Abstract
The usage of veterinary antibacterial drugs in dogs and cats in Sweden and Norway for the period 1990–1998 was investigated by use of drug wholesalers' statistics. Additionally, usage of human antibacterial drugs in these species in Sweden was investigated by use of prescription data for the period 1996–1998. On average, more than 50% of the prescribed veterinary antibacterials in Sweden were beta-lactam antibiotics. In Norway, about 75% of the preparations prescribed for dogs and cats contained sulfonamides and trimethoprim. Furthermore, the prescription data from Sweden showed a reduced usage of human antibacterials prescribed for dogs and cats since the beginning of the 1980s. Approximately 20% of the prescribed packages for dogs in the years 1996–1998 were human approved drugs. The corresponding figure for cats was 13%. The differences between the countries in the choice of antibacterial drugs can be explained by differences in the availability of approved preparations during the study period. The consumption of veterinary antibacterials in dogs and cats in Sweden during the period was in the range of 3% to 8% of the total use of veterinary antibacterials. The corresponding figures in Norway were in the range of 3% to 7%. It is of vital importance to study usage patterns of antibacterial drugs in dogs and cats in surveillance and control of bacterial resistance, but also in discussions of therapeutic appropriateness. Therefore, further research is needed in this area.
Keywords: antimicrobial, antibiotics, antibacterials, drug statistics, prescription data, wholesalers' statistics
Introduction
The major route of transmission of resistant bacteria or genes from animals to humans is thought to be via the food chain [18]. Published drug statistics have therefore focused on antibacterial drug use in food producing animals because of the fear of food-borne transmission of antibacterial drug resistant organisms to humans and the resulting human health implications. However, because companion small animals, i.e. dogs and cats, live in close contact with their owners, the potential for transmission of resistant bacteria to humans and of resistance genes to human commensals and pathogenes should not be overlooked. Therefore, information about the consumption of antibacterial drugs in companion small animals should be included in assessing the risk of the spread of bacterial resistance.
Information on the choice and usage of antibacterials is also of interest from a clinical viewpoint in terms of efficacy, appropriateness and limiting the development of resistance in veterinary pathogenes, and might initiate therapy discussions both nationally and internationally. In comparison with most other countries, Sweden and Norway have the advantage of having access to overall sales statistics of veterinary drugs from the national drug wholesalers. In addition, detailed veterinary prescription data have been available in Sweden since 1996. Such data can be utilised to interpret the wholesalers' figures.
The main aims of the present study were to estimate the usage and analyse trends in the usage pattern of antibacterial drugs in dogs and cats in Sweden and Norway during 1990–1998 by use of wholesalers' statistics. The usage pattern and proportional usage in dogs and cats of human antibacterial preparations in Sweden in the period 1996–1998 were also investigated by use of prescription data.
Materials and methods
In both Sweden and Norway, all antibacterial preparations used in animals are prescription drugs. Moreover, drugs intended for use in animals have to be sold by pharmacies, which in turn are supplied by authorised drug wholesalers, 2 in Sweden and 3 in Norway.
Wholesaler data 1990–1998
In both countries, wholesalers' data have a very high degree of completeness. This is explained by the fact that the wholesalers represent the entire drug distribution network (i.e. there are no other sources of antibacterials for use or prescription by veterinarians) and that all pharmaceutical companies use the wholesalers to distribute their products.
In Norway, overall sales data from the Norwegian drug wholesalers are collected by the stateowned drug wholesaler Norwegian Medicinal Depot AS on behalf of the Directorate of Health. In Sweden, the corresponding data are available from Apoteket AB (The National Corporation of Swedish Pharmacies, Stockholm). Relevant data were collected from both sources for the years 1990–1998. Because overall sales figures of drugs from wholesalers provide a good approximation of the prescribing of these drugs, the terms usage patterns, use, usage and consumption are used synonymously with sales figures in this paper.
Classification system
In Norway and Sweden, the Anatomical Therapeutic Chemical veterinary classification system (ATCvet) is used to classify veterinary medicinal products [13] and was the system employed in this study.
All antibacterial veterinary preparations approved only for use in dogs and/or cats were included in the study (ATCvet code = QJ01), thus excluding otic, ophtalmic and topical preparations. However, only oral antibacterial preparations were recorded, because some injectable antibacterial veterinary drugs approved for use in dogs and/or cats are also approved for other animal species and, therefore, their exact use cannot be determined from the ATCvet system. "Veterinary" drugs are preparations approved only for use in animals and have "vet" included in their brand names. "Human" drugs are preparations originally approved for use in humans, but may also be approved for use in some animal species.
Unit of measurement
Annual sales figures, in kg and in number of packages sold, for the preparations included in this study were calculated based on sales figures collected from the wholesalers in both countries for 1990–1998.
Swedish prescription data 1996–1998
Both in Sweden and Norway, electronic records of veterinary prescriptions, i.e. species, prescribed drug, strength, formulation, package size, and number of packages dispensed, are kept by the pharmacies as part of the dispensing process. Moreover, since January 1st 1996, data on all veterinary prescriptions dispensed in Sweden have been recorded in a centralised data system owned by Apoteket AB. It should be emphasised that the information in the database does not include the name either of the animal owner or of the veterinarian, therefore, confidentiality is maintained. The proportional usage of human approved antibacterial drugs in dogs and cats was derived from the Apoteket data system, number of packages being the unit of measurement. A corresponding centralised prescription database has not yet been established in Norway.
Results
Wholesaler data
The amounts, in kg active substance, of oral veterinary antibacterial drugs approved for use in dogs and/or cats sold by wholesalers to pharmacies in Sweden are presented in Table 1. The corresponding data for Norway is shown in Table 2. In Norway, the usage increased gradually from 262 kg active substance in 1990 to 468 kg in 1998. The figures for Sweden for the same period were 863 kg and 1612 kg, the usage peaking in 1994 with 1716 kg active substance.
Table 1.
The amounts, in kg active substance, of oral veterinary antibacterial drugs (QJ01) approved for use in dogs and/or cats sold by wholesalers to pharmacies in Sweden in 1990–1998.
| ATC-group | Class of drug | Active substances | 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 |
| QJ01A | Tetracyclines | Doxycycline, oxytetracycline | 63 | 75 | 72 | 77 | 69 | 66 | 69 | 70 | 78 |
| QJ01C | Beta-lactam antibiotics | Ampicillin, amoxycillin Phenoxymethylpenicillin Amoxycillin + clavulanic acid | 724 | 757 | 821 | 1115 | 1373 | 1257 | 1182 | 1108 | 1020 |
| QJ01D | Cephalosporins | Cefadroxil, cefalexin | - | - | - | - | - | - | - | 53 | 133 |
| QJ01E | Sulfonamides + trimethoprim | Sulfadiazine + trimethoprim | 67 | 44 | 73 | 93 | 95 | 88 | 124 | 137 | 151 |
| QJ01F | Lincosamides | Clindamycin | - | - | 9 | 102 | 151 | 172 | 163 | 179 | 191 |
| QJ01M | Quinolones | Enrofloxacin | 9 | 15 | 18 | 21 | 28 | 30 | 34 | 35 | 39 |
| QJ01R | Macrolides + imidazoles | ||||||||||
| TOTALS | 863 | 891 | 993 | 1408 | 1716 | 1613 | 1572 | 1582 | 1612 | ||
Table 2.
The amounts, in kg active substance, of oral veterinary antibacterial drugs (QJ01) approved for use in dogs and/or cats sold by wholesalers to pharmacies in Norway in 1990–1998.
| ATC-group | Class of drug | Active substances | 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 |
| QJ01A | Tetracyclines | Doxycycline | - | - | - | - | - | <1 | <1 | <1 | <1 |
| QJ01C | Beta-lactam antibiotics | Amoxycillin Amoxycillin + clavulanic acid | - | - | - | - | 3 | 13 | 11 | 22 | 47 |
| QJ01D | Cephalosporins | ||||||||||
| QJ01E | Sulfonamides + trimethoprim | Sulfadiazine + trimethoprim Sulfadimetoxine + baquiloprim | 235 | 187 | 266 | 258 | 278 | 295 | 391 | 401 | 385 |
| QJ01F | Lincosamides | Lincomycin | 27 | 36 | 42 | 61 | 69 | 70 | 46 | 35 | 27 |
| QJ01M | Quinolones | Enrofloxacin | - | - | <1 | 2 | 3 | 4 | 4 | 4 | 5 |
| QJ01R | Macrolides + imidazoles | Spiramycin + metronidazole | - | - | - | - | - | - | 1 | 7 | 4 |
| TOTALS | 262 | 223 | 308 | 321 | 353 | 382 | 453 | 469 | 468 | ||
Figs. 1 and 2 illustrate the number of packages of veterinary oral antibacterials approved for dogs and/or cats sold by wholesalers in Sweden and Norway respectively for the period 1990–1998. In both countries, the number of packages sold increased gradually during the study period.
Figure 1.
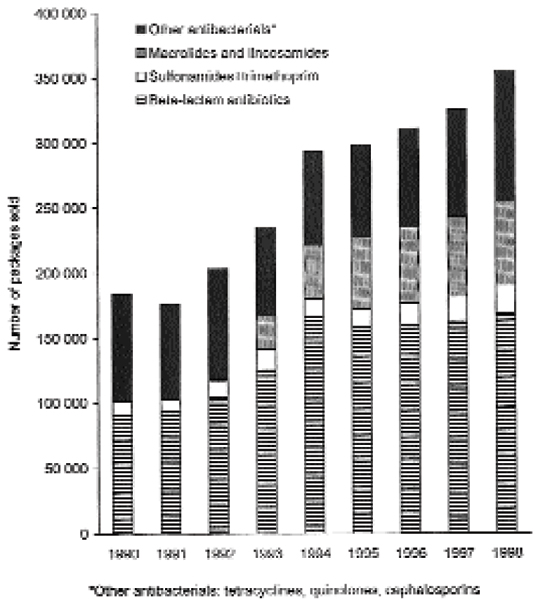
Number of packages of veterinary oral antibacterial drugs approved for dogs and/or cats sold by the drug wholesalers to pharmacies in Sweden in 1990–1998.
Figure 2.
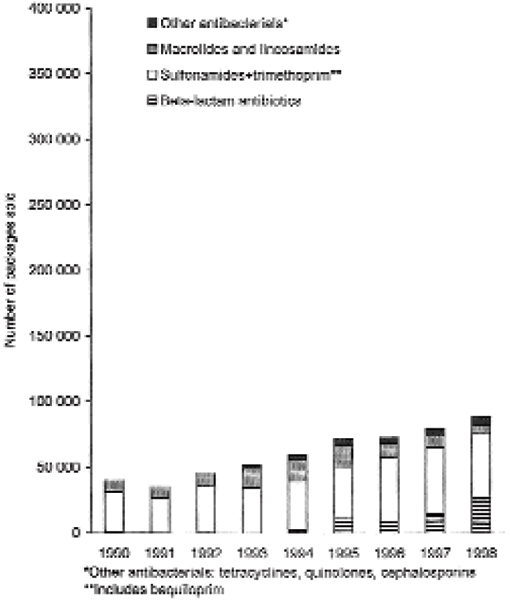
Number of packages of veterinary oral antibacterial drugs approved for dogs and/or cats sold by the drug wholesalers to pharmacies in Norway in 1990–1998.
In Sweden, the beta-lactams (QJ01C) were the main veterinary antibacterial drugs prescribed during the study period, both when the figures were expressed as kg active substance and as number of packages. On a percentage basis, use of veterinary beta-lactam antibacterials (kg) fell from 84% in 1990 to 63% in 1998 (Table 1). In number of packages, the proportional use of beta-lactam antibiotics was ranged from 48% to 57% during 1990–1998, with the lowest use in 1998 (Fig. 1).
In Norway, combination preparations of sulfonamides and trimethoprim or baquiloprim (QJ01E) were the main veterinary preparations sold by the drug wholesalers during the study period, both when measured as kg active substance and in number of packages. On a percentage basis, use (kg) of these combination preparations for dogs and cats varied slightly during the study period (range: 77%–90%) (Table 2). In number of packages, the proportional use of sulfonamides and trimethoprim or baquiloprim ranged between 64% to 79%, the highest figure being in 1998.
During the 9-year study period, an increase was observed in the number of approved veterinary oral antibacterial preparations (brand names and strengths) for dogs and/or cats in both Sweden and Norway (Table 3).
Table 3.
Number of substances or combinations of substances/number of veterinary antibacterial preparations approved for oral use in dogs and/or cats in Sweden and Norway, respectively, in the period 1990–1998.
| 1990 | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | |
| Sweden | 9/14 | 9/15 | 10/16 | 11/16 | 10/14 | 12/17 | 12/18 | 13/19 | 13/19 |
| Norway | 2/3 | 2/3 | 3/5 | 3/5 | 4/7 | 6/10 | 7/13 | 8/15 | 8/16 |
Prescription data
In Sweden, the number of prescriptions of veterinary and human antibacterial drugs for use in dogs was approximately 211 000, 213 000 and 226 000, in 1996, 1997 and 1998, respectively. For cats the corresponding figures were 72 000, 72 000 and 79 000 prescriptions.
Furthermore, in Sweden the prescribing of human approved antibacterial drugs to dogs in the years 1996–1998 was approximately 20% of all antibacterial packages prescribed for dogs, while the corresponding figure for cats was 13% (Table 4). The prescribing of human antibacterial drugs in dogs and cats, split into classes of substances, is shown in Table 5. For both species, the most frequently prescribed human drugs were beta-lactam antibiotics.
Table 4.
The distribution (%) of prescribed packages of antibacterial drugs to dogs and cats in Sweden, split into human drugs (J01) and veterinary drugs (QJ01), in 1996–1998. J01 = General antiinfectives for systemic use in humans; QJ01 = General antiinfectives for systemic use in animals.
| Dogs | Cats | |||||
| 1996 | 1997 | 1998 | 1996 | 1997 | 1998 | |
| Human preparations (J01) | 21 | 20 | 19 | 13 | 12 | 13 |
| Veterinary preparations (QJ01) | 79 | 80 | 81 | 87 | 88 | 87 |
| TOTALS | 100 | 100 | 100 | 100 | 100 | 100 |
Table 5.
Human antibacterial drugs (J01) prescribed for dogs and cats in Sweden during 1996–1998. The figures represent the number of packages dispensed.
| Dogs | Cats | ||||||
| ATC-group | Class of drugs | 1996 | 1997 | 1998 | 1996 | 1997 | 1998 |
| J01A | Tetracyclines | 10 583 | 8 996 | 9 696 | 1 120 | 1 248 | 1 218 |
| J01B | Amphenicols | 2 | 2 | 1 | 0 | 0 | 0 |
| J01C | Beta-lactam antibiotics | 18 804 | 16 953 | 17 296 | 7 906 | 6 740 | 9 225 |
| J01D | Cephalosporins | 14 296 | 17 504 | 19 417 | 267 | 327 | 378 |
| J01E | Sulfonamides + trimethoprim | 4 707 | 3 951 | 3 277 | 158 | 163 | 164 |
| J01F | Macrolides and lincosamides | 9 293 | 7 135 | 4 358 | 926 | 1 023 | 1 234 |
| J01G | Aminoglycosides | 18 | 10 | 12 | 2 | 12 | 2 |
| J01M | Quinolones | 211 | 186 | 192 | 2 | 1 | 1 |
| J01X | Others* | 42 | 42 | 58 | 8 | 4 | 12 |
| TOTALS | 57 956 | 54 779 | 54 307 | 10 389 | 9 518 | 12 234 | |
* includes e.g. fusidic acid
The average number of packages prescribed per prescription of antibacterial drugs in Sweden was found to be stable during 1996–1998. Number of packages of veterinary antibacterial preparations per prescription for dogs was 1.3 for each year of the study period, while for human preparations this figure was 1.1. For cats these figures were 1.2 and 1.0 respectively.
Discussion
In both countries, all antibacterial drugs are prescription-only and must be dispensed through pharmacies. The figures used in the present study are based on annual sales of the antibacterial drugs from national drug wholesalers to pharmacies. The pharmacies stock drugs on a short-term basis. Thus, annual sales figures are reasonable estimates of the consumption of the drugs in each of the years.
Wholesaler data
The use of wholesaler statistics to investigate the use of antibacterial drugs in animals has certain limitations. In both countries, a selection of antibacterial drugs, which are approved for human use, are also approved for use in dogs and cats. However, wholesalers' statistics cannot differentiate whether or not these drugs are sold for use in humans or animals. Consequently, these drugs are included in the wholesalers' statistics of drugs for human use. Prescription data are necessary when monitoring the extent of usage of human antibacterials for use in dogs and cats and extra-label use of human approved antibacterials not approved for use in dogs and cats.
Injectable veterinary preparations approved for use in dogs and cats in Sweden and Norway are approved for other animal species as well. It is not possible to estimate the use of such drugs in dogs and cats by use of wholesalers' statistics.
Injectable antibacterial drugs are almost exclusively administered in connection with the veterinary consultation and are prescribed as ad usum proprium preparations. Additionally, no injectable preparations are prescribed to outpatients. Therefore, these drugs are not identified as prescribed to specific species within the centralised database of Apoteket AB. It is, however, thought that the use of injectable antibacterial drugs in dogs and cats is proportionally small compared to oral use.
Population size
When interpreting the usage of antibacterial preparations in dogs and cats, it is necessary to have an approximate knowledge of the population size of the species of concern. The population of dogs in Sweden increased from 700 000 in 1990 to 800 000 dogs in 1998; the cat population declined from 1.1 million in 1990 to 1 million cats in 1998 [12,9]. In Norway, the population of dogs has been roughly estimated to 400 000 and the cat population to 300 000 (Personal communication, W. Bredal), however, these figures should be interpreted with caution.
Human drugs
A cross-sectional prescription study performed in Sweden in 1981 found that 36% and 39%, respectively, of the packages of antibacterial drugs prescribed for use in dogs and cats were human drugs [1]. In the present study it was found that 20% and 13%, respectively, of the total number of prescribed packages of antibacterials for systemic use (i.e. excluding otic, ophtalmic and topical preparations) in dogs and cats in Sweden in the period 1996–1998, were human preparations. During the period 1990–1998, the number of veterinary antibacterial drug preparations (brand names and strengths) approved in Sweden increased gradually. This likely accounts for the substantial decrease in the usage of human drugs in dogs and cats in Sweden since the early 1980s.
A cross-sectional prescription study performed in Norway in 1987–1988 found that 60% and 70% of prescriptions of antibacterial drugs prescribed for use in dogs and cats respectively were human approved drugs [3]. This can easily be explained by the fact that at that time only 3 oral veterinary antibacterial preparations (brand names and strengths) were approved for use in dogs and cats. Since 1988, the number of approved veterinary antibacterial preparations has increased from 3 to 16. The use of human antibacterial drugs in dogs and cats is thus thought to be much lower in 1998 than in 1987–1988. However, in order to accurately estimate the usage of human antibacterial drugs in Norway a centralised database is needed.
The differences in the type and number of veterinary antibacterials approved for dogs and cats in Sweden and Norway may, in part, explain the differences in the usage patterns between the 2 countries. The number of substances and veterinary antibacterial preparations (brand names and strengths) approved for dogs and cats in Norway was lower than in Sweden during the study period. For example, veterinary preparations containing cephalosporins, ampicillin or phenoxymethylpenicillin are approved for dogs and/or cats in Sweden, but not in Norway. The veterinarians in Norway thus have to prescribe a human approved product when they want an equivalent antibacterial.
Units of measurements
In the present study, sales figures were presented as kg active substance. Antibacterial drugs are used in different dosages depending on their potency, rate of absorption, and the size of the animal in question. Therefore, sales figures of antibacterial drugs given as kg active substances must be interpreted with caution when the aim is to analyse prescription patterns of these drugs.
To correct for the differences in dosages when interpreting drug sales statistics, defined daily dose (DDD) is used in human medicine as a unit of measurement [2,17]. This unit of measurement allows for comparison of drug use in a medical context. DDD is defined as the assumed average maintenance dose per day for the drug used in its main indication in adults. DDD is considered as the "gold standard" in drug consumption studies (Capellà 1993, WHO 1998). Unfortunately, the number of DDD prescribed for dog cannot easily be estimated based on sales statistics, because of the wide range of weights, especially of dogs «at risk» of being treated with an antibacterial drug.
However, sales statistics, expressed as kg active substance, may be utilised to estimate the contribution of use in dogs and cats of veterinary antibacterial drugs to the total use of these drugs. In 1990, overall sales of veterinary antibacterial drugs for therapeutic use in Sweden was 30.3 tonnes active substance. This figure declined to 19.3 tonnes in 1998 [14,15]. Sales figures (kg) in Sweden, of veterinary antibacterials approved only for dogs and cats, increased from 3% of the total use in 1990 to 8% in 1998.
In Norway, overall sales figures of veterinary antibacterials for therapeutic use decreased from 9.5 tonnes in 1990 to 6.8 tonnes in 1998 [4-6]. During the same period, the usage of veterinary antibacterials for dogs and cats, as a percentage of all veterinary antibacterials, increased from 3% to 7%.
The policies taken to promote prudent use of antibacterials, especially in food producing animals, may have contributed to the total decrease seen in both countries in the consumption of antibacterials prescribed to animals.
As a comparison, in Norway, consumption of antibacterial drugs in human medicine was estimated to be 35 tonnes both in 1992 and in 1996 [7]. Therefore, the contribution to the total environmental load in Norway of use of antibacterial drugs in dogs and cats is proportionally small. Corresponding consumption data for antibacterial drug use in human medicine in Sweden is not published.
Consideration of the use of antibacterial drugs in terms of sold packages may give a more precise idea of the prescribing patterns than kg active substance [2]. This is especially true when the number of packages prescribed per treatment is thought to be constant for specific groups of diseases, e.g. infectious diseases. In the present study, it was found that in Sweden number of packages per prescriptions of veterinary antibacterial drugs for dogs and cats remained constant from 1996 to 1998. Based on information about the package size and the recommended dosage regimen for veterinary preparations approved for dogs and cats in Norway [16] it is reasonable to believe that this is true for Norway as well.
Usage pattern
The usage in dogs and cats in Sweden, measured by the number of sold packages, was mainly beta-lactam antibacterials during 1990–1998. The number of packages sold of sulfonamides in combination with trimethoprim, lincosamides (i.e. clindamycin) and quinolones (i.e. enrofloxacin) increased during the period. In contrast to Norway, Sweden had no approved preparation in the ATCvet group QJ01R i.e. spiramycin in combination with metronidazole. In Norway, the principle antibacterial drugs used during the study period, measured as number of packages sold, were trimethoprim in combination with sulfonamides (QJ01E). The beta-lactam antibacterials (QJ01C) were approved in Norway as veterinary preparations for the first time in 1994 and the number of packages sold of this group has since then increased, while the use of lincosamides has decreased.
In the present study it was found that the usage of human and veterinary antibacterial drugs for dogs and cats in Sweden during the period 1996–1998 increased only slightly. However, the number of animals "at risk" also increased slightly during the period, suggesting that the use of antibacterials in dogs and cats remained relatively constant on a per animal basis.
In Norway, the number of packages sold of veterinary antibacterial drugs for dogs and/or cats almost doubled during the study period. As we do not have information about the consumption of human antibacterial drugs by dogs and cats we cannot conclude whether or not the incidence of treatment of bacterial diseases has increased in Norway. The data presented in the present study only gives information of the trends of use of veterinary antibacterial drugs. In addition to the previously discussed need for a centralised database like the one in Sweden, reliable statistics about the number of dogs and cats at risk of being treated in Norway are necessary to estimate the incidence of treatment with antibacterial drugs.
Bacterial resistance
In the efforts to contain antibacterial drug resistance, both from the veterinary and human public health viewpoints, joint monitoring of use and resistance is a crucial component. In the present study, an increase in sales of lincosamides for veterinary use was noted in both Sweden and Norway. Interestingly, a parallel increase in resistance to lincosamides in staphylococci isolated from pyoderma in dogs has been reported from both countries [11,8,10]. However, the observation should be interpreted with caution, as data on sales of human lincosamides and macrolides are not available for the whole period of observation. Nonetheless, information of this type is needed as a basis for discussions about current prescription policies and practices. If interventions designed to change antibacterial drug use behaviour are implemented, baseline data on use and resistance will be essential for evaluations of the effectiveness of those interventions.
It is crucial to be able to study prescription/usage patterns of antibacterial drugs in dogs and cats not only in the surveillance of bacterial resistance, but also in discussions of the therapeutic appropriateness. Therefore, further research is needed in the area. Moreover, information about the frequency of prescribing of human drugs may encourage the pharmaceutical industry to apply for approval of human drugs as veterinary drugs for companion small animals.
Acknowledgments
Acknowledgements
This study was in part supported by a grant from the Research Council of Norway. Apoteket AB, Sweden, and Norwegian Medicinal Depot AS are acknowledged for providing sale statistics.
References
- Bingefors K. The use in animals of drugs licensed for human use: The situation in Sweden. Proceedings of the 3rd Congress of the European Association for Veterinary Pharmacology and Toxicology, Ghent, Belgium, 25–29 August, 1985, Comparative Veterinary Pharmacology, Toxicology and Therapy. pp. 513–520.
- Capellà D. Descriptive tools and analysis. In: Dukes MNG, editor. Drug utilization studies Methods and uses, 1993, No 45. WHO Regional Publications, European Series, WHO Regional Office for Europe, Copenhagen, Denmark; pp. 55–78. [PubMed] [Google Scholar]
- Grave K, Bangen M, Engelstad M, Søli NE. Prescribing of veterinary and human preparations for animals in Norway. Was the preparation approved for the animal species for which it was prescribed? J vet Pharmacol Therap. 1992;15:45–52. doi: 10.1111/j.1365-2885.1992.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Grave K, Rønning M. Forskrivningsmønsteret for antibakterielle midler registrert til veterinærmedisinsk bruk i Norge i 1996 (Prescribing patterns of veterinary antibacterial drugs in Norway in 1996) Nor Vet Tidsskr. 1997;109:242–243. (Norwegian Veterinary Journal). [Google Scholar]
- Grave K, Rønning M. Forskrivningen av antibakterielle midler til dyr redusert med 23% fra 1995 til 1997 (The prescribing of veterinary antibacterial drugs in Norway was reduced by 23% in the period 1995–1997) Nor Vet Tidsskr. 1998;110:205–206. (Norwegian Veterinary Journal). [Google Scholar]
- Grave K, Rønning M. Forbruket av antibakterielle midler til husdyr har gått ned 28% fra 1995 til 1998 (The consumption of veterinary antibacterial drugs was reduced by 28% in the period 1995–1998) Nor Vet Tidsskr. 1999;111:344–345. (Norwegian Veterinary Journal). [Google Scholar]
- Grave K, Lingaas E, Bangen M, Rønning M. Surveillance of the overall consumption of antibacterial drugs in humans, domestic animals and farmed fish in Norway in 1992 and 1996. J Antimicrob Chemotherapy. 1999;43:243–252. doi: 10.1093/jac/43.2.243. [DOI] [PubMed] [Google Scholar]
- Hansson L, Sternberg S, Greko C. Antimicrobial susceptibility in isolates from Swedish dogs – a retrospective study. 4th International Meeting on Bacterial Epidemiological Markers, Elsinore, Denmark. 1997.
- Hedhammar Å, Egenvall A, Olson P, Sallander M, Uddman U, Bonnett B. Hund i Sverige (Dogs in Sweden) Svensk VetTidn. 1999;51:355–362. (Swedish Veterinary Journal). [Google Scholar]
- Holm B, Raue H, Bergström K, Petterson U, Mörner A. Antibiotic sensitivity of staphylococci isolated from cases of canine pyoderma. 14th ESVD-ECVD Annual Congress, Pisa, Italy; 1997. [Google Scholar]
- Kruse H, Hofshagen M, Thoresen SI, Bredal WP, Vollset I, Søli NE. The antimicrobial susceptibility of Staphylococcus species isolated from canine dermatitis. Vet Res Com. 1996;20:205–214. doi: 10.1007/BF00366918. [DOI] [PubMed] [Google Scholar]
- Moore A. Proceedings of the Annual Meeting of Swedish Veterinarians. Västerås, Sweden; 1991. Pet population in Europe; pp. 39–42. [Google Scholar]
- Nordic Council on Medicines . Guidelines on ATCvet classification. 3. NLN Publication No 50, Uppsala, Sweden; 1999. [Google Scholar]
- Odensvik K, Greko C. Antibakteriella läkemedel för djur – en uppdatering (Antibacterials for animals – an update) Svensk VetTidn. 1998;50:313–316. (Swedish Veterinary Journal). [Google Scholar]
- Odensvik K. Antibakteriella läkemedel för djur – 1998 års siffror (Antibacterials for animals – the figures of 1998) Svensk VetTidn. 1999;51:369–371. (Swedish Veterinary Journal). [Google Scholar]
- Tørisen HM, Ed . The Norwegian Compendium of Veterinary Medicines. 11th–15th. Felleskatalogen AS, Oslo, Norway; 1992, 1994, 1996, 1998. [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment. 2. Oslo, Norway; 1998. [Google Scholar]
- Witte W. Impact of antibiotic use in animal feeding on resistance of bacterial pathogens in humans. Antibiotic resistance: origins, evolution, selection and spread. Wiley, Chichester (Ciba foundation symposium); 1996. pp. 61–70. [DOI] [PubMed] [Google Scholar]


