Abstract
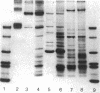
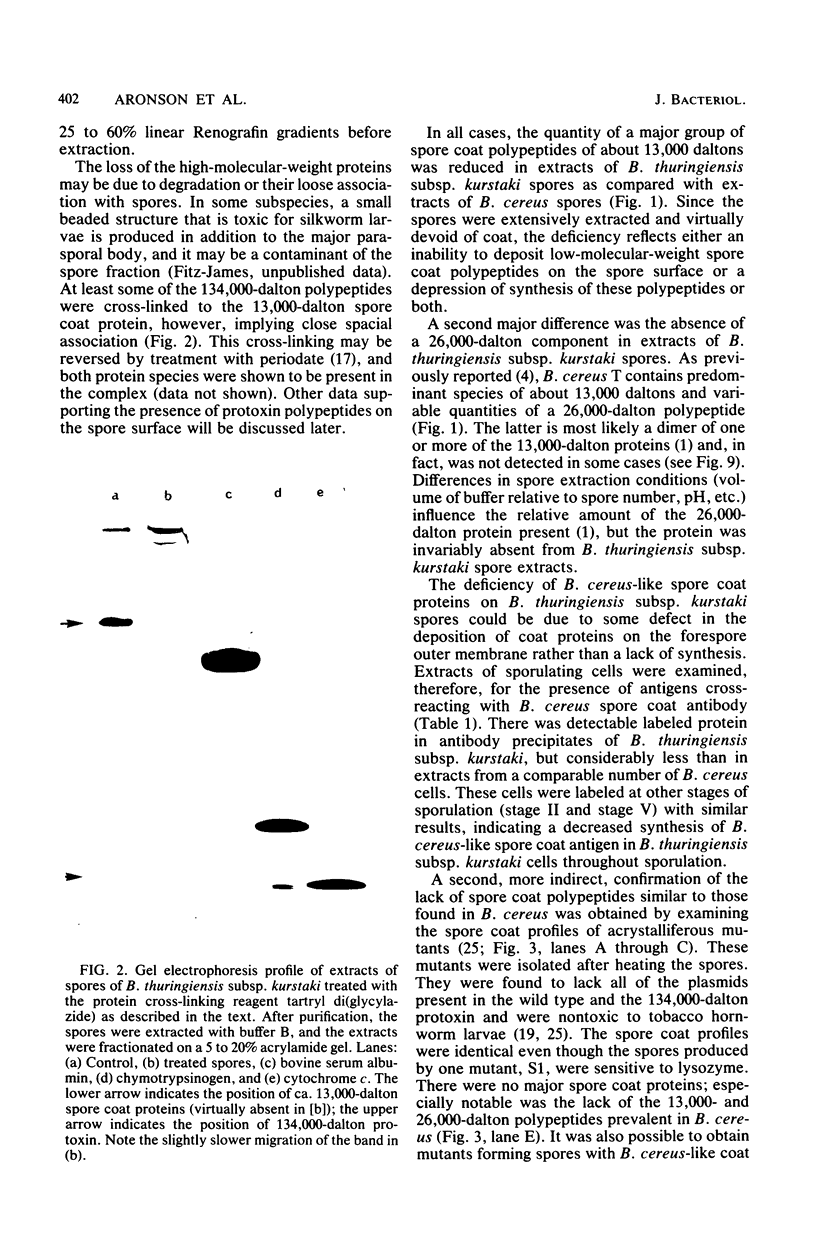
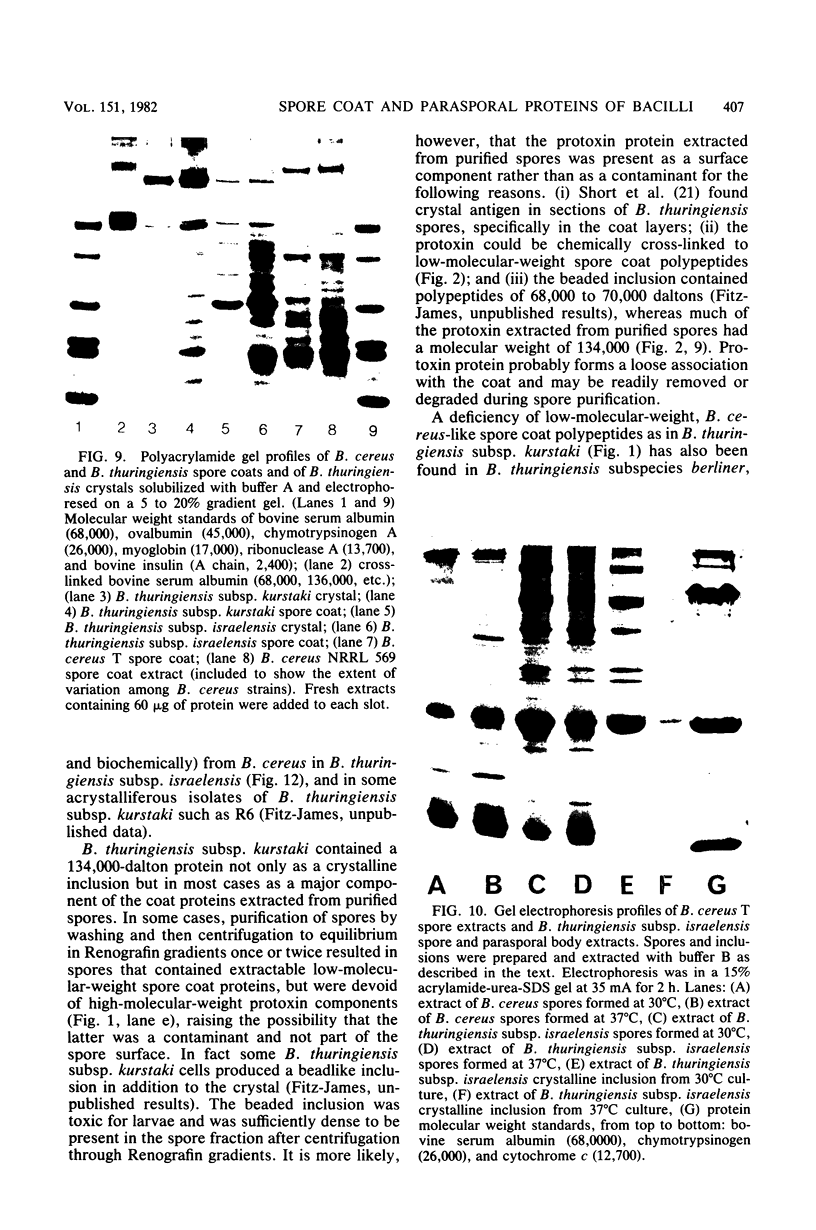
Two major classes of polypeptides were extracted from the spore surface of Bacillus thuringiensis subsp. kurstaki: the 134,000-dalton protoxin that is the major component of the crystalline inclusion and spore coat polypeptides very similar to those found on Bacillus cereus spores. The quantity of spore coat polypeptides produced was reduced when compared with that produced by certain acrystalliferous mutants or by B. thuringiensis subsp. israelensis. The latter organism produced an inclusion toxic to mosquito larvae, but deposited very little of the inclusion protein on the spore surface. The reduction in spore coat protein in B. thuringiensis subsp. kurstaki was also seen in freeze-etched electron micrographs of spores. B. thuringiensis subsp. kurstaki spores germinated rather slowly when compared with related species, a property previously correlated with a deficiency or defect of the spore coat. Many mutants of B. thuringiensis subsp. kurstaki unable to form a crystalline inclusion were nontoxic and lacked a well-defined spore coat. Other mutants isolated either directly from the wild type or from coat-deficient mutants produced spores that were identical to those produced by the closely related species. Bacillus cereus, on the basis of morphology, germination rate, and the size and antigenicity of the spore coat polypeptides. Most of the protein extractable from the inclusion produced by B. thuringiensis subsp. israelensis was about 26,000 daltons, considerably smaller than the major polypeptide extractable from other inclusions. Some of the B. thuringiensis subsp. israelensis inclusion protein was found on the spore surface, but the majority of the extractable spore coat protein was the same size and antigenicity as that found on B. cereus spores. The B. thuringiensis subsp. israelensis spores germinated at a rate close to that of B. cereus, especially when the spores were formed at 37 degrees C, and the morphology of the spore surface was very similar to that of B. cereus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Angelo N., Holt S. C. Regulation of extracellular protease production in Bacillus cereus T: characterization of mutants producing altered amounts of protease. J Bacteriol. 1971 Jun;106(3):1016–1025. doi: 10.1128/jb.106.3.1016-1025.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Properties of Bacillus cereus spore coat mutants. J Bacteriol. 1975 Jul;123(1):354–365. doi: 10.1128/jb.123.1.354-365.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976 Jun;40(2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I. Synthesis of Bacillus cereus spore coat protein. J Bacteriol. 1981 Jan;145(1):541–547. doi: 10.1128/jb.145.1.541-547.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulla L. A., Jr, Bechtel D. B., Kramer K. J., Shethna Y. I., Aronson A. I., Fitz-James P. C. Ultrastructure, physiology, and biochemistry of Bacillus thuringiensis. Crit Rev Microbiol. 1980;8(2):147–204. doi: 10.3109/10408418009081124. [DOI] [PubMed] [Google Scholar]
- Bulla L. A., Jr, Davidson L. I., Kramer K. J., Jones B. L. Purification of the insecticidal toxin from the parasporal crystal of Bacillus thuringiensis subsp. kurstaki. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1123–1130. doi: 10.1016/0006-291x(79)91997-1. [DOI] [PubMed] [Google Scholar]
- Bulla L. A., Jr, Kramer K. J., Davidson L. I. Characterization of the entomocidal parasporal crystal of Bacillus thuringiensis. J Bacteriol. 1977 Apr;130(1):375–383. doi: 10.1128/jb.130.1.375-383.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Aronson A. I. Alterations of spore coat processing and protein turnover in a Bacillus cereus mutant with a defective postexponential intracellular protease. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1254–1258. doi: 10.1073/pnas.74.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J. M., Jr, Dulmage H. T., Carlton B. C. Correlation between specific plasmids and delta-endotoxin production in Bacillus thuringiensis. Plasmid. 1981 May;5(3):352–365. doi: 10.1016/0147-619x(81)90010-x. [DOI] [PubMed] [Google Scholar]
- Horn D., Aronson A. I., Golub E. S. Development of a quantitative immunological assay for the study of spore coat synthesis and morphogenesis. J Bacteriol. 1973 Jan;113(1):313–321. doi: 10.1128/jb.113.1.313-321.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lecadet M. M., Chevrier G., Dedonder R. Analysis of a protein fraction in the spore coats of Bacillus thuringiensis. Comparison with crystal protein. Eur J Biochem. 1972 Feb 15;25(2):349–358. doi: 10.1111/j.1432-1033.1972.tb01703.x. [DOI] [PubMed] [Google Scholar]
- Lutter L. C., Ortanderl F., Fasold H. The use of a new series of cleavable protein-crosslinkers on the Escherichia coli ribosome. FEBS Lett. 1974 Nov 15;48(2):288–292. doi: 10.1016/0014-5793(74)80488-6. [DOI] [PubMed] [Google Scholar]
- Schesser J. H., Bulla L. A., Jr Toxicity of Bacillus thuringiensis spores to the tobacco hornworm, Manduca sexta. Appl Environ Microbiol. 1978 Jan;35(1):121–123. doi: 10.1128/aem.35.1.121-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schesser J. H., Kramer K. J., Bulla L. A., Jr Bioassay for homogeneous parasporal crystal of Bacillus thuringiensis using the tobacco hornworm, Manduca sexta. Appl Environ Microbiol. 1977 Apr;33(4):878–880. doi: 10.1128/aem.33.4.878-880.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe E. S., Nickerson K. W., Bulla L. A., Jr, Aronson J. N. Separation of spores and parasporal crystals of Bacillus thuringiensis in gradients of certain x-ray contrasting agents. Appl Microbiol. 1975 Dec;30(6):1052–1053. doi: 10.1128/am.30.6.1052-1053.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. A., Walker P. D., Thomson R. O., Somerville H. J. The fine structure of Bacillus finitimus and Bacillus thuringiensis spores with special reference to the location of crystal antigen. J Gen Microbiol. 1974 Oct;84(2):261–276. doi: 10.1099/00221287-84-2-261. [DOI] [PubMed] [Google Scholar]
- Somerville H. J., Jones M. L. DNA competition studies within the Bacillus cereus group of bacilli. J Gen Microbiol. 1972 Nov;73(2):257–265. doi: 10.1099/00221287-73-2-257. [DOI] [PubMed] [Google Scholar]
- Somerville H. J., Pockett H. V. An insect toxin from spores of Bacillus thuringiensis and Bacillus cereus. J Gen Microbiol. 1975 Apr;87(2):359–369. doi: 10.1099/00221287-87-2-359. [DOI] [PubMed] [Google Scholar]
- Stahly D. P., Dingman D. W., Bulla L. A., Jr, Aronson A. I. Possible origin and function of the parasporal crystal in Bacillus thuringiensis. Biochem Biophys Res Commun. 1978 Oct 16;84(3):581–588. doi: 10.1016/0006-291x(78)90745-3. [DOI] [PubMed] [Google Scholar]
- Stelma G. N., Jr, Aronson A. I., Fitz-James P. C. A Bacillus cereus mutant defective in spore coat deposition. J Gen Microbiol. 1980 Jan;116(1):173–185. doi: 10.1099/00221287-116-1-173. [DOI] [PubMed] [Google Scholar]
- Stelma G. N., Jr, Aronson A. I., Fitz-James P. Properties of Bacillus cereus temperature-sensitive mutants altered in spore coat formation. J Bacteriol. 1978 Jun;134(3):1157–1170. doi: 10.1128/jb.134.3.1157-1170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrell D. J., Bulla L. A., Jr, Andrews R. E., Jr, Kramer K. J., Davidson L. I., Nordin P. Comparative biochemistry of entomocidal parasporal crystals of selected Bacillus thuringiensis strains. J Bacteriol. 1981 Feb;145(2):1052–1062. doi: 10.1128/jb.145.2.1052-1062.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]