Abstract
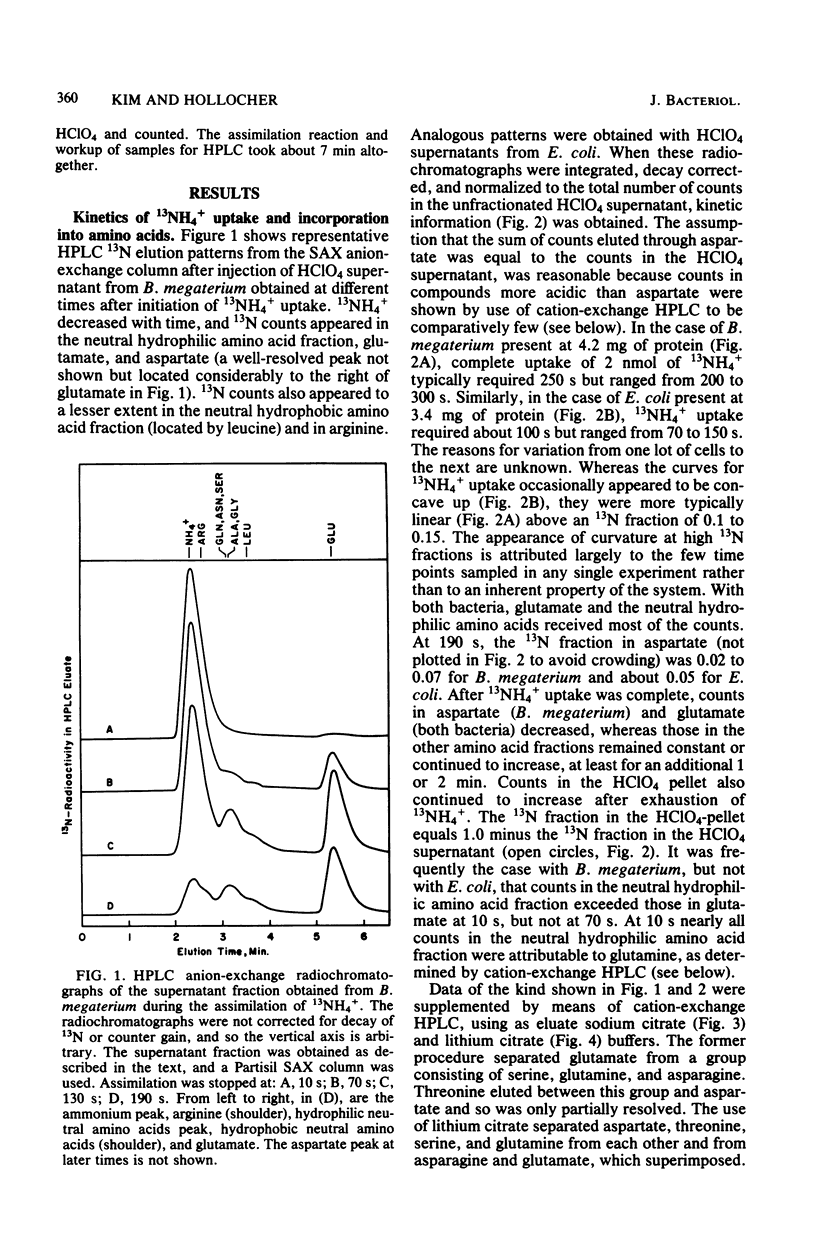
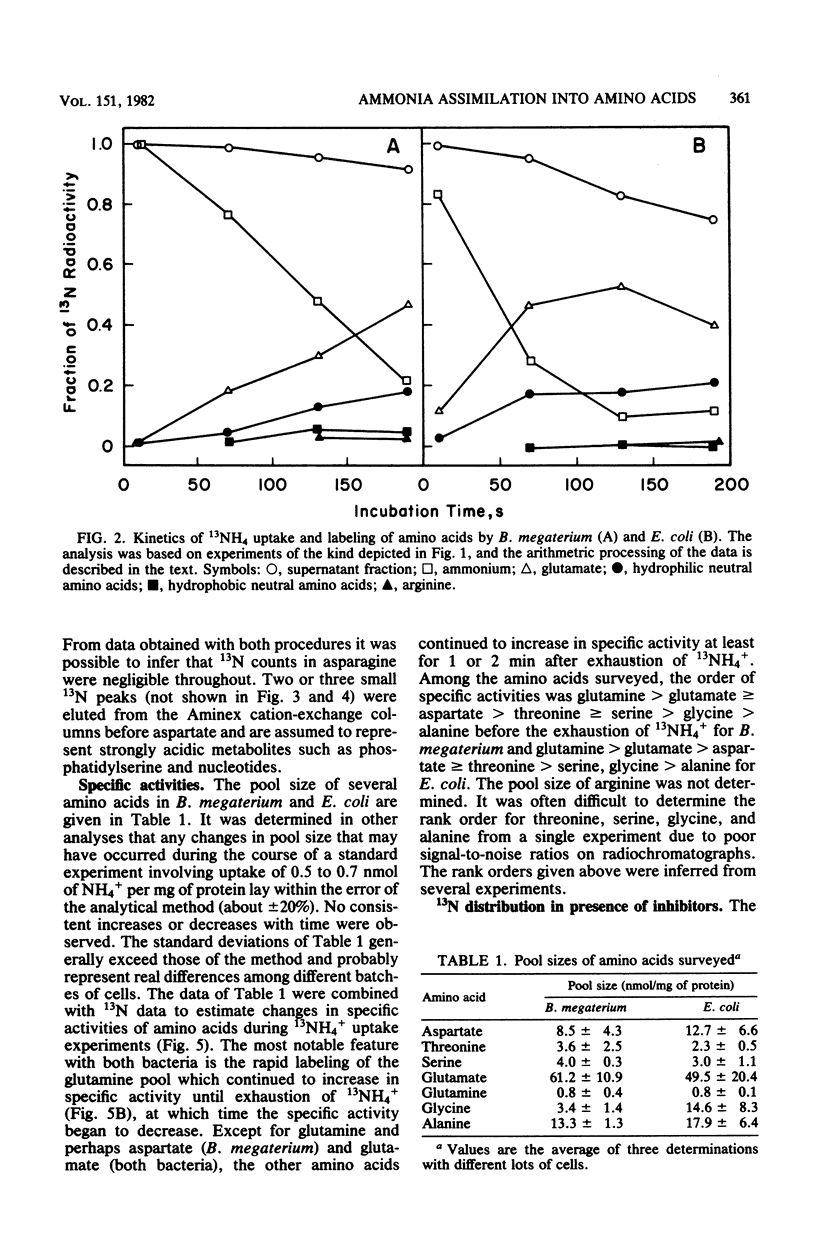
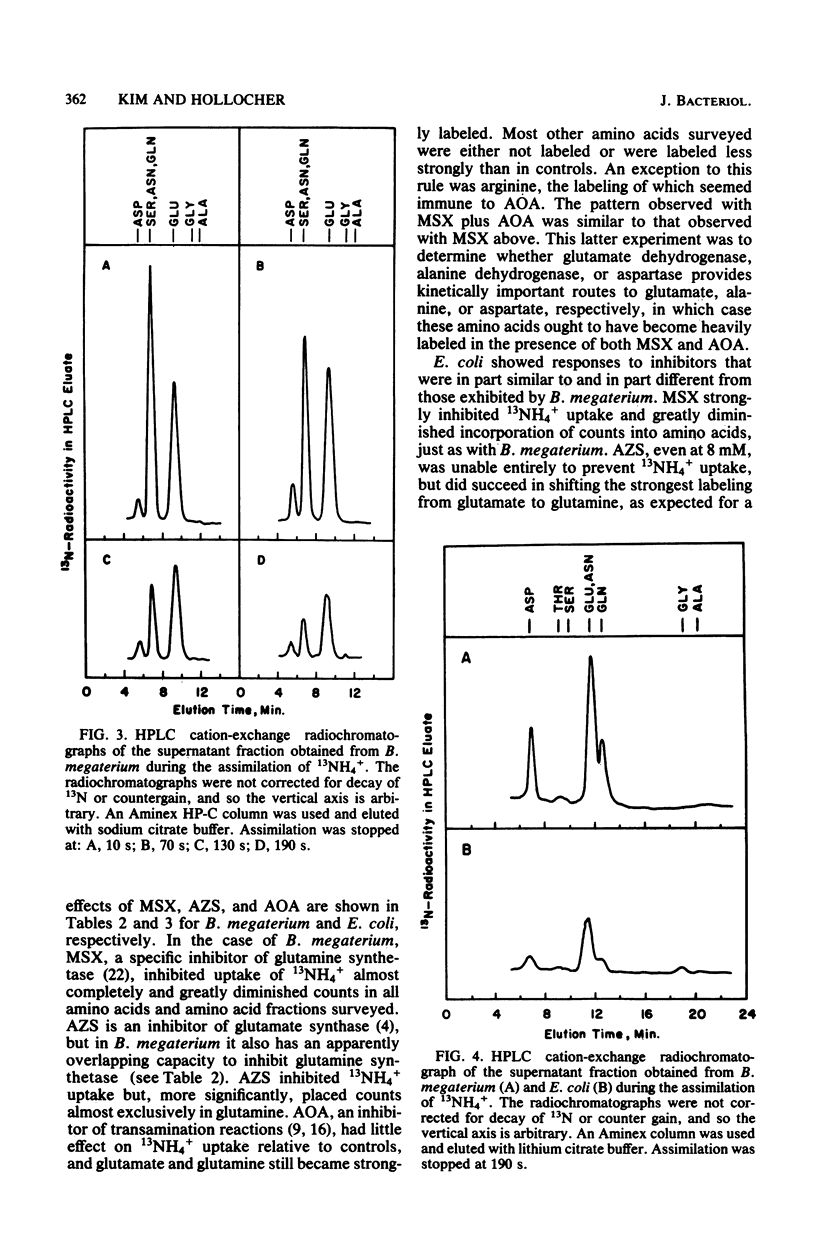
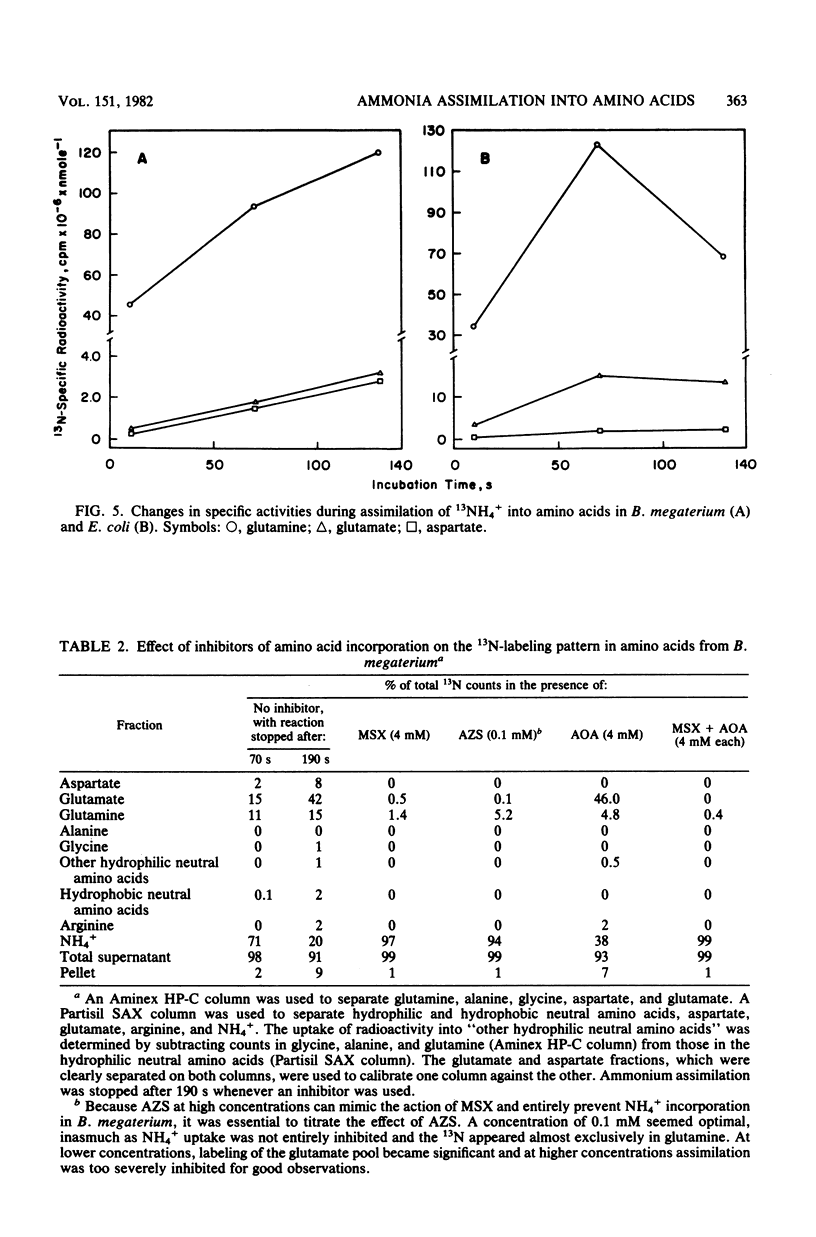
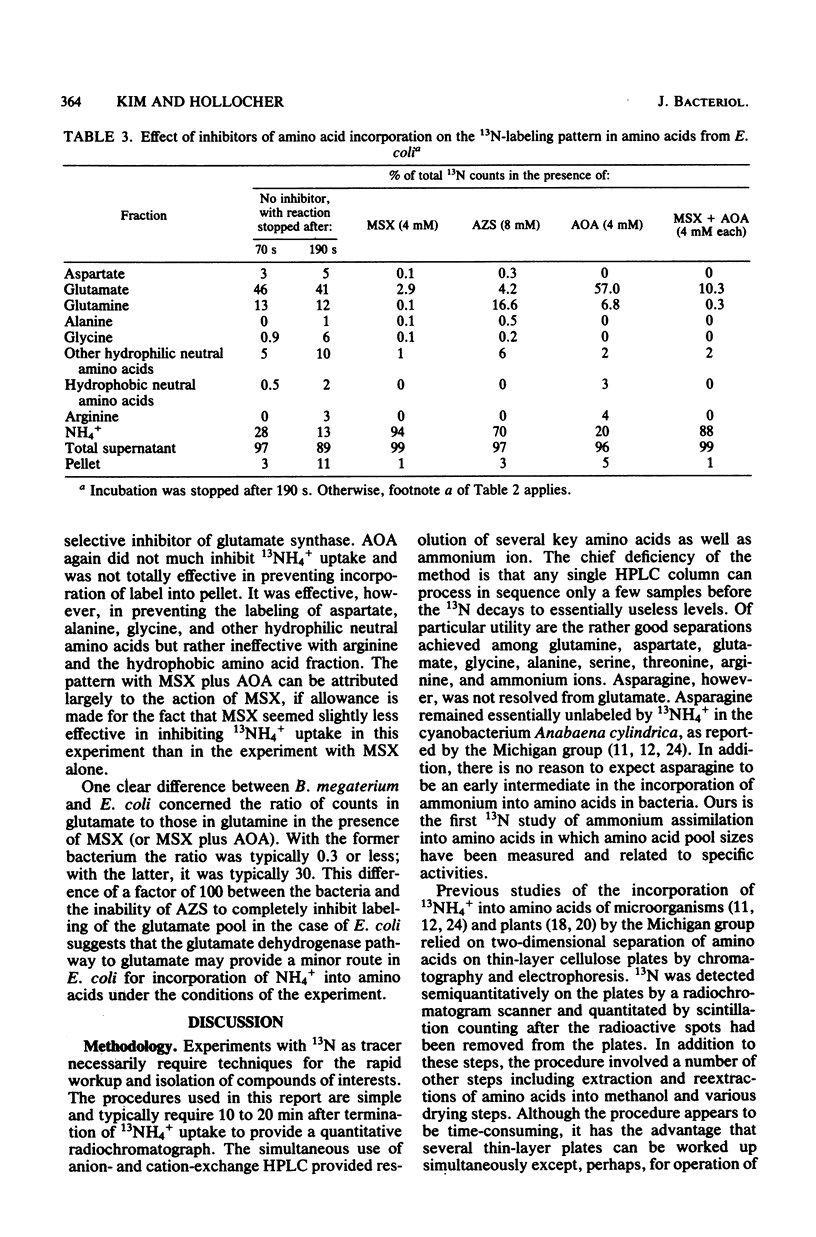
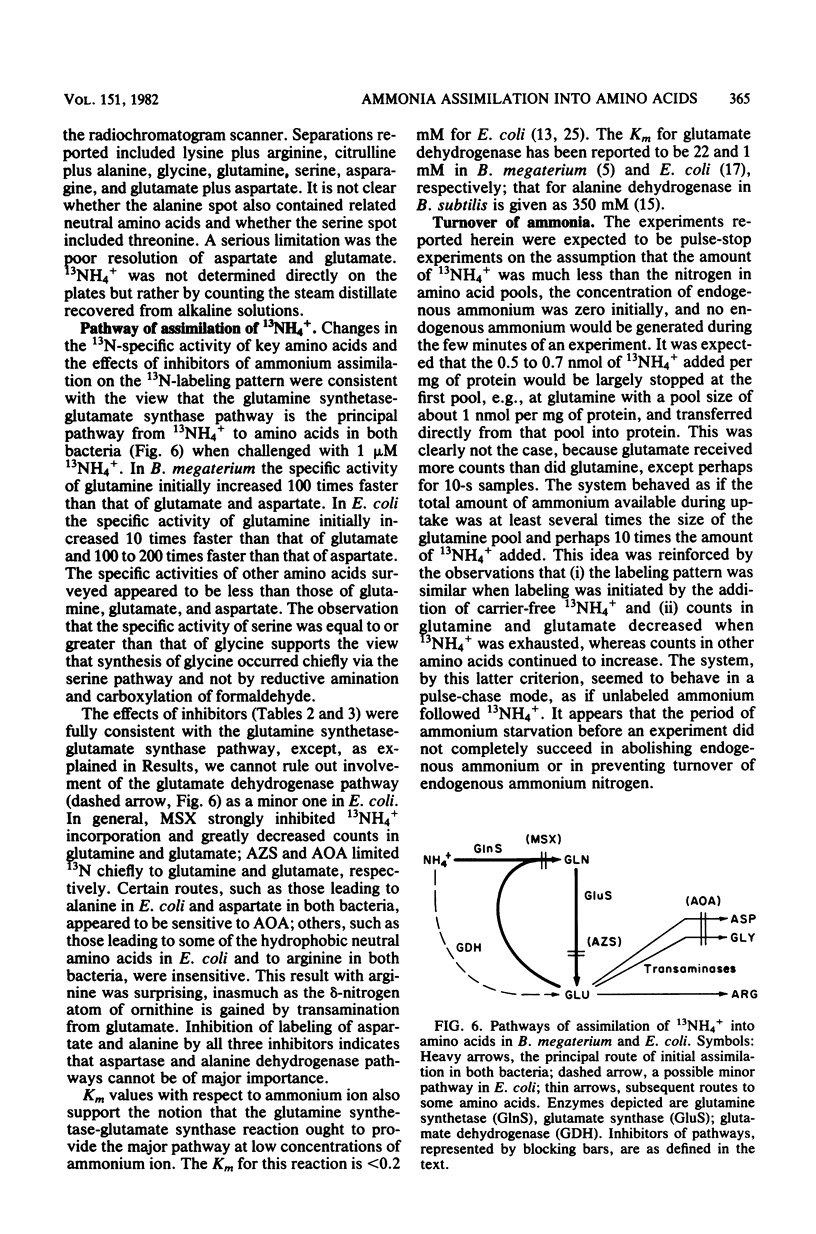
The pathway of ammonia incorporation into amino acids was studied by use of 13N-ammonium ions in Bacillus megaterium and Escherichia coli that had been grown aerobically on a minimal salts medium containing NH4Cl as the source of nitrogen. Anion- and cation-exchange high-pressure-liquid chromatography was used to separate amino acids relevant to the several possible pathways for ammonia assimilation in bacteria. At an initial concentration of added NH4+ of 1 microM, the glutamine synthetase-glutamate synthase pathway represented the major pathway in both bacteria on the basis of the effects of inhibitors of that pathway (L-methionine-DL-sulfoximine and azaserine) and of transamination (aminooxy-acetate) and the observation that the specific activity of glutamine was greater initially than that of any other amino acid likely to be the first product of an assimilation pathway. The study provides (i) a new analytical method for 13N-tracer investigation of amino acids, (ii) confirmation of conclusions from enzymological studies on the pathway of ammonia assimilation in B. megaterium and E. coli, and (iii) proof that alanine dehydrogenase and aspartate ammonia lyase (aspartase) are not important pathways in B. megaterium at low NH4+ concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DEPUE R. H., MOAT A. G. Factors affecting aspartase activity. J Bacteriol. 1961 Sep;82:383–386. doi: 10.1128/jb.82.3.383-386.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmerich C., Aubert J. P. Synthesis of glutamate by a glutamine: 2-oxo-glutarate amidotransferase (NADP oxidoreductase) in Bacillus megaterium. Biochem Biophys Res Commun. 1971 Feb 5;42(3):371–376. doi: 10.1016/0006-291x(71)90380-9. [DOI] [PubMed] [Google Scholar]
- FREESE E., PARK S. W., CASHEL M. THE DEVELOPMENTAL SIGNIFICANCE OF ALANINE DEHYDROGENASE IN BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1964 Jun;51:1164–1172. doi: 10.1073/pnas.51.6.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG M. M., SHEN S. C., BRAUNSTEIN A. E. Distribution of L-alanine dehydrogenase and L-glutamate dehydrogenase in Bacilli. Biochim Biophys Acta. 1959 Nov;36:288–289. doi: 10.1016/0006-3002(59)90111-8. [DOI] [PubMed] [Google Scholar]
- Hemmilä I. A., Mäntsälä P. I. Purification and properties of glutamate synthase and glutamate dehydrogenase from Bacillus megaterium. Biochem J. 1978 Jul 1;173(1):45–52. doi: 10.1042/bj1730045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocher T. C., Garber E., Cooper A. J., Reiman R. E. 13N,15N isotope and kinetic evidence against hyponitrite as an intermediate in dentrification. J Biol Chem. 1980 Jun 10;255(11):5027–5030. [PubMed] [Google Scholar]
- Hoop B., Jr, Smith T. W., Burnham C. A., Correll J. E., Brownell G. L., Sanders C. A. Myocardial imaging with 13 NH 4+ and a multicrystal positron camera. J Nucl Med. 1973 Mar;14(3):181–183. [PubMed] [Google Scholar]
- Hotta S. S. Oxidative metabolism of isolated brain mitochondria: changes caused by aminooxyacetate. Arch Biochem Biophys. 1968 Sep 20;127(1):132–139. doi: 10.1016/0003-9861(68)90209-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meeks J. C., Wolk C. P., Lockau W., Schilling N., Shaffer P. W., Chien W. S. Pathways of assimilation of [13N]N2 and 13NH4+ by cyanobacteria with and without heterocysts. J Bacteriol. 1978 Apr;134(1):125–130. doi: 10.1128/jb.134.1.125-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks J. C., Wolk C. P., Thomas J., Lockau W., Shaffer P. W., Austin S. M., Chien W. S., Galonsky A. The pathways of assimilation of 13NH4+ by the cyanobacterium, Anabaena cylindrica. J Biol Chem. 1977 Nov 10;252(21):7894–7900. [PubMed] [Google Scholar]
- Miller R. E., Stadtman E. R. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem. 1972 Nov 25;247(22):7407–7419. [PubMed] [Google Scholar]
- PIERARD A., WIAME J. M. [Properties of L(d)-alanine dehydrogenase]. Biochim Biophys Acta. 1960 Jan 29;37:490–502. doi: 10.1016/0006-3002(60)90506-0. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Clark D. G. Effects of aminooxyacetate on the metabolism of isolated liver cells. Arch Biochem Biophys. 1974 Apr 2;161(2):638–646. doi: 10.1016/0003-9861(74)90348-8. [DOI] [PubMed] [Google Scholar]
- SHEN S. C., HONG M. M., BRAUNSTEIN A. E. The main path of nitrogen assimilation in Bacillus subtilus. Biochim Biophys Acta. 1959 Nov;36:290–291. doi: 10.1016/0006-3002(59)90112-x. [DOI] [PubMed] [Google Scholar]
- Sakamoto N., Kotre A. M., Savageau M. A. Glutamate dehydrogenase from Escherichia coli: purification and properties. J Bacteriol. 1975 Nov;124(2):775–783. doi: 10.1128/jb.124.2.775-783.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Coker G. T. Ammonia Assimilation in Alnus glutinosa and Glycine max: SHORT-TERM STUDIES USING [N]AMMONIUM. Plant Physiol. 1981 Apr;67(4):662–665. doi: 10.1104/pp.67.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokut T. A., Wolk C. P., Thomas J., Meeks J. C., Shaffer P. W. Initial organic products of assimilation of [N]ammonium and [N]nitrate by tobacco cells cultured on different sources of nitrogen. Plant Physiol. 1978 Aug;62(2):299–304. doi: 10.1104/pp.62.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]
- Weisbrod R. E., Meister A. Studies on glutamine synthetase from Escherichia coli. Formation of pyrrolidone carboxylate and inhibition by methionine sulfoximine. J Biol Chem. 1973 Jun 10;248(11):3997–4002. [PubMed] [Google Scholar]
- Wolk C. P., Thomas J., Shaffer P. W., Austin S. M., Galonsky A. Pathway of nitrogen metabolism after fixation of 13N-labeled nitrogen gas by the cyanobacterium, Anabaena cylindrica. J Biol Chem. 1976 Aug 25;251(16):5027–5034. [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]


