Abstract
Secondary copper (Cu) deficiency, chromium (Cr) deficiency and molybdenosis (Mo) has been suggested to cause the "mysterious" moose disease in the southwest of Sweden. The present experiment was performed on goats to investigate the clinical, chemical, and pathological alterations after 20 months feeding of a semi-synthetic diet deficient in Cu and Cr. Four groups were included in the study: control group (n = 4), Cu-deficient group (group 1, n = 4), Cr-deficient group (group 2, n = 2) and Cu+Cr-deficient group (group 3, n = 3). Group 3 was additionally supplemented with tetrathiomolybdate during the last 2 months of the experiment. Main histopathological findings in groups 1 and 3 were the lesions in the liver, characterised by a severe active fibrosis, bile duct proliferation, haemosiderosis and mild necroses. Additionally, degenerative alterations of the exocrine pancreas were prominent in groups 1 and 3. Lesions in group 3 were more pronounced than in group 1. In group 3, the skin showed an atrophic dermatosis, while in group 2 a crusty dermatitis caused by Candida spp. was observed. This study shows that liver, pancreas and skin are mainly affected by a long term deficiency of copper and the findings are complicated by molybdenum application while chromium deficiency produced no histomorphological effects in our study.
Keywords: molybdenosis, histopathology, liver, pancreas, moose.
Introduction
The aetiology of the "mysterious" moose disease observed in southwestern Sweden is not clearly understood. Secondary copper (Cu) deficiency, chromium (Cr) deficiency and molybdenosis (Mo) have been suggested to cause this disease [20,17].
The presence of Cu and its key role in vertebrate metabolism is well established. Several reviews concerning the role of Cu [29,7,21,44] and molybdenum [22,31] in animals are available. Deficiency of cuproenzymes have been linked to a variety of disease syndromes [17]. Defects which affect the cardiovascular system [6] and the skeleton [45] of Cu-deficient animals are biochemically related to disordered cross-linking of connective tissue proteins caused by a deficiency of lysyl oxidase [24]. Disorders of the nervous system [46] and achromotrichia [45] have been linked to a lack of cuproenzymes dopamine-β-hydroxylase and tyrosinase respectively. Cytochrome c oxidase catalyses the final step of the mitochondrial electron transport chain. It is important in energy production, and a deficiency can cause alterations in the gut [11], heart [49], pancreas [15], brain [46], and other energy dependent mechanisms. Ceruloplasmin is an oxidase that catalyses the oxidation of amines and ferrous iron. It is synthesised in the liver and its deficiency leads to anaemia and deposition of iron as haemosiderin in tissues, especially in the liver [29,23].
The effect of excessive Mo intake and Cu deficiency and an imbalance of these 2 metals and sulphur (S) in the feed is a complex nutritional problem in ruminants dependent on a number of variables, including species and age of the animal [22,31]. Biochemical interactions between Cu, Mo and S that can lead to Cu deficiency and/or molybdenosis occur predominantly in the gastrointestinal tract [53,43]. This may explain the differences in Mo toxicity seen between ruminants and non-ruminants that have been fed the same amount of dietary Mo. However, the cause of the species differences in ruminants are not fully understood until now.
Chromium deficiency is rare in animals and can lead to impaired glucose tolerance, hyperglycaemia, relative insulin resistance, peripheral neuropathy, and metabolic encephalopathy [52,5].
The present study on goats was performed to investigate the clinical, chemical and pathological alterations in an experimental model imitating the conditions suspected to initiate the "mysterious" moose disease [34,41,30].
More specifically, the aim of this long-term (20 months) study in goats was to investigate the morphological findings of Cu deficiency with and without supplementation of Mo, and the effects of Cr deficiency.
Materials and methods
The design of this experiment has been described in detail elsewhere [18]. Briefly, 20 male goats, (14 white and 6 brown, German Noble breed) 3 months of age at the start of the experiment, were used. Thirteen of the animals were available for pathological investigations at the end of the feeding period. The experiment was carried out with 4 groups of animals fed a semi-synthetic ration described by [1] containing different concentrations of Cu, Cr, Mo [18]: control group (11 mg Cu/kg DM, 1.4 mg Cr/kg DM, 0.54 mg Mo/kg DM) (n = 4), group 1 Cu-deficient (11 mg Cu/kg DM) (n = 4), group 2 Cr-deficient (0.355 mg Cr/kg DM) (n = 2), and group 3 Cu and Cr-deficient (0.96 mg Cu/kg DM, 0.31 mg Cr/kg DM) (n = 3). Group 3 was additionally fed tetrathiomolybdate (TTM) (10–40 mg Mo/kg DM) per day during the last 10 weeks of the experiment to accentuate the Cu deficiency. After 20 months, the animals were euthanised and pathological investigations performed. Macroscopical findings were documented, and the fore- and hind-legs were x-rayed. Specimens of liver, kidneys, spleen, intestinal lymphnodes, rumen (8 locations from different ruminal pillars and sacs of rumen), abomasum, gut (duodenum, jejunum, ileum), pancreas, adrenal glands, testes, lungs, heart, aorta, thyroid gland, skin (head, back), muscles (m. gluteus, m. semimembranosus), synovial capsule and joints (artt. genus, tarsi, cubiti, carpi), bones (femur, tibia, humerus, ulna), brain (cerebrum, cerebellum, medulla, brain stem), spinal cord and peripheral nerves (n. ischiadicus, n. femoralis) were fixed in formalin and embedded in paraplast. Bones were decalcified with Ossa fixona® (Diagonal, Münster, Germany) before embedding, and 2–3 μm sections were stained with Haematoxylin-Eosin, Picrosirius-Red, Turnbull-blue and Periodic Acid Schiff (PAS)-reaction according to McManus (Romeis 1989) was carried out.
Immunohistochemistry
Tissue sections of the skin were mounted on SuperFrost® slides (Menzel Gläser, Braunschweig, Germany). The PAP-method (peroxidase anti-peroxidase) was used to detect Candida spp. with a polyclonal rabbit anti-candida spp. antibody (1:400) (Biodesign, Dunn Labortechnik GmbH, Asbach, Germany) as the primary antibody. Parallel sections were incubated with rabbit serum for negative control. The slides were finally developed in DAB (diaminobenzidine tetrahydrochloride) and counterstained with Papanicolaous fluid.
The APAAP-method (alkaline phosphatase anti-alkaline phosphatase; Dako, Hamburg, Germany) was used to detect insulin, glucagon and NSE (neuron specific enolase) in the pancreas. The antibodies used were guinea-pig anti-insulin (1:1000, Dako, Hamburg, Germany), rabbit anti-glucagon (1:10, Dako, Hamburg, Germany) and mouse anti-NSE (1:20, Dako, Hamburg, Germany). Parallel sections were incubated with rat or rabbit serum, respectively, for negative control. The slides were developed in newfuchsin (Chroma-Gesellschaft, Köngen, Germany) and counterstained with haematoxylin (Merck, Darmstadt, Germany).
TUNEL -technique
Apoptotic cells in the pancreas were detected by TUNEL-technique (TdT-mediated dUTP-biotin nick end labelling). The slides were treated with proteinase K (Fluka, Neu Ulm, Germany) (3 μl/ml TBS) for 15 min. at room temperature. As specific positive control, caprine ovarian tissue was used. For nonspecific positive control, samples were incubated with DNase I (Boehringer Mannheim, Mannheim, Germany). For negative control, the slides were incubated with biotin only. All samples were incubated for 60 min. at 37°C with a mixture consisting of Terminal-deoxynucleotide transferase (TdT: 25U/25 μl, Boehringer Mannheim) and Biotin-dUTP (5 nM/50 ml, Boehringer Mannheim) followed. The Avidin-Biotin-Complex (Vectastain®, Vector Laboratories Inc., Burlingame, CA, USA) detection system was used.
Electronmicroscopy
Specimens of jejunum, colon and aorta were fixed in 3% glutaraldehyde, embedded in glycidether 100 and stained with uranylacetatelead. To demonstrate elastic and collagen fibers, the modified tannin-uranylactate-technique [40] was used.
Histometry
Morphometric results of the endocrine pancreas were evaluated with the colourimetric system Quantimet 500 (Leica, Wetzlar, Germany).
Results
Body weight
The most obvious differences between the 4 groups were the marked differences in body weight. The body weight (mean ± SD) of the animals in the control group was 34.8 ± 4.8 kg. In group 1 the body weight was 31.3 ± 7.0 kg, in group 2 it was 47.5 ± 14.9 kg and in group 3 it was 24.0 ± 4.5 kg. The post mortem examination showed that the higher body weight of the animals in group 2 was due to an increase of the subcutaneous adipose tissue and the fat tissue in the carcasses (omentum majus, omentum minus, mesenterium). Weight gain and feed consumption for the animals are presented elsewhere [18].
Macroscopic findings
The animals in group 2 showed a crusty dermatitis affecting the head but the hair colour and structure were normal.
In all animals except the control group, a red-yellowish coloured liver and a largely filled gall bladder were the main gross findings.
The gastrointestinal tracts of all animals contained smooth grey ingesta as usually found when feeding the present semi-synthetic diet. The intestinal mucosa and the mesenteric lymph nodes appeared normal. The lungs and the associated lymph nodes showed mild anthracosis. Macroscopic lesions were not seen in pancreas, kidneys, spleen, testes, eyes, heart, bones, joints and muscles in any group. X-ray examination of the fore and hind legs did not reveal any lesions in the bone structure.
Histopathological findings
Unless otherwise stated, the histomorphology of the tissues in the control group and group 2 revealed no histopathological findings.
Liver
A variety of histopathological changes was observed in goats in groups 1 and 3 but they were most pronounced in group 3 goats. The lesions diagnosed included a mild microvesicular steatosis and moderate bile duct proliferation (Fig. 1), multifocal mild periportal hepatocellular necroses accompanied by a moderate periportal bridging fibrosis (Fig. 2), moderate periportal deposits of haemosiderin within macrophages and hepatocytes (Fig. 3) and spread of collagen fibers between the irregular and pleomorphic hepatocytes.
Figure 1.
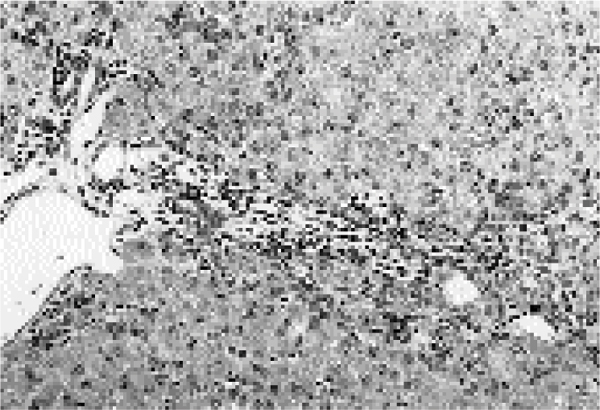
Liver from an animal in group 1 (Cu deficiency) showing a moderate proliferation of the bile ducts (b) and a portal fibrosis, a mild portal haemosiderosis (arrows). H.-E., magnification 62.5x
Figure 2.
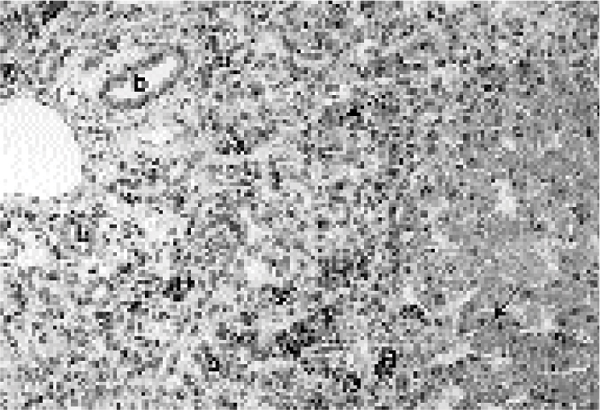
Liver from an animal in group 3 (Cu and Cr deficiency, supplemented with Mo) showing severe bile duct proliferation (b), signs of multifocal hepatocellular degeneration (arrows), an irregular arrangement of the hepatocytes, and moderate portal haemosiderosis (H). H.-E., magnification 62.5x
Figure 3.
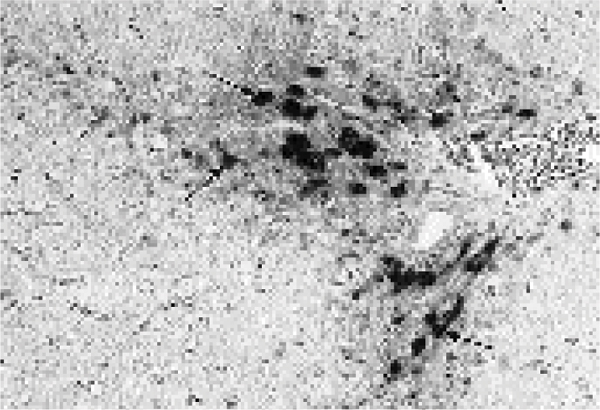
Liver from an animal in group 3 (Cu and Cr deficiency, supplemented with Mo) showing a moderate portal haemosiderosis (arrows). Turnbull staining, magnification 62.5x
Pancreas
Structural irregularity of the exocrine pancreas was observed in groups 1 and 3; but was most marked in group 3 animals (Fig. 4). Single cell necroses were accompanied by alterations of the acinar structure but the number of apoptotic bodies detected by the TUNEL-technique was not significantly increased. Many acini were significantly smaller and disorganised. PAS-reaction revealed a patchy discontinuity of the basement membranes in these acini. The pleomorphic cells showed a decreased number of cymogen granules and a diminished basophilic staining reaction.
Figure 4.

Pancreas from an animal in group 3 (Cu and Cr deficiency, supplemented with Mo) showing an irregular arrangement of the acinar structures, signs of a multifocal eosinophilic degeneration affecting the acinar cells (arrows), but a regular structure of the endocrine islets (i). H.-E., magnification 62.5x
The endocrine islets of the goats in the control group and groups 1 and 2 contained 30% glucagon and 70% insulin producing cells. The proportion of glucagon-producing cells in the islets was slightly diminished in group 3 (about 25% glucagon and 75% insulin producing cells). Apart from this, pancreatic islets of group 3 goats were morphologically normal.
Cardiovascular system
No major histopathological changes were observed in the myocardium, endocardium, epicardium, coronary vessels or conduction system.
However the histological and ultrastructural findings in the aortal walls varied individually as well as within the control group: The normal findings, regular bands of mature elastic fibers, surrounded by small amounts of collagen, and numerous myocytes within the media, were seen in some individuals of each group. However, in several animals from every group (control: 2/4, group 1: 1/4, group 2: 1/2, group 3: 1/3) swollen and fragmentated immature elastic fibres were observed within the intima. For the most part, the media appeared regular, but in some areas, the myocytes were shrunken and widely separated from each other by accumulation of elastin and collagen fibres. In some animals (control: 1/4, group 1: 2/4, group 2: 0/2, group 3: 2/3) the number of collagen fibres was moderately increased, forming whirly bundles.
Skin
In the white goats it was impossible to detect any signs of achromotrichia. But also in the 6 coloured animals no signs of achromotrichia or an irregular structure of the hair appeared.
Histopathologically, the goats in group 1 showed a moderate orthokeratotic hyperkeratosis of the epidermis at the abdomen and the back, but no signs of inflammation.
The dermatitis of the head of the animals in group 2 was histopathologically characterised by a severe orthokeratotic hyperkeratosis, a moderate perivascular infiltration of eosinophilic granulocytes and a moderate interstitial edema. Immunohistologically, masses of Candida spp. were detected on the epidermal surface and within the infundibula of the hairs. The skin at the abdomen and the back was without histopathological alterations.
In group 3, a severe orthokeratotic hyperkeratosis and a moderate atrophy of the epidermis and the integumentary appendages was observed at the abdomen and the back. The hair follicles mostly stayed in the telogen phase.
Gastrointestinal tract
In general the rumenal epithelium was covered by a thick layer of completely keratinised long slender eosinophilic cells, except in group 3 goats that showed incomplete keratinisation.
In the small intestine, no histopathological alterations were seen in any group.
Ultrastructurally, the enterocytes of the small intestine appeared normal. The enterocytes of the colon of some animals (control: 2/4, group 1: 2/4, group 2: 1/2, group 3: 1/3) showed dilated intercellular spaces and mildely to moderately swollen mitochondria containing condensed amorphous material but vacuolated endoplasmatic reticula were not observed. These findings occurred in some of the controls as well and therefore were not interpreted as group specific.
Kidneys
In the animals of group 2, a mild, moderate or severe proliferation of mesangial cells was observed. In group 3 goats, the glomerula appeared partially atrophic, and numerous proximal tubuli showed a mild vacuolar epithelial degeneration.
Testes
The histomorphological findings of the testes varied individually within the groups. The seminiferous tubules in most animals (control: 3/4, group 1: 2/4, group 2: 1/2, group 3: 2/3) consisted of normal germinal epithelium with several cell layers. But according to the time of the year (March: outside the breeding season) the germinal epithelium was not very active and showed signs of mild testicular degeneration, characterised by vacuolated germinal epithelium and few multinucleated giant cells deriving from spermatogonia. In each group, severe testicular degeneration and atrophy was observed in one animal, indicating that these findings are not group specific.
Lymphatic tissues
All animals showed a low to moderate activity of the lymphatic tissues (spleen, lymph nodes) characterised by activated secondary lymphfollicles. The main histopathological finding of the spleen was an individually varying degree of diffuse haemosiderosis.
Adrenal glands, thyroid glands, and parathyroid glands
No histomorphologic alterations were observed in these tissues.
Lungs
Histologically, a mild hyperaemia, alveolar emphysema and anthracosis was seen in the animals of all groups.
Bones, joints, muscle
Histologically, the bones from all animals showed a regular structure and proportions with a normal degree of mineralisation. Osteoporosis was not observed in any case.
Eyes, brain, spinal cord, peripheral nerves
A mild to moderate satellitosis was seen in the cerebrum of animals from groups 1 and 2. No degenerative processes were obvious in the eyes, brain, spinal cord or peripheral nerves of any animal.
It can be summarized that specific pathological findings were made in the liver, pancreas and skin, while alterations observed in vessels, testes and gut were not specific for any group or the control.
Discussion
This study presents the results of a histopathological examination of 13 male goats fed a semi-synthetic diet containing different amounts of Cu, Cr and Mo for 20 months.
Several studies have reported effects of Cu deficiency and/or molybdenosis affecting skin, skeleton, cardiovascular system, liver, pancreas, gut and nervous system, in a variety of species, with different ages and durations of exposure [28,37,38,17,31,7].
In contrast to sheep [46] and cattle [45] very few pathological lesions were seen in the Cu and Cr deficient goats of this study although they had been subjected to a rather severe trace element deficiency and/or imbalance. This is, however, in agreement with the findings of [43] and [37,38].
In our experiment changes were observed in liver, skin and pancreas of the animals in groups 1 and 3. In general, the lesions in the goats in group 3 were more marked probably due to a synergistic effect of Cu deficiency and molybdenosis.
A striking difference between the experimental groups was the increase in body weight of Cr-deficient animals (group 2) caused by an accumulation of subcutaneous fat and adipose tissue in the carcasses [18]. Although feed consumption, weight development and some of the clinical chemical findings were aberrant in group 2 [18,19], no conclusive changes were observed from a morphological point of view.
Reports on morphological alterations of the liver caused by Cu deficiency and/or molybdenosis are limited [14]. In the groups 1 and 3, a mild fatty degeneration of the liver, hepatocellular necrosis, periportal fibrosis, bile duct proliferation and haemosiderosis were observed. These findings are in accordance with the results in an earlier experiment with Mo exposure of goats for 235 days [37,38]. In the rat, molybdenum stimulates accumulation of lipids and phospholipids within the hepatocytes by inhibiting mitochondrial phosphorylation [33] leading to hepatocellular degeneration and focal necroses [2].
The hepatic alterations detected in our study were supported by clinical chemistry findings with high bilirubin levels and glutamate-dehydrogenase activities. But also elevated levels of aluminium, iron and lead were detected in the liver [19], mostly in Cu deficient animals of group 3. The lesions in the liver may have been responsible for the anaemia and maldigestion observed in animals in group 3 [19]. Similar symptoms are described in goats suffering from cobalt deficiency [3]. It can be excluded in our study because [19] found increased levels of cobalt in the liver of the goats of groups 1, 2 and 3 compared with the control.
In the exocrine parts of pancreas, degeneration, acinar irregularity and loss of basement membranes were seen in groups 1 and 3 animals. Similar pancreatic lesions have been described in copper deficient rats [15,9,26], in cattle [13] and in guinea pigs with molybdenosis [25]. It may be speculated that these alterations contributed to the maldigestion and malnutrition and also the decreased feed intake and loss of body weight in the goats [18].
[16] reported that plasma insulin levels were increased in rats with Cu deficiency, more in female than in male animals. Morphological and functional alterations of the islets of Langerhans cells have not been previously described. Correlations between the clinically diagnosed hyperinsulinaemia in the present groups 2 and 3 [19] and the histopathological findings in the endocrine pancreas were not seen in group 2 animals, but in group 3 the number of glucagon-producing cells was slightly diminished.
In the gut histopathological or ultrastructural changes were not evident. This is in accordance with earlier studies in goats [38,39] and cattle [8,45] suffering from molybdenosis or Cu deficiency [12]. However, the incomplete keratinisation of the rumen wall observed in group 3 has not to our knowledge been reported earlier.
Although the metal concentrations were severely altered in the kidneys of all experimental groups [19], very mild non-specific degenerative lesions were observed only in the kidneys of animals in group 1 and 3. [10] stated that the kidney lesions in Cu deficient rats were most likely caused by vascular malfunction and not a direct effect of Cu deficiency. Chronic exposure to high doses of Mo have been found to induce mild chronic renal failure characterised by decreased glomerular filtration rates in rats [4].
Morphological findings of the cardiovascular system showed a very high degree of individual variation in the present study, and were not specific for any group. In earlier studies, vascular lesions like haemorrhages and blood vessel rupture have been described in Cu deficient cattle [45], piglets [32,6,50], chicken [39] and moose [41].
It has been reported that goats suffering from molybdenosis exhibit testicular degeneration [37]. A decreased volume of ejaculate, decreased sperm concentration, and sperm motility have also been reported in Cu deficient rats [47,48]. But in the present study, no such lesions were observed.
Copper deficiency leads to achromotrichia in cattle [45] and molybdenosis results in depigmentation of the skin in cows [8], goats [37,38] and defective hair structure in sheep [28]. None of these changes were found in our study. However, the goats belonging to group 3 were characterised by a moderate atrophy of the skin, similar to findings induced by several endocrinological disturbances [36]. [19] described lowered T4 levels in the group 3 goats, which may explain these findings. However, we did not find any morphological alterations of the thyroid gland with the methods used in our study. Additionally the symptoms may have been induced by an alteration of the Cu-dependent enzyme lysyl oxidase which is responsible for the maturing of collagen fibers and elastin [24,29,17].
Group 2 animals were characterised by a crusty dermatitis of the head caused by Candida spp. There is no direct explanation for this observation.
No x-ray or histological changes were seen in the bones of any of the present goats. Growth arrest at the growth plates of the distal extremities have been reported in goats fed high doses of Mo for 70 days but no further lesions were reported after 235 days [37,38]. In Cu-deficient calves, growth arrest and osteoporosis are typical findings [27,45]. The absence of lesions in the present study could possibly be related to the age of the goats and/or the duration of the Mo exposure, as described by [37,38].
Neurological symptoms are prominent signs of severe Cu deficiency induced during pregnancy in weaned lambs (sway back) [46] and rats [42]. However, Cu deficiency in our study was induced in 3-month-old animals and not in their mothers, so this type of neurological lesions could not be expected.
It can be assumed that the pathological findings in this long term study of Cu deficiency and the effect of Mo supplementation in goats mainly are caused by effects of an insufficient activity of lysyloxidase (skin), cytochrome-oxidase (liver, pancreas) and caeruloplasmin activity (haemosiderosis) [17]. The clinical chemistry findings showed that molybdenosis will not only aggravate the lesions of Cu deficiency, but also has effects of its own [19]. Group 2 goats with Cr deficiency, showed very little morphological changes apart from the very obvious accumulation of body fat. Achromotrichia and nervous symptoms were not evident indicating that probably tyrosinase and dopamin β-hydroxylase may be less affected in the goats of our experiment. The age of the animals, the duration of the experiment and the fact that goats are less sensitive to Cu deficiency and molybdenosis than other ruminants [17] may explain the differing results found in our study compared with other reports.
Acknowledgments
Acknowledgements
The study was performed with an economic support from the Swedish Environmental Protection Agency, Stockholm, Sweden.
References
- Anke M, Groppel B. Flourmangelerscheinungen bei der Ziege. (Signs of flourdeficiency in the goat.) In: Anke M et al, editor. Mengen- und Spurenelemente Friedrich-Schiller-Universität, Jena, Germany. Leipzig: VEB Kongress- und Werbedruck Oberlungwitz; 1989. pp. 346–363. [Google Scholar]
- Bandyopadhyay SK, Chatterjee K, Tiwari RK, Mitra A, Banerjee A, Ghosh KK, Chatterjee GC. Biochemical studies on molybdenum toxicity in rats: effects of high protein feeding. Internat. J Vit Nutr Res. 1981;51:401–409. [PubMed] [Google Scholar]
- Black H, Hutton JB, Sutherland RJ, James MP. White liver disease in goats. N Z Vet J. 1988;36:15–17. doi: 10.1080/00480169.1988.35465. [DOI] [PubMed] [Google Scholar]
- Bompart G, Pecher C, Precot D, Girolami J-P. Mild renal failure induced by subchronic exposure to molybdenum: urinary kallikrein excretion as a marker of distal tubular effect. Tox Lett. 1990;52:293–300. doi: 10.1016/0378-4274(90)90039-O. [DOI] [PubMed] [Google Scholar]
- Brown RO, Forloines-Lynn S, Cross RN, Heizer WD. Chromium deficiency after long-term total parenteral nutrition. Dig Dis Sci. 1986;31:661–664. doi: 10.1007/BF01318699. [DOI] [PubMed] [Google Scholar]
- Coulson WF, Carnes WH. Cardiovascular studies on copper-deficient swine. V. The histogenesis of the coronary artery lesions. Am J Pathol. 1963;43:945–954. [PMC free article] [PubMed] [Google Scholar]
- Davis GK, Mertz W. Copper. In: Mertz W, editor. Trace elements in human and animal nutrition. Vol. 1. San Diego, New York, Berkeley, Boston, London, Sydney, Tokyo, Toronto: Academic Press; 1987. pp. 301–364. [Google Scholar]
- Dollahite JW, Rowe LD, Cook LM, Hightower D, Mailey EM, Kyzar JR. Copper deficiency and molybdenosis intoxication associated with grazing near a uranium mine. Southw Vet. 1972;26:47–50. [Google Scholar]
- Dubick GS, Yu M, Majumdar APN. Morphological and biochemical changes in the pancreas of the copper-deficient female rat. J Nutr. 1989;119:1165–1172. doi: 10.1093/jn/119.8.1165. [DOI] [PubMed] [Google Scholar]
- Fell BF. In: The pathology of copper deficiency of animals. Howell JMcC, Gawthorne JM, editor. Vol. 2. Copper in animals and man. Boca Raton, CRC Press; 1987. pp. 1–28. [Google Scholar]
- Fell BF, Dinsdale D, El-Gallad TT. Gut pathology of rats dosed with tetrathiomolybdate. J Comp Pathol. 1979;89:495–513. doi: 10.1016/0021-9975(79)90042-2. [DOI] [PubMed] [Google Scholar]
- Fell BF, Dinsdale D, Mills CF. Changes in enterocyte mitochondria associated with deficiency of copper in cattle. Res Vet Sci. 1975;18:274–281. [PubMed] [Google Scholar]
- Fell BF, Farmer LJ, Farquharson C, Bremner I, Graca DS. Observations on the pancreas of cattle deficient in copper. J Comp Pathol. 1985;95:573–590. doi: 10.1016/0021-9975(85)90027-1. [DOI] [PubMed] [Google Scholar]
- Fell BF, Farquharson C, Riddoch GI. Kidney lesions in copper-deficient rats. J Comp Pathol. 1987;97:186–196. doi: 10.1016/0021-9975(87)90039-9. [DOI] [PubMed] [Google Scholar]
- Fell BF, King TP, Davies NT. Pancreatic atrophy in copper-deficient rats: histochemical and ultra-structural evidence of a selective effect on acinar cells. Histochem J. 1982;14:665–680. doi: 10.1007/BF01011899. [DOI] [PubMed] [Google Scholar]
- Fields M, Lewis CG. Impaired endocrine and exocrine pancreatic functions in copper-deficient rats: the effect of gender. J Am Coll Nutr. 1997;16:346–351. doi: 10.1080/07315724.1997.10718696. [DOI] [PubMed] [Google Scholar]
- Frank A. "Mysterious" moose disease in Sweden. Similarities to copper deficiency and/or molybdenosis in cattle and sheep. Biochemical background of clinical signs and organ lesions. Sci Total Environ. 1998;209:17–26. doi: 10.1016/S0048-9697(97)00303-3. [DOI] [PubMed] [Google Scholar]
- Frank A, Anke M, Danielsson R. Experimental copper and chromium deficiency and additional molybdenum supplementation in goats. I. Feed consumption and weight development. Sci Total Environ. 2000;249:133–142. doi: 10.1016/S0048-9697(99)00517-3. [DOI] [PubMed] [Google Scholar]
- Frank A, Danielsson R, Jones B. Experimental copper and chromium deficiency and additional molybdenum supplementation in goats. II. Concentrations of trace and minor elements in liver, kidneys and ribs: haematology and clinical chemistry. Sci Total Environ. 2000;249:143–170. doi: 10.1016/S0048-9697(99)00518-5. [DOI] [PubMed] [Google Scholar]
- Frank A, Galgan V, Petersson LR. Secondary copper deficiency, chromium deficiency and trace element imbalance in the moose (Alces alces L.) Effect of anthropogenic activity. Ambio. 1994;23:315–317. [Google Scholar]
- Gooneratne SR, Buckley WT, Christensen DA. Review of copper deficiency and metabolism in ruminants. Can J Anim Sci. 1989;69:819–845. [Google Scholar]
- Hainline BE, Rajagopalan KV. In: Molybdenum in animal and human health. in: Trace elements in health, a review of current sites. Rose J, editor. London Butterworth; 1983. pp. 150–166. [Google Scholar]
- Harris ED. The iron – copper connection: The link to ceruloplasmin grows stronger. Nutr Rev. 1995;53:170–173. doi: 10.1111/j.1753-4887.1995.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Hill CH, Starcher B, Kim C. Role of copper in the formation of elastin. Fed Proc. 1967;26:129–133. [PubMed] [Google Scholar]
- Howell JM, Shunxiang Y, Gawthorne JM. Effect of thiomolybdate and ammonium molybdate in pregnant guinea pigs and their offspring. Res Vet Sci. 1993;55:224–230. doi: 10.1016/0034-5288(93)90085-t. [DOI] [PubMed] [Google Scholar]
- Ide H, Yelandi AV, Reddy JK, Rao MS. Increased expression of sulfated glycoprotein-2 and DNA fragmentation in the pancreas of copper-deficient rats. Toxicol Appl Pharm. 1994;126:174–177. doi: 10.1006/taap.1994.1104. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Poulos PW, Smith BP, Fisher GL. Radiology and histopathology of lameness in young cattle with secondary copper deficiency. J Comp Pathol. 1974;84:611–621. doi: 10.1016/0021-9975(74)90052-8. [DOI] [PubMed] [Google Scholar]
- Ishida N, Kawashima R. Histochemical studies of the skin and wool in sheep reared on high molybdenum diet. Jap J Zootech Sci. 1974;45:352–360. [Google Scholar]
- Kay RG. Zinc and copper in human nutrition. J Hum Nutr. 1981;35:25–36. doi: 10.3109/09637488109143483. [DOI] [PubMed] [Google Scholar]
- Merza M, Larsson E, Stéen M, Morein B. Association of a retrovirus with a wasting condition in the Swedish moose. Virology. 1994;202:956–961. doi: 10.1006/viro.1994.1418. [DOI] [PubMed] [Google Scholar]
- Mills CF, Davis GK. Molybdenum. In: Mertz W, editor. Trace elements in human and animal nutrition. Vol. 1. San Diego, New York, Berkeley, Boston, London, Sydney, Tokyo, Toronto: Academic Press; 1987. pp. 429–457. [Google Scholar]
- Pletcher JM, Banting LF. Copper deficiency in piglets characterized by spongy myelopathy and degenerative lesions in the great blood vessels. J South Afr Vet Assoc. 1983;3:43–46. [PubMed] [Google Scholar]
- Rana SVS, Kumar A, Bhardwaj NG, Kumar A. Lipids in the liver and kidney of rats, fed various heavy metals. Acta Anat. 1980;108:402–412. doi: 10.1159/000145323. [DOI] [PubMed] [Google Scholar]
- Rehbinder C, Gimeno E, Belák K, Belák S, Stéen M, Rivera E, Nikkilä T. A bovine viral diarrhoea/mucosal disease-like syndrom in moose (Alces alces L.): investigations on the central nervous system. Vet Rec. 1991;129:552–554. [PubMed] [Google Scholar]
- Romeis B. In: Mikroskopische Technik. (Microscopic technique) P Böck, editor. Urban und Schwarzenberg, München, Wien; 1987. [Google Scholar]
- Scott DW. Endocrine diseases. in: Large animal Dermatology, W.B. Saunders Company; 1988. pp. 374–387. [Google Scholar]
- Sharma AK, Parihar NS. Pathology of experimental molybdenosis in goats. Ind J Anim Sci. 1994;64:114–119. [Google Scholar]
- Sharma AK, Parihar NS. Clinicopathology of induced molybdenum toxicity in young goats. Ind J Anim Sci. 1994;64:120–125. [Google Scholar]
- Simpson CF, Boucek RJ, Noble NL. Similarity of aortic pathology in Marfan's syndrome, copper deficiency in chicks and B-Aminopropionitrile toxicity in turkeys. Exp Mol Path. 1980;32:81–90. doi: 10.1016/0014-4800(80)90045-3. [DOI] [PubMed] [Google Scholar]
- Stadler R. Elektronenmikroskopische Untersuchung der elastischen Fasern der Haut in Abhängigkeit vom Alter. (Electronmicroscopic studies of elastic fibers of the skin related to the age) Köln, Univ Fachber Medizin Diss. 1978.
- Stéen M, Diaz R, Faber WE. An erosive/ulcerative alimentary disease of undetermined etiology in Swedish moose (Alces alces L.) Rangifer. 1993;13:149–156. [Google Scholar]
- Sun SH-H, O'Dell BL. Elevated striated levels of glial fibrillary acidic protein associated with neuropathology in copper-deficient rats. J Nutr Biochem. 1992;3:503–509. doi: 10.1016/0955-2863(92)90071-P. [DOI] [Google Scholar]
- Suttle NF. The role of comparative pathology in the study of copper and cobalt deficiencies in ruminants. J Comp Pathol. 1988;99:241–258. doi: 10.1016/0021-9975(88)90048-5. [DOI] [PubMed] [Google Scholar]
- Suttle NF. In: Trace element disorders. Andrews AH, Blowey RW, Boyed H, Eddy RG, editor. Bovine Medicine, Diseases and husbandry of cattle. London: Blackwell Scientific Publ; 1992. pp. 263–265. [Google Scholar]
- Suttle NF, Angus KW. Experimental copper deficiency in the calf. J Comp Pathol. 1976;86:595–608. doi: 10.1016/0021-9975(76)90068-2. [DOI] [PubMed] [Google Scholar]
- Suttle NF, Field AC, Barlow RM. Experimental copper deficiency in sheep. J Comp Pathol. 1970;80:151–162. doi: 10.1016/0021-9975(70)90042-3. [DOI] [PubMed] [Google Scholar]
- Van Niekerk FE, Van Niekerk CH. The influence of experimentally induced copper deficiency on the fertility of rams. I. Semen parameters and peripheral plasma androgen concentration. J South Afr Vet Assoc. 1989;60:28–31. [PubMed] [Google Scholar]
- Van Niekerk FE, Van Niekerk CH. The influence of experimentally induced copper deficiency on the fertility of rams. II. Macro- and Microscopic changes in the testes J. South Afr Vet Assoc. 1989;60:32–35. [PubMed] [Google Scholar]
- Waisman J. The ultrastructure and histochemistry of the myocardium in copper-deficient pigs. Fed proc. 1972;31:627. [Google Scholar]
- Waisman J, Carnes WH. Cardiovascular studies on copper-deficient swine. Am J Pathol. 1967;51:117–135. [PMC free article] [PubMed] [Google Scholar]
- Waisman J, Carnes WH, Weissman N. Some properties of the microfibrils of vascular elastic membranes in normal and copper-deficient swine. Am J Pathol. 1969;54:107–119. [PMC free article] [PubMed] [Google Scholar]
- Wallach S. Clinical and biochemical aspects of chromium deficiency. J Am College Nutr. 1985;4:107–120. doi: 10.1080/07315724.1985.10720070. [DOI] [PubMed] [Google Scholar]
- Ward GM. Molybdenum toxicity and hypocuprosis in ruminants: a review. J Anim Sci. 1978;46:1078–1085. doi: 10.2527/jas1978.4641078x. [DOI] [PubMed] [Google Scholar]


