Abstract
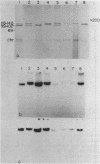
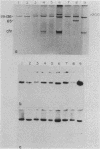
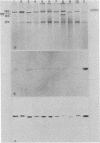
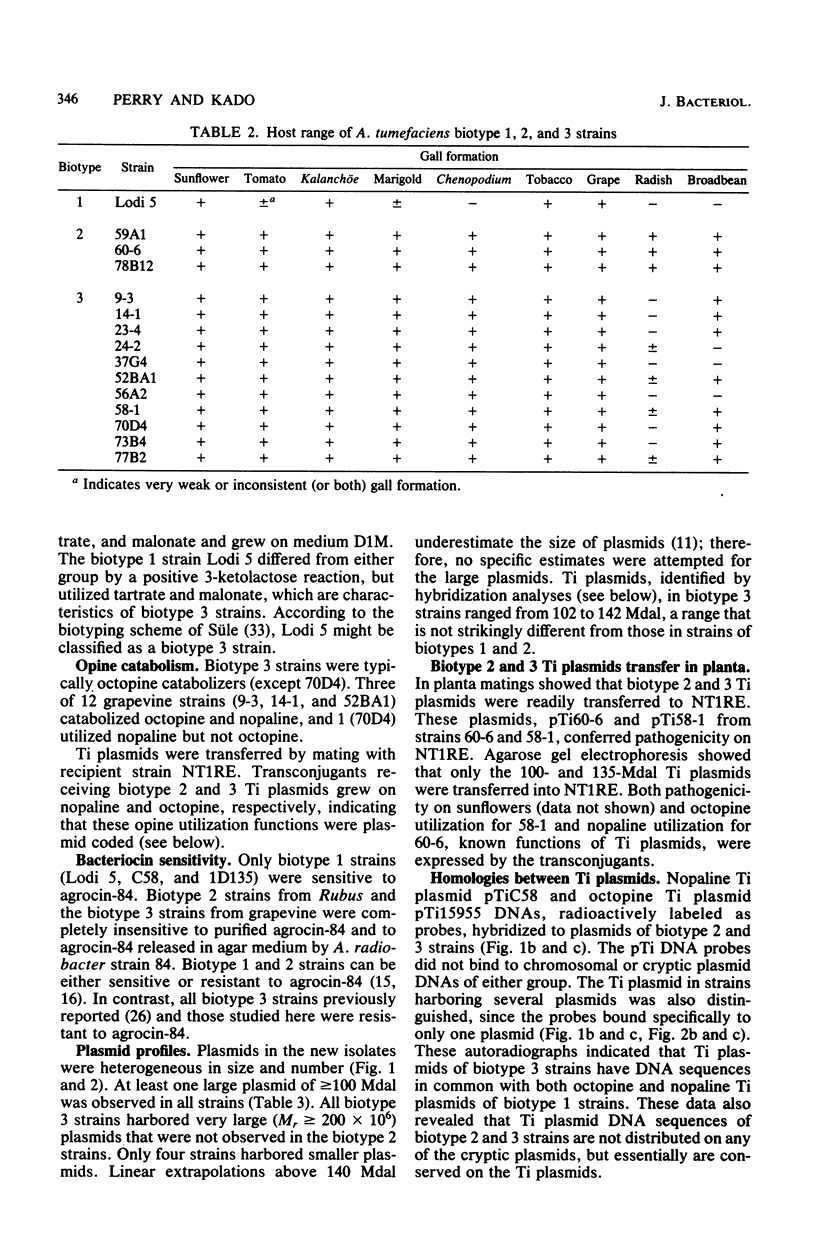
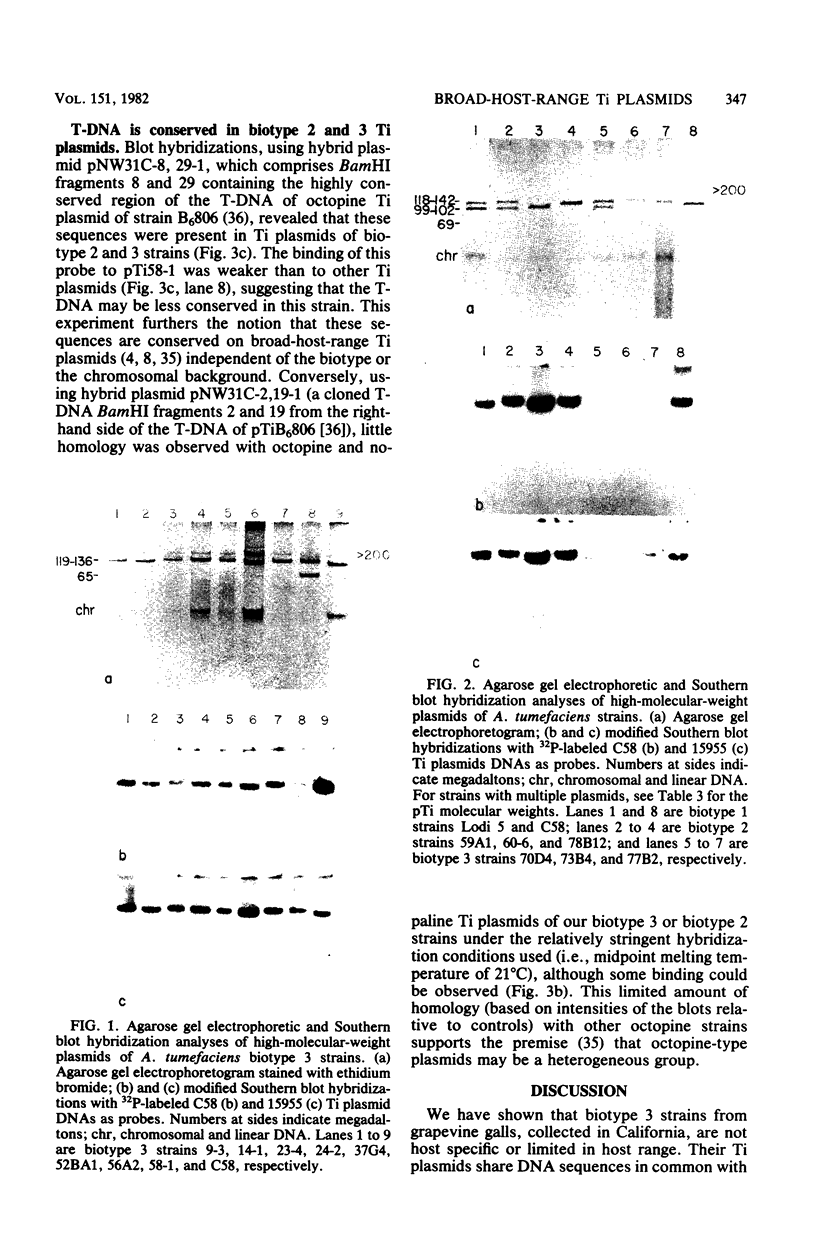
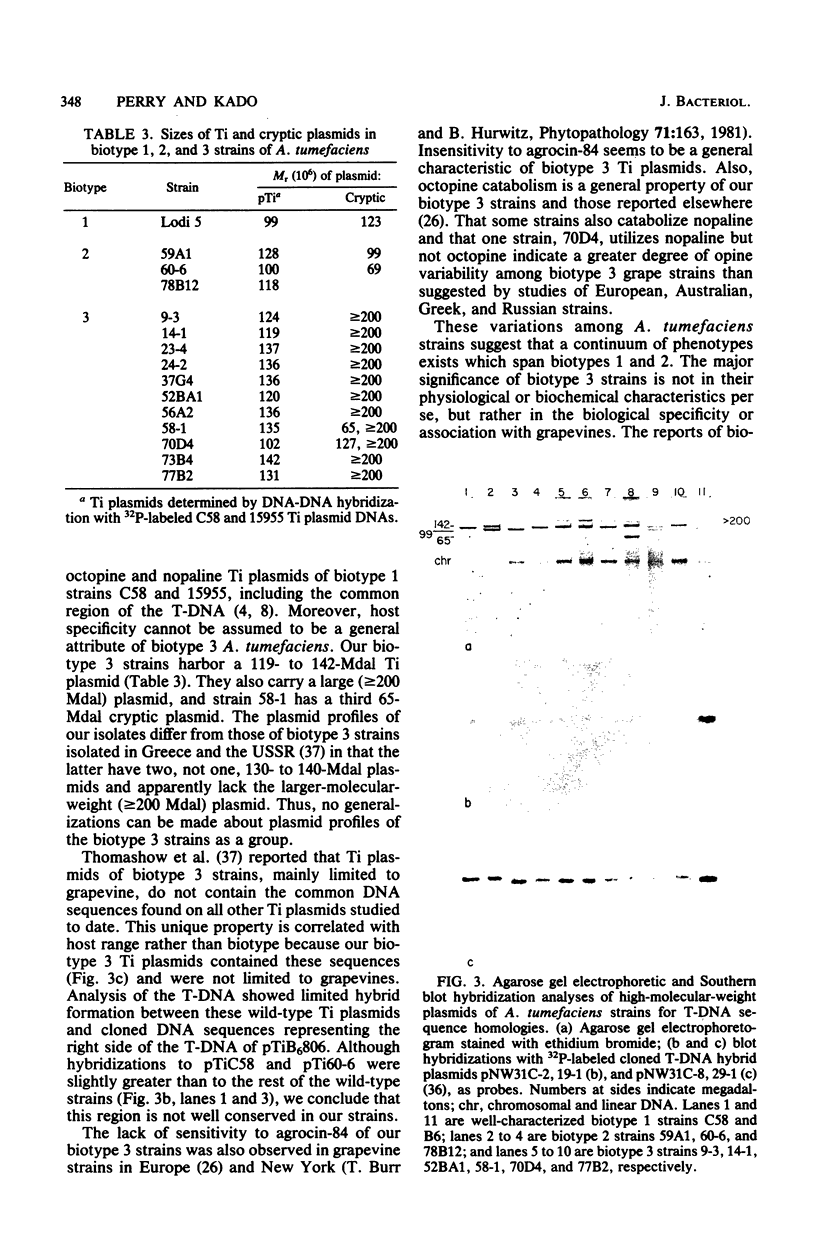
Agrobacterium tumefaciens strains isolated from crown gall tumors on grapevines in California were consistently of the biotype 3 group. All 11 of these strains were limited in their host range and harbored Ti plasmids with molecular masses between 119 and 142 megadaltons (Mdal) as well as a larger cryptic plasmid of greater than 200 Mdal; occasionally a smaller cryptic plasmid of 65 Mdal was also present. Ti plasmids o these strains have DNA sequences in common with Ti plasmids of octopine and nopaline strains belonging to the biotype 1 group and exhibited sequence homologies with the conserved region of the T-DNA. Ten of the 11 strains utilized octopine as a sole source of carbon and nitrogen and 3 strains catabolized both octopine and nopaline, whereas 1 strain catabolized only nopaline. All of these strains were resistant to the bacteriocin agrocin-84, except one grapevine strain that belonged to the biotype 1 group and was agrocin sensitive; it is also differed in its plasmid and virulence characteristics. Isolations from Rubus ursinus ollalieberry galls yielded exclusively biotype 2 strains. These strans were insensitive to agrocin-84, utilized nopaline as a sole carbon and nitrogen source, and were highly virulent on all host plants tested. They contained Ti plasmids ranging between 100 and 130 Mdal and occasionally a cryptic plasmid of 69 Mdal. Their Ti plasmids have DNA sequences in common with Ti plasmids of biotype 1 strains and with the conserved region of the T-DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrés I., Slocombe P. M., Cabello F., Timmis J. K., Lurz R., Burkardt H. J., Timmis K. N. Plasmid replication functions. II. Cloning analysis of the repA replication region of antibiotic resistance plasmid R6-5. Mol Gen Genet. 1979 Jan 5;168(1):1–25. doi: 10.1007/BF00267929. [DOI] [PubMed] [Google Scholar]
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Coplin D. L., Rowan R. G., Chisholm D. A., Whitmoyer R. E. Characterization of plasmids in Erwinia stewartii. Appl Environ Microbiol. 1981 Oct;42(4):599–604. doi: 10.1128/aem.42.4.599-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Evidence for diverse types of large plasmids in tumor-inducing strains of Agrobacterium. J Bacteriol. 1976 Apr;126(1):157–165. doi: 10.1128/jb.126.1.157-165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Engler G., Holsters M., Van Montagu M., Schell J., Hernalsteens J. P., Schilperoort Agrocin 84 sensitivity: a plasmid determined property in Agrobacterium tumefaciens. Mol Gen Genet. 1975 Jul 10;138(4):345–349. doi: 10.1007/BF00264804. [DOI] [PubMed] [Google Scholar]
- Guyon P., Chilton M. D., Petit A., Tempé J. Agropine in "null-type" crown gall tumors: Evidence for generality of the opine concept. Proc Natl Acad Sci U S A. 1980 May;77(5):2693–2697. doi: 10.1073/pnas.77.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. E., Zdybak W. T., Yasuda K., Chilton W. S. A useful synthesis of nopaline, a crown gall tumor metabolite. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1066–1070. doi: 10.1016/0006-291x(77)91490-5. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B. C., Kado C. I. Studies on Agrobacterium tumefaciens. VIII. Avirulence induced by temperature and ethidium bromide. Can J Microbiol. 1977 Nov;23(11):1554–1561. doi: 10.1139/m77-229. [DOI] [PubMed] [Google Scholar]
- Liu S. T., Kado C. I. Indoleacetic acid production: a plasmid function of Agrobacterium tumefaciens C58. Biochem Biophys Res Commun. 1979 Sep 12;90(1):171–178. doi: 10.1016/0006-291x(79)91605-x. [DOI] [PubMed] [Google Scholar]
- Loper J. E., Kado C. I. Host range conferred by the virulence-specifying plasmid of Agrobacterium tumefaciens. J Bacteriol. 1979 Aug;139(2):591–596. doi: 10.1128/jb.139.2.591-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos C. G., Psallidas P. G. Characteristics of Greek Isolates of Agrobacterium tumefaciens (E. F. Smith & Townsend) Conn. J Appl Bacteriol. 1973 Jun;36(2):233–240. doi: 10.1111/j.1365-2672.1973.tb04096.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Knauf V. C., Nester E. W. Relationship between the limited and wide host range octopine-type Ti plasmids of Agrobacterium tumefaciens. J Bacteriol. 1981 May;146(2):484–493. doi: 10.1128/jb.146.2.484-493.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]