Abstract
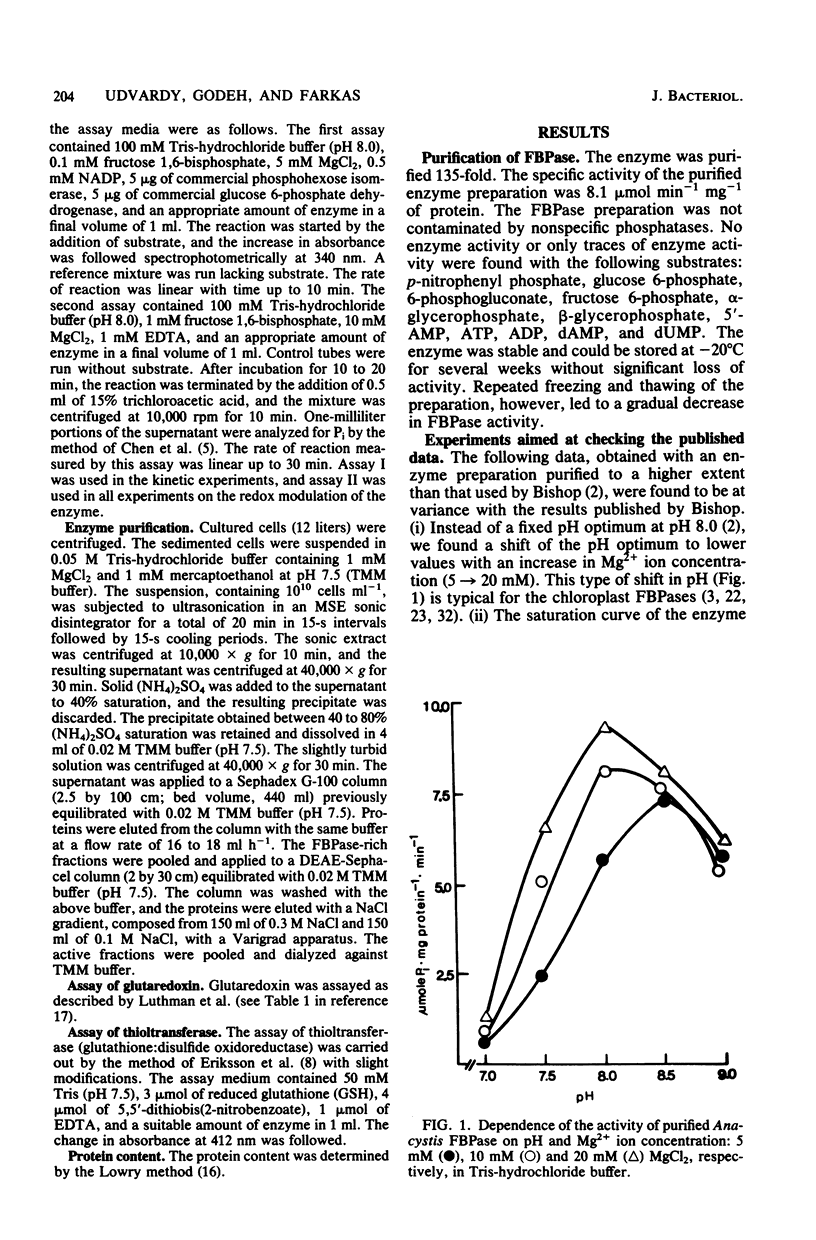
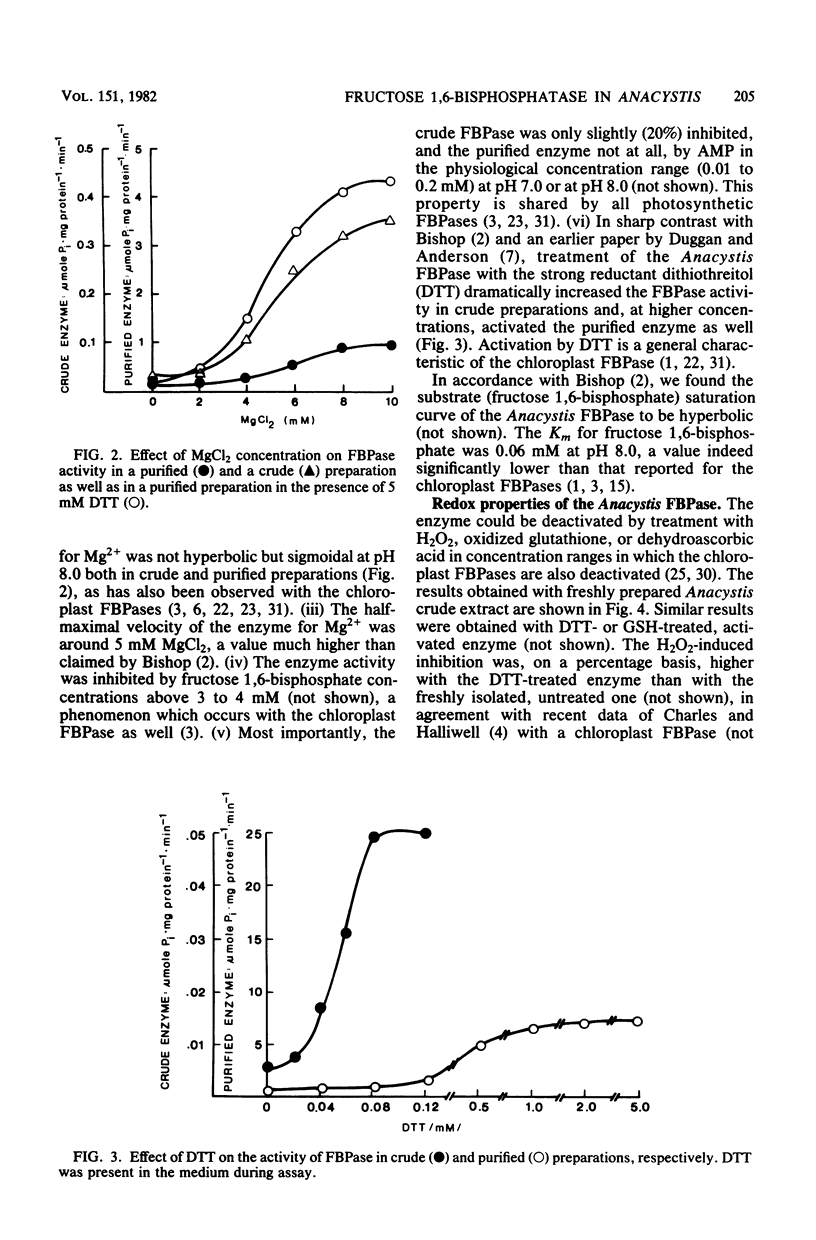
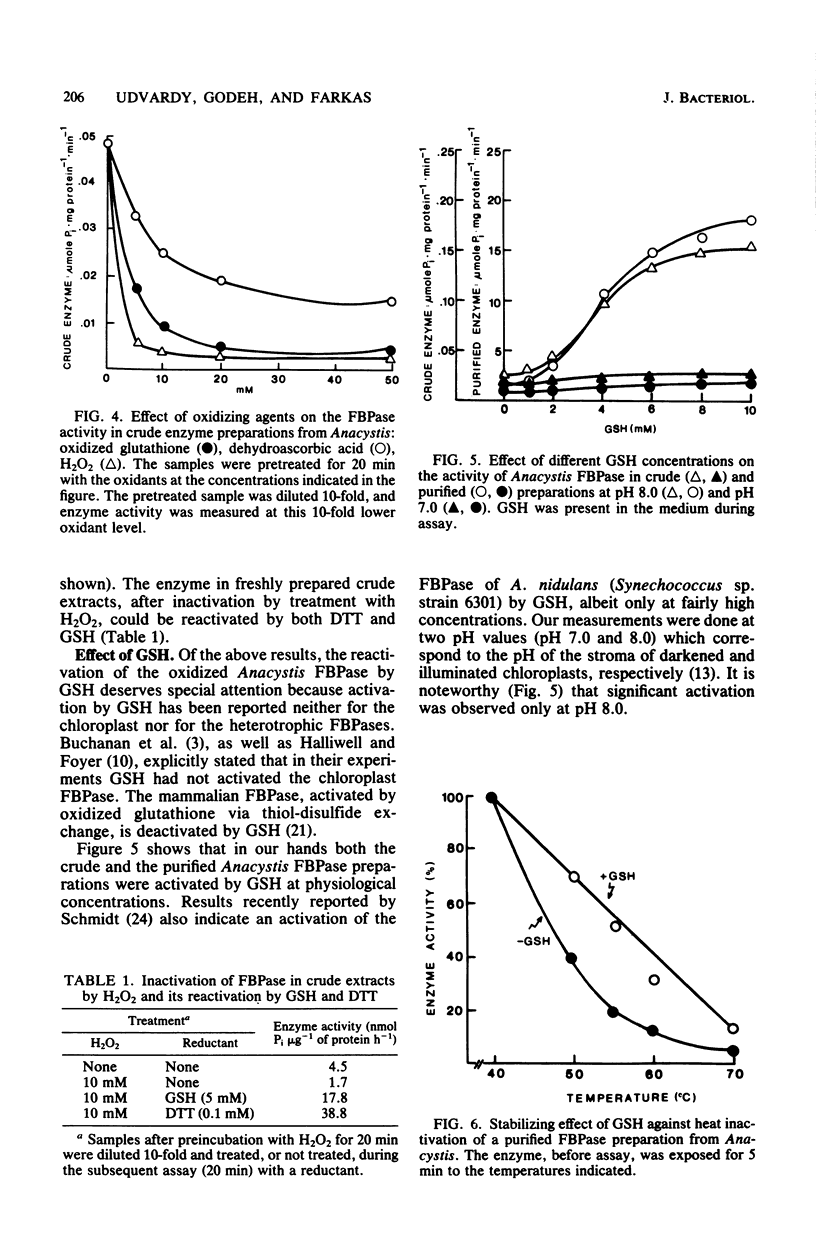
A fructose 1,6-bisphosphatase (EC 3.1.3.11) (FBPase) was purified over 100-fold from Anacystis nidulans. At variance with a previous report (R. H. Bishop, Arch. Biochem. Biophys. 196:295-300, 1979), the regulatory properties of the enzyme were found to be like those of chloroplast enzymes rather than intermediate between chloroplast (photosynthetic) and heterotrophic FBPases. The pH optimum of Anacystis FBPase was between 8.0 and 8.5 and shifted to lower values with increasing Mg2+ concentration. Under the experimental conditions used by Bishop, we found the saturation curve of the enzyme to be sigmoidal for Mg2+ ions and hyperbolic for fructose 1,6-bisphosphate. The half-maximal velocity of the Anacystis FBPase was reached at concentrations of 5 mM MgCl2 and 0.06 mM fructose 1,6-bisphosphate. AMP did not inhibit the enzyme. The activity of the FBPase was found to be under a delicate control of oxidizing and reducing conditions. Oxidants like O2, H2O2, oxidized glutathione, and dehydroascorbic acid decreased the enzyme activity, whereas reductants like dithiothreitol and reduced glutathione increased it. The oxido-reductive modulation of FBPase proved to be reversible. Reduced glutathione stimulated the enzyme activity at physiological concentrations (1 to 10 mM).l The reduced glutathione-induced activation was higher at pH 8.0 than at pH 7.0.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baier D., Latzko E. Properties and regulation of C-1-fructose-1,6-diphosphatase from spinach chloroplasts. Biochim Biophys Acta. 1975 Jul 8;396(1):141–148. doi: 10.1016/0005-2728(75)90197-8. [DOI] [PubMed] [Google Scholar]
- Bishop R. H. Regulatory characteristics of a fructose bisphosphatase from the blue-green bacterium Anacystis nidulans. Arch Biochem Biophys. 1979 Aug;196(1):295–300. doi: 10.1016/0003-9861(79)90579-4. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Chehebar C., Wolosiuk R. A. Studies on the regulation of chloroplast fructose-1,6-bisphosphatase. Activation by fructose 1,6-bisphosphate. Biochim Biophys Acta. 1980 Jun 13;613(2):429–438. doi: 10.1016/0005-2744(80)90097-2. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Askelöf P., Axelsson K., Carlberg I., Guthenberg C., Mannervik B. Resolution of glutathione-linked enzymes in rat liver and evaluation of their contribution to disulfide reduction via thiol--disulfide interchange. Acta Chem Scand B. 1974;28(8):922–930. doi: 10.3891/acta.chem.scand.28b-0922. [DOI] [PubMed] [Google Scholar]
- Freedman R. B. How many distinct enzymes are responsible for the several cellular processes involving thiol:protein-disulphide interchange? FEBS Lett. 1979 Jan 15;97(2):201–210. doi: 10.1016/0014-5793(79)80085-x. [DOI] [PubMed] [Google Scholar]
- Horecker B. L., Melloni E., Pontremoli S. Fructose 1,6-bisphosphatase: properties of the neutral enzyme and its modification by proteolytic enzymes. Adv Enzymol Relat Areas Mol Biol. 1975;42:193–226. doi: 10.1002/9780470122877.ch4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lara C., de la Torre A., Buchanan B. B. Ferralterin: an iron-sulfur protein functional in enzyme regulation in photosynthesis. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1337–1344. doi: 10.1016/0006-291x(80)90566-5. [DOI] [PubMed] [Google Scholar]
- Luthman M., Eriksson S., Holmgren A., Thelander L. Glutathione-dependent hydrogen donor system for calf thymus ribonucleoside-diphosphate reductase. Proc Natl Acad Sci U S A. 1979 May;76(5):2158–2162. doi: 10.1073/pnas.76.5.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Axelsson K. Role of cytoplasmic thioltransferase in cellular regulation by thiol-disulphide interchange. Biochem J. 1980 Jul 15;190(1):125–130. doi: 10.1042/bj1900125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelroy R. A., Bassham J. A. Photosynthetic and dark carbon metabolism in unicellular blue-green algae. Arch Mikrobiol. 1972;86(1):25–38. doi: 10.1007/BF00412397. [DOI] [PubMed] [Google Scholar]
- Pradel J., Soulié J. M., Buc J., Meunier J. C., Ricard J. On the activation of fructose-1,6-bisphosphatase of spinach chloroplasts and the regulation of the Calvin cycle. Eur J Biochem. 1981 Jan;113(3):507–511. doi: 10.1111/j.1432-1033.1981.tb05092.x. [DOI] [PubMed] [Google Scholar]
- Preiss J., Biggs M. L., Greenberg E. The effect of magnesium ion concentration on the pH optimum of the spinach leaf alkaline fructose diphosphatase. J Biol Chem. 1967 May 10;242(9):2292–2294. [PubMed] [Google Scholar]
- Schürmann P., Wolosiuk R. A. Studies on the regulatory properties of chloroplast fructose-1,6-bisphosphatase. Biochim Biophys Acta. 1978 Jan 12;522(1):130–138. doi: 10.1016/0005-2744(78)90329-7. [DOI] [PubMed] [Google Scholar]
- Soulié J. M., Buc J., Meunier J. C., Pradel J., Ricard J. Molecular properties of chloroplastic thioredoxin f and the photoregulation of the activity of fructose 1,6-bisphosphatase. Eur J Biochem. 1981 Oct;119(3):497–502. doi: 10.1111/j.1432-1033.1981.tb05635.x. [DOI] [PubMed] [Google Scholar]
- Vita A., Kido H., Pontremoli S., Horecker B. L. Inhibition of rabbit liver fructose 1,6-biphosphatase by AMP: effect of temperature and physiological concentrations of cations and anions. Arch Biochem Biophys. 1981 Jul;209(2):598–605. doi: 10.1016/0003-9861(81)90318-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase. Eur J Biochem. 1976 Nov 15;70(2):361–367. doi: 10.1111/j.1432-1033.1976.tb11025.x. [DOI] [PubMed] [Google Scholar]


