate this possible recruitment of a repressor protein, we utilized a two-hybrid screening in yeast (20), from which we have identified a protein, denoted repressor of estrogen receptor activity (REA), that interacts preferentially with the dominant negative ER and with the antiestrogen-liganded ER. In this report, we show that this protein markedly represses ER transcriptional activity, but not that of other steroid and nonsteroid nuclear receptors, and that it selectively potentiates the inhibitory effectiveness of the antiestrogen-occupied ER and the dominant negative ER complex.¶
MATERIALS AND METHODS
Chemicals and Materials.
The antiestrogens ICI182,780 (ICI) and trans-hydroxytamoxifen (TOT) were kindly provided by Alan Wakeling and Zeneca (Wilmington, DE). Custom oligonucleotides were purchased from GIBCO.
Plasmids.
pBD-GAL4-EF was constructed by subcloning the blunted EagI/BamHI insert from pCMV5-ER (wild type or L540Q mutant) into the EcoRI-digested and blunted pBD-GAL4 (Stratagene). pCMV-REA was constructed by releasing the REA cDNA from the pT7T3D-PAC vector by XhoI/NotI digest. The insert then was blunted and subcloned into SalI/SmaI-digested and blunted CMV5. The pCMV5 expression vector for the wild-type human ER (WT-ERα), the dominant negative ER L540Q (9, 11), human ERβ (530 residues) (21, 22), and human progesterone receptor, pCMV5-PRB (23), have been described. The expression vectors pRSV-RARα and pBK-CMV-SRC-1 (15), pCMV-androgen receptor, and human thyroid receptor β1 were obtained from Ron Evans (Salk Institute, La Jolla, CA), Bert O’Malley (Baylor College of Medicine, Houston), Michael McPhaul (Southwestern Medical School, Dallas), and William Chin (Harvard Medical School, Boston).
The estrogen response element (ERE)-containing reporters (ERE)2-TATA-chloramphenicol acetyltransferase (CAT) and (ERE)2-pS2-CAT, and the CMV-ERE-CAT promoter interference plasmids have been described previously (11, 24). Mouse mammary tumor virus (MMTV-CAT) was obtained from Steven Nordeen (University of Colorado Medical Center, Denver). SV-DR5-CAT, the retinoic acid receptor (RAR)-responsive reporter, was obtained from Ronald Evans. G5-E1b-CAT reporter construct containing the Gal4 upstream activating sequences (UAS) was obtained from Michael Green (University of Massachusetts, Worcester, MA). The plasmids pCH110 (Pharmacia) and pCMVβ (CLONTECH) were used as β-galactosidase internal controls for transfection efficiency, and all CAT activity measurements were corrected for β-galactosidase activity (11, 18).
To make truncated REA constructs, deletions of the REA ORF were generated by PCR using the full-length REA plasmid, pCMV-REA, as template. Reactions with constructed forward and reverse PCR primers were performed by using VENT DNA polymerase from New England Biolabs. Each forward primer contained an ATG and identical Kozac sequence, and each reverse primer contained a stop codon. PCR fragments were purified, digested with EcoRI and XbaI, and cloned into the EcoRI/XbaI sites of pCMV5.
cDNA Library Construction.
cDNA was prepared from MCF-7 cells and ligated into the HybriZAP vector (Stratagene) to generate a primary λ library. This library was amplified and converted by in vivo excision to a pAD-GAL4 phagemid library. The average insert size in the phagemid library is 1.4 kb.
Yeast Two-Hybrid Screening.
The yeast strain YRG2 (Stratagene) containing pBD-GAL-EF (wild type or L540Q, amino acids 313–595) was transformed with the human MCF-7 cDNA library in pAD-GAL4 and plated on medium lacking histidine and supplemented with 10−5 M TOT. HIS3+ colonies were measured for β-galactosidase activity by using the filter-lift assay (25). HIS3+ colonies exhibiting high β-galactosidase activity (LacZ+ colonies) were characterized further. To recover library plasmids, total DNA from HIS3+, LacZ+ colonies was isolated and used to transform Escherichia coli (XLI-Blu MRF′ strain from Stratagene). To ensure that the correct cDNAs were identified, as well as to establish ligand-dependent interaction of promising clones with the ER, library plasmids isolated were transformed into YRG2 containing pBD-GAL-EF and plated into medium lacking histidine and supplemented with control vehicle, estradiol (10−5 M), or TOT (10−5 M). β-Galactosidase activity was determined from HIS3+ colonies by using both the filter-lift assay or liquid assay (25).
Cell Culture and Transfection.
MCF-7 human breast cancer cells, Chinese hamster ovary (CHO) cells, and MDA-MB-231 human breast cancer cells were maintained in cell culture and transfected by the CaPO4 coprecipitation method exactly as described previously (11, 26). CHO cells were plated at 1.8 × 105 per 60-mm plate and transfected 48 h later with 2 μg (ERE)2-TATA-CAT, 0.4 μg pCH110, 5 ng pCMV5-ER expression vector, and carrier DNA to 8 μg total DNA per plate. MDA-MB-231 cells in 100-mm dishes were transfected with 6 μg of the ERE-containing reporter construct [(ERE)2-pS2-CAT], 100 ng CMV5-ER expression vector, 1 μg pCMVβ, β-galactosidase internal control plasmid, and carrier DNA to adjust total DNA to 15 μg. Cells were harvested 24 h after hormone treatment, and cell extracts were prepared. β-Galactosidase activity, which was measured to normalize for transfection efficiency, and CAT activity were assayed as described (11).
In Vitro Translation.
In vitro translation of REA, ER, or other receptors was performed (18) by using the Promega TNT kit.
In Vitro Interaction Assays.
Five hundred micrograms of E. coli bacterial crude extract containing glutathione S-transferase (GST)-ER hormone-binding domain (amino acids 282–595) or GST-REA fusion proteins was incubated at 4°C with 25 μl of glutathione-Sepharose beads (50% slurry; Pharmacia) for 2.5 h as described (18) with minor modifications. After two washes with 1 ml NET (20 mM Tris, pH 7.9/100 mM NaCl/1 mM EDTA/0.5% Nonidet P-40/0.5% milk) and two washes with 1 ml binding buffer (20 mM Hepes, pH 7.9/10% vol/vol glycerol/60 mM NaCl/1 mM DTT/6 mM MgCl2/1 mM EDTA), the beads were incubated with 5 μl of in vitro translated product for 2.5 h at 4 C. The beads then were washed three times with 1 ml NET and two times with 1 ml binding buffer. After washing, bound protein was eluted with 10 mM reduced glutathione in 50 mM Tris⋅HCl, pH 8.0, and boiled in SDS sample buffer. One-fourth of each protein sample was analyzed by SDS/PAGE. The gel was dried, and [35S]methionine-radiolabeled protein was detected by autoradiography.
RESULTS
REA Interacts with the ER in the Yeast Two-Hybrid System.
To identify potential repressors of the estrogen receptor, we used the C-terminal E-F domains of the dominant negative ER L540Q as bait in two-hybrid screening in yeast to identify clones from an MCF-7 breast cancer cell cDNA library that express protein(s) that interacts with the mutated AF-2 domain of the dominant negative ER.
A cDNA library from MCF-7 breast cancer cells was constructed and introduced as a translational fusion with the GAL4 transactivating domain [GAL(AD)-cDNA] into the YRG2 yeast strain. This yeast strain contains two reporter genes, a histidine auxotrophic marker (HIS3) and a LacZ reporter gene, under the control of the GAL4 UAS (UASGAL4). A plasmid encoding a chimeric protein consisting of the GAL4 DNA-binding domain (DBD) and the ER activation domain 2 with a mutation at amino acid 540, GAL(DBD)-ER (EFL540Q), was used as the bait for interacting clones.
ER-interacting clones were identified by their ability to activate reporter constructs containing the UASGAL4 when cotransformed with GAL(DBD)-ER (EFL540Q) and then were isolated from HIS+ clones or clones that showed increased transcription from the LacZ reporter gene (LacZ+). The transformed YRG2 yeast cells were selected for growth on plates in the presence of estradiol or the antiestrogen TOT.
In this way, we isolated a clone, which we named REA. The interaction of REA with the ER fusion protein was confirmed by backcrossing. The interaction was found to be ligand-dependent, in that the activation of transcription from LacZ or HIS3 reporter genes was observed only in the presence of antiestrogen or estradiol, and it occurred preferentially in the presence of antiestrogen.
The REA insert in GAL(AD) was sequenced, and this sequence (≈700 bp from the 3′ and coding regions) was compared with the gene databank by using the blast search program. An expressed sequence tag clone, which contains a larger fragment of the REA cDNA, was identified and obtained from the IMAGE Consortium. Sequence analysis of this 1,500-bp cDNA clone indicated an ORF of 897 bp (299 aa; Fig. 1). The clone shares nearly complete identity (99%) with the gene for a murine protein named B cell receptor-associated protein (BAP-37), a soluble protein originally isolated through its physical association with the B lymphocyte IgM antigen receptor (27).
Figure 1.
Amino acid and nucleotide sequence of human REA. Potential protein kinase A (PKA) and protein kinase C (PKC) phosphorylation sites and a nuclear receptor-interaction box (NR, LXXLL) are underlined, and a nuclear localization sequence (NLS) in REA is boxed.
REA Enhances the Potency of Dominant Negative ER and Antiestrogens as Suppressors of ER Activity.
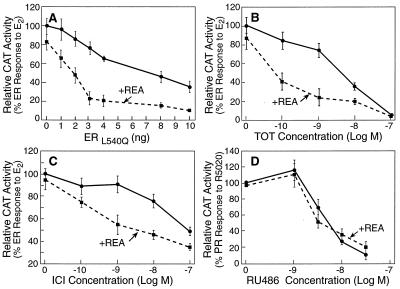
As shown in Fig. 2A, REA markedly potentiated the inhibitory effectiveness of the dominant negative L540Q ER. For these experiments, wild-type ER was expressed in CHO cells with increasing amounts of the dominant negative ER in the presence or absence of REA. A low concentration of REA (10 ng expression plasmid) reduced by 4-fold the amount of dominant negative ER required to suppress 50% of wild-type ER activity. Similar effects of REA were observed with the S554fs dominant negative ER (data not presented). As shown in Fig. 2 B and C, REA also markedly potentiated the inhibitory activity of antiestrogens. Intriguingly, REA resulted in a 50-fold increase in the inhibitory potency of the antiestrogen TOT and the antiestrogen ICI182,780 (ICI). Thus, 50-fold less antiestrogen is needed for equal inhibitory effectiveness in the presence of low amounts of REA.
Figure 2.
REA enhances the potency of antiestrogens and dominant negative ER as suppressors of ER activity, but has no affect on the potency of antiprogestin as a progesterone receptor antagonist. In A– C, CHO cells were transfected with an expression vector for the wild-type ER (5 ng) and the reporter construct (ERE)2-TATA-CAT in the absence or presence of an expression vector for REA (10 ng). In A, cells were cotransfected with increasing amounts of an expression vector for the dominant negative L540Q ER and were treated with 10−8 M E2 for 24 h. In B and C, cells were treated with 10−8 M E2 along with increasing concentrations of the antiestrogen TOT (B) or ICI182,780 (ICI) (C) for 24 h. (D) The effect of REA on the repressive action of antiprogestin on R5020-mediated activation of the PR also was tested. CHO cells were transfected with an expression vector for human PR-B (5 ng), the PR responsive reporter construct MMTV-CAT in the absence or presence of REA expression vector (10 ng), and were treated with 10−8 M progestin R5020 plus increasing concentrations of the antiprogestin RU486 for 24 h. All cells in A– D also were transfected with a β-galactosidase internal control reporter to correct for transfection efficiency. Cell extract CAT activity values, normalized for β-galactosidase activity, are the means ± SD from three separate experiments.
The amounts of REA that elicited this marked enhancement of L540Q ER dominant negative effectiveness and suppression by antiestrogens caused little, if any, decrease in activity of the wild-type ER (see zero point values in Fig. 2 A, B, or C), indicating that the potentiation of inhibitory activity of the dominant negative ER or antiestrogen-liganded ER cannot be attributed to a decrease in the activity of the wild-type ER alone. The data in Fig. 2D show that REA did not enhance the suppressive effects of antiprogestin on progesterone receptor (PR) transcriptional activity, suggesting that the effects of REA are selective for the ER.
REA Is an ER-Selective Coregulator; Mapping of the Regions of REA Required for Repression.
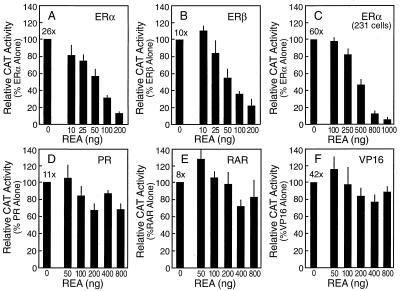
Transcriptional activity experiments shown in Fig. 3 indicate that REA is an ER-selective coregulator. When the estrogen responsive (ERE)2-TATA-CAT reporter gene construct was transfected into ER-negative CHO cells along with an expression vector for ERα or ERβ, addition of estradiol resulted in a 26- or 11-fold increase, respectively, in ER transcriptional activity. When REA and the ER were coexpressed, transcriptional activation by the ER was suppressed in a dose-dependent fashion by REA (Fig. 3 A and B). REA had no effect on reporter activity in the absence of liganded ER (data not shown). REA-mediated repression of ER transcriptional activity also was observed in other cells, including MDA-MB-231 breast cancer cells, and in the context of another promoter–reporter construct, (ERE)2-pS2-CAT, indicating that REA does not appear to be a cell- or promoter-specific repressor (Fig. 3C).
Figure 3.
REA is an ER-selective coregulator, suppressing transcriptional activation of ERα and ERβ, but not that of PR, RAR, or Gal4-VP16. CHO cells (A and B and D–F) were transfected with 5 ng of the expression vector for various activators and 2 μg of the reporter construct indicated. (A) ERα/(ERE)2-TATA-CAT. (B) ERβ/(ERE)2-TATA-CAT. (D) PR/MMTV-CAT. (E) RAR/DR-5-CAT. (F) Gal4-VP-16/G5-E1-CAT. In C, MDA-MB-231 breast cancer cells were transfected with 100 ng of the expression vector for ERα and 6 μg of the (ERE)2-pS2-CAT reporter. The cells were cotransfected with increasing concentrations of an expression vector for REA as indicated and with a β-galactosidase internal control reporter to correct for transfection efficiency. Cells then were treated for 24 h with 10−8 M ligands (ER, E2; PR, R5020; RAR, all-trans retinoic acid). Cell extracts were prepared, and CAT activity, normalized for β-galactosidase activity, is shown. Values are the means ± SD from three separate experiments. Numbers at the top of the leftmost bar show the fold induction in CAT activity by hormone (receptor-transfected, plus vs. minus hormone) in the absence of added REA.
In contrast to what was observed with ERα and ERβ, REA had little or no effect on the transcriptional activity of other hormone receptors, including PR (Fig. 3D), RAR (Fig. 3E), and androgen or thyroid hormone receptors (data not shown), and it had no effect on the transcriptional activity of an unrelated transcriptional activator, Gal4-VP16 (Fig. 3F), even when amounts of REA much greater than those giving nearly full inhibition of ER activity were used in the same cells (Figs. 3 A and B).
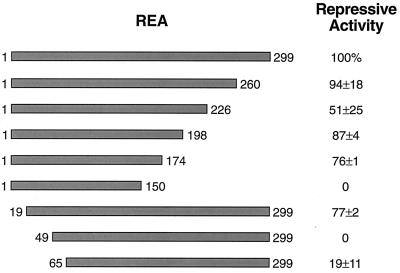
To map the region(s) of REA required for ER repression, amino- and carboxyl-terminal truncations of REA were generated. As shown in Fig. 4, deletion of the first 18 or the last 125 aa from REA had almost no effect of repression of ER activity. However, further N-terminal deletion to amino acid 49 or C-terminal deletion to amino acid 150 resulted in complete loss of repression by REA. Thus, repressive activity appears to require two regions of REA encompassing amino acids 19–49 and 150–174.
Figure 4.
Mapping the regions of REA required for repression of ER activity. The regions of REA required for repression of ER activity were monitored by using the N- and C-terminal truncated REAs indicated. Repressive activity was monitored by the ability of the cotransfected REA to repress ER-stimulated transcription as measured by CAT assay from the estrogen-responsive reporter (ERE)2-pS2-CAT. MDA-MB-231 human breast cancer cells were cotransfected with 100 ng pCMV-ERα, 500 ng of pCMV-REA construct, and internal control β-galactosidase plasmid. Cells were treated with 10−8 M E2 for 24 h. The level of repression of ER activity by full-length REA is set at 100%. The effectiveness of the truncated forms of REA in repressing ER activity is listed as a percentage of full-length REA. Values are the means ± SD of three to eight determinations. REA (1–226) was found to suppress ER activity to the same level as full-length REA when higher amounts of REA (1–226) expression plasmid were utilized.
REA Interacts Directly with ER in in Vitro GST “Pull-Down” Assays.
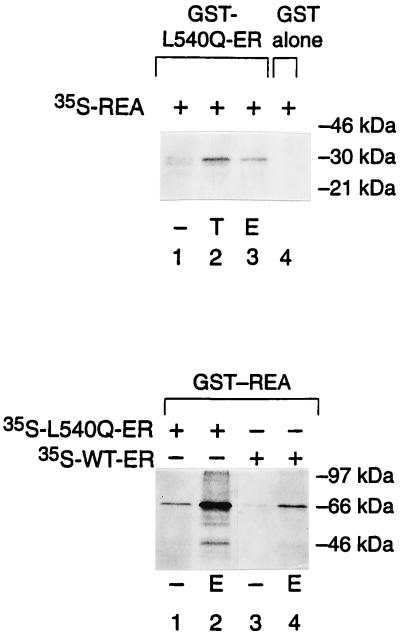
The interaction between REA and ER was verified in vitro by using a protein–protein interaction assay in which the affinity matrix was a GST fusion protein with ER. In vitro transcribed and translated [35S]REA was retained by the GST-ER affinity column in the presence of TOT or estradiol (Fig. 5, T and E, respectively). REA was not retained on the GST column without ER (or with GST alone). Likewise, REA did not interact with the ligand-binding domain of human PR-B (GST-PR) in the presence or absence of ligand (data not presented). We compared the interaction of wild-type ER and the L540Q mutant ER with REA in GST pull-down assays wherein GST was fused to REA (GST-REA). As shown in Fig. 5, higher levels of the dominant negative L540Q ER were retained on the GST-REA column when compared with the wild-type ER, and the interaction with both receptors was increased greatly by hormone [estradiol (E2); or TOT, not shown].
Figure 5.
Direct interaction of REA with ER. (Upper) In vitro translated, [35S]methionine-labeled REA was incubated with GST-L540Q-ER (lanes 1–3) or GST alone bound to Sepharose beads (lane 4) in the presence of 0.1% ethanol control vehicle (−), 10−6 M TOT (T), or 10−6 M estradiol (E). (Lower) In vitro translated, [35S]methionine-labeled L540Q ER (lanes 1 and 2) or wild-type ER (lanes 3 and 4) were incubated with GST-REA (REA bound to GST-Sepharose beads) in the presence of control vehicle (−) or estradiol (E). Bound protein was eluted and analyzed by 12.5% SDS/PAGE. Typically, 12–15% of input-radiolabeled wild-type ER was bound to GST-REA in the presence of ligand. The numbers at the right indicate molecular size markers in kDa.
REA Does Not Interfere with the Ability of ER to Bind to ERE DNA, but Does Compete with the Coactivator Steroid Receptor Coactivator 1 (SRC-1) for Modulation of ER Transcriptional Activity.
We examined the ability of the ER to bind to ERE DNA, as tested in vivo in intact cells by using a promoter interference assay (24) or in vitro in gel mobility-shift assays (24) and found that REA did not reduce ER DNA-binding activity (data not shown). We therefore determined whether REA affects steps subsequent to DNA binding in the ER response pathway, such as the functional interaction of the ER with necessary adapter or coactivator proteins. As expected (15, 28), the coactivator SRC-1 enhanced the estradiol-mediated transcriptional activity of ER up to 4- to 5-fold (Fig. 6). Coexpression of REA suppressed the enhancement of ER transcriptional activity by SRC-1 very effectively, and it did so in a concentration-dependent manner (Fig. 6), implying mutual functional competition between these two coregulatory proteins.
Figure 6.
REA suppresses SRC-1-mediated enhancement of ER transcriptional activity. CHO cells were transfected with 5 ng of expression vector for ER and 2 μg of (ERE)2-TATA-CAT reporter construct. The cells were cotransfected with increasing concentrations of an expression vector for SRC-1 in the presence or absence of REA as indicated. The cells also were transfected with a β-galactosidase internal control reporter to correct for transfection efficiency. Cells then were treated for 24 h with 10−8 M E2. Cell extracts were prepared and analyzed for CAT activity and β-galactosidase activity. Values are the means ± SD from three separate experiments.
DISCUSSION
Using a dominant negative ER as a bait for interaction with other protein factors, we have identified an ER-selective coregulator protein. This repressor of ER activity, REA, markedly potentiates the inhibitory effectiveness of dominant negative ERs as well as the inhibitory activity of antiestrogens. Our experiments show that there is a direct interaction of REA with ER and that this interaction is ligand-dependent and is observed preferentially with the dominant negative ER and with the antiestrogen-liganded ER. Interestingly, REA enhances antiestrogen and dominant negative ER potency at concentrations that are lower than those at which it suppresses estradiol-ER activity. At higher concentrations of REA, where estradiol-stimulated activity of the wild-type ER is suppressed, similar efficacy of REA is observed with ERα and ERβ. Mapping experiments indicate the involvement of two regions of REA in ER repression.
Of note, we observed mutual functional competition between REA and the steroid receptor coactivator SRC-1, suggesting that these proteins compete for modulation of ER biological activity. That REA may function as an anticoactivator is consistent with our observations that REA does not have intrinsic transcription-repression activity. Even very high amounts of a Gal4 DBD-REA fusion protein (up to 30 μg plasmid transfected) failed to repress activity of a (Gal4)5-SV40-Luc reporter in transfection experiments conducted in MDA-MB-231 breast cancer or 293T cells (data not shown). Repression of ER activity by REA thus is likely to involve competition with coactivators for interaction with ER. It is known that SRC-1 binds to the AF-2 region of nuclear receptors (2, 15, 18), and our yeast two-hybrid screening pulled out REA by using the portion of the ER encompassing AF-2. The observation that the L540Q dominant negative ER shows preferential interaction with REA is consistent with this hypothesis, because we find that this dominant negative ER fails to interact with coactivators such as SRC-1 (G. Lazennec, T. Ediger, D. Schodin, and B.S.K., unpublished data). Also, REA does not interfere with the ability of ER to bind to ERE-containing DNA in intact cells, implying that it does not act at this point in the ER-response pathway, nor does it act by keeping the ER out of the nucleus.
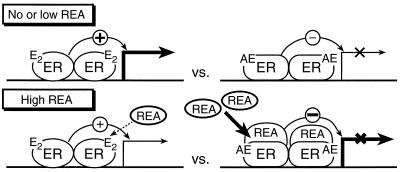
In Fig. 7, we present a working model for REA potentiation of antiestrogen inhibitory effectiveness and for repression of ER activity. This model predicts that the cellular level of REA will be an important determinant of the effectiveness (potency) of antiestrogens in inhibiting estrogen activity. In cells with no or low levels of REA, estradiol will effectively activate ER-mediated transcription and antiestrogens will suppress estrogen action, whereas at high cellular levels of REA, antiestrogen potency will be enhanced markedly, resulting in a much greater inhibitory effectiveness of low concentrations of antiestrogen. With high levels of REA, estradiol-occupied ERs also will have their stimulatory effectiveness suppressed, thereby further reducing estrogen activity in cells. REA thus sensitizes cells to the antagonist activity of antiestrogens over a concentration range where it has minimal effect on desensitizing cells to estrogens, but at high levels, reduced response to estradiol also would be expected.
Figure 7.
Model for REA potentiation of antiestrogen-inhibitory effectiveness. See text for description.
REA bears no structural resemblance to three known corepressors of the nuclear receptor subfamily of nonsteroid hormone receptors, namely N-CoR, SMRT, and SUN-CoR (29–33), and as opposed to these corepressors, which interact with thyroid hormone or RARs, REA shows great selectivity for estrogen receptors. A major difference between REA and N-CoR/SMRT and SUN-CoR is that REA interacts preferentially with a liganded ER, whereas SMRT, N-CoR, and SUN-CoR interact with an unliganded receptor and dissociate from the receptor upon ligand binding. Thus, repressor interaction with unliganded thyroid receptor or RAR correlates well with the repressive activity of thyroid receptor or RAR in the absence of their ligand. In contrast, the ER when unliganded is inactive and does not have repressive activity; repression through ER requires that ER bind an antagonist ligand, which, with wild-type ER, is an antiestrogen but, with a dominant negative ER, may be an estrogen. Although SMRT and N-CoR have been shown to suppress the agonistic activity of the tamoxifen-occupied ER (34), these corepressors do not enhance the inhibitory effectiveness of antiestrogens, nor do they alter the activity of the estrogen-liganded ER.
Of note, human REA shows near identity with a soluble murine B cell receptor-associated protein named BAP-37. Although first identified (27, 35) in B cells, BAP-37 also was shown to be present in other cells and tissues examined, clearly indicating that it functions in cells that do not contain the B cell receptor, although its activities have not been characterized (27). Its role in inhibiting activity of the ER is an example of the increasingly common theme in functional genomics of new functions for previously known proteins. The human ortholog of BAP-37 recently has been localized on chromosome 12 (36). Intriguingly, BAP-37 is related to prohibitin (49% amino acid identity), a highly conserved protein that is reported to play roles in diverse processes, including development, tumor suppression, and senescence (37–43).
REA represents an example of a protein that enhances the potency of two inhibitors of ER action, antiestrogens and dominant negative ERs, implying that interaction with REA may represent an important point of convergence in the mechanism of action pathway through which these two factors function. The activities of REA suggest that it may play an important role in determining the sensitivity of estrogen target cells, including breast cancer cells, to antiestrogens and estrogens.
Acknowledgments
This work was supported by National Institutes of Health Grants CA18119 and CA60514 and by a postdoctoral fellowship from The Susan G. Komen Breast Cancer Foundation. We thank Ron Evans, Bert O’Malley, Steve Nordeen, Michael Green, William Chin, Michael McPhaul, and Mitch Lazar for providing plasmids.
ABBREVIATIONS
- ER
estrogen receptor
- E2
estradiol
- ERE
estrogen response element
- TOT
trans-hydroxytamoxifen
- REA
repressor of estrogen receptor activity
- AF
activation function
- GST
glutathione S-transferase
- CAT
chloramphenicol acetyltransferase
- SRC-1
steroid receptor coactivator 1
- UAS
upstream activating sequences
- CHO
Chinese hamster ovary
- PR
progesterone receptor
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF150962).
Portions of this work were presented at the 80th Endocrine Society Meeting, Abstract OR 34-2, p. 96, 1998.
References
- 1.Katzenellenbogen J A, O’Malley B W, Katzenellenbogen B S. Mol Endocrinol. 1996;10:119–131. doi: 10.1210/mend.10.2.8825552. [DOI] [PubMed] [Google Scholar]
- 2.Shibata H, Spencer T E, Onate S A, Jenster G, Tsai S Y, Tsai M-J, O’Mally B W. Recent Prog Horm Res. 1997;52:141–165. [PubMed] [Google Scholar]
- 3.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz K B, Jackson T A, Bain D L, Richer J K, Takimoto G S. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 5.Katzenellenbogen B S, Montano M M, Ekena K, Herman M E, McInerney E M. Breast Cancer Res Treat. 1997;44:23–38. doi: 10.1023/a:1005835428423. [DOI] [PubMed] [Google Scholar]
- 6.Dickson R B, Lippman M E. Endocr Rev. 1987;8:29–43. doi: 10.1210/edrv-8-1-29. [DOI] [PubMed] [Google Scholar]
- 7.Santen R, Manni A, Harvey H, Redmond C. Endocr Rev. 1990;11:221–265. doi: 10.1210/edrv-11-2-221. [DOI] [PubMed] [Google Scholar]
- 8.Jordan V C, Murphy C S. Endocr Rev. 1990;11:578–610. doi: 10.1210/edrv-11-4-578. [DOI] [PubMed] [Google Scholar]
- 9.Ince B A, Zhuang Y, Wrenn C K, Shapiro D J, Katzenellenbogen B S. J Biol Chem. 1993;268:14026–14032. [PubMed] [Google Scholar]
- 10.Ince B A, Schodin D J, Shapiro D J, Katzenellenbogen B S. Endocrinology. 1995;136:3194–3199. doi: 10.1210/endo.136.8.7628351. [DOI] [PubMed] [Google Scholar]
- 11.Schodin D J, Zhuang Y, Shapiro D J, Katzenellenbogen B S. J Biol Chem. 1995;270:31163–31171. doi: 10.1074/jbc.270.52.31163. [DOI] [PubMed] [Google Scholar]
- 12.Lazennec, G., Alcorn, J. L. & Katzenellenbogen, B. S. (1999) Mol. Endocrinol. 13, in press. [DOI] [PubMed]
- 13.Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. Science. 1994;264:1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- 14.Cavaillés V, Dauvois S, Danielian P S, Parker M G. Proc Natl Acad Sci USA. 1994;91:10009–10013. doi: 10.1073/pnas.91.21.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onate S A, Tsai S Y, Tsai M J, O’Malley B W. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 16.Hong H, Kohli K, Trivedi A, Johnson D L, Stallcup M R. Proc Natl Acad Sci USA. 1996;93:4948–4952. doi: 10.1073/pnas.93.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voegel J J, Heine M J S, Zechel C, Chambon P, Gronemeyer H. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 18.Lazennec G, Ediger T R, Petz L N, Nardulli A M, Katzenellenbogen B S. Mol Endocrinol. 1997;11:1375–1386. doi: 10.1210/mend.11.9.9983. [DOI] [PubMed] [Google Scholar]
- 19.Brzozowski A M, Pike A C W, Dauter Z, Hubbard R E, Bonn T, Engstrom O, Ohman L, Greene G L, Gustafsson J A, Carlquist M. Nature (London) 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 20.Fields S, Song O K. Nature (London) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 21.Mosselman S, Polman J, Dijkema R. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 22.McInerney E M, Weiss K E, Sun J, Mosselman S, Katzenellenbogen B S. Endocrinology. 1998;139:4513–4522. doi: 10.1210/endo.139.11.6298. [DOI] [PubMed] [Google Scholar]
- 23.Kraus W L, Weis K E, Katzenellenbogen B S. Mol Cell Biol. 1995;15:1847–1857. doi: 10.1128/mcb.15.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese J C, Katzenellenbogen B S. Mol Cell Biol. 1992;12:4531–4538. doi: 10.1128/mcb.12.10.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wrenn C K, Katzenellenbogen B S. J Biol Chem. 1993;268:24089–24098. [PubMed] [Google Scholar]
- 26.Montano M M, Katzenellenbogen B S. Proc Natl Acad Sci USA. 1997;94:2581–2586. doi: 10.1073/pnas.94.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terashima M, Kim K-M, Adachi T, Nielsen P J, Reth M, Kohler G, Lamers M C. EMBO J. 1994;13:3782–3792. doi: 10.1002/j.1460-2075.1994.tb06689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McInerney E M, Tsai M J, O’Malley B W, Katzenellenbogen B S. Proc Natl Acad Sci USA. 1996;93:10069–10073. doi: 10.1073/pnas.93.19.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J D, Evans R M. Nature (London) 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 30.Horlein A J, Naar A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass C K, et al. Nature (London) 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 31.Chen J D, Umesono K, Evans R M. Proc Natl Acad Sci USA. 1996;93:7567–7571. doi: 10.1073/pnas.93.15.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy L, Kao H Y, Chakravarti D, Lin R J, Hassig C A, Ayer D E, Schreiber S L, Evans R M. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 33.Zamir I, Dawson J, Lavinsky R M, Glass C K, Rosenfeld M G, Lazar M A. Proc Natl Acad Sci USA. 1997;94:14400–14405. doi: 10.1073/pnas.94.26.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson T A, Richer J, Bain D L, Takimoto G S, Tung L, Horwitz K B. Mol Endocrinol. 1997;11:693–705. doi: 10.1210/mend.11.6.0004. [DOI] [PubMed] [Google Scholar]
- 35.Kim K-M, Adachi T, Nielsen P J, Terashima M, Lamers M C, Kohler G, Reth M. EMBO J. 1994;13:3793–3800. doi: 10.1002/j.1460-2075.1994.tb06690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ansari-Lari M A, Shen Y, Muzny D M, Lee W, Gibbs R A. Genome Res. 1997;7:268–290. doi: 10.1101/gr.7.3.268. [DOI] [PubMed] [Google Scholar]
- 37.McClung J K, Jupe E R, Liu X-T, Dell’Orco R T. Exp Gerontol. 1995;30:99–124. doi: 10.1016/0531-5565(94)00069-7. [DOI] [PubMed] [Google Scholar]
- 38.Jupe E R, Liu X-T, Kiehlbauch J L, McClung J K, Dell’Orco R T. Cell Growth Differ. 1996;7:871–878. [PubMed] [Google Scholar]
- 39.Asamoto M, Cohen S M. Cancer Lett. 1994;83:201–207. doi: 10.1016/0304-3835(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 40.Thompson W E, Sanbuissho A, Lee G Y, Anderson E. J Reprod Fertil. 1997;109:337–348. doi: 10.1530/jrf.0.1090337. [DOI] [PubMed] [Google Scholar]
- 41.Liu X-T, Stewart C A, King R L, Danner D A, Dell-Orco R T, McClung J K. Biochem Biophys Res Commun. 1994;201:409–414. doi: 10.1006/bbrc.1994.1716. [DOI] [PubMed] [Google Scholar]
- 42.Nuell M J, Stewart D A, Walker L, Friedman V, Wood C M, Owens G A, Smith J R, Schneider E L, Orco R D, Lumpkin C K, et al. Mol Cell Biol. 1991;11:1372–1381. doi: 10.1128/mcb.11.3.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato T, Sakamoto T, Takita K-I, Saito H, Okui K, Nakamura Y. Genomics. 1993;17:762–764. doi: 10.1006/geno.1993.1402. [DOI] [PubMed] [Google Scholar]