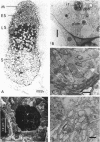
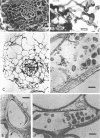
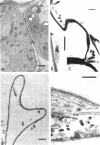
Abstract
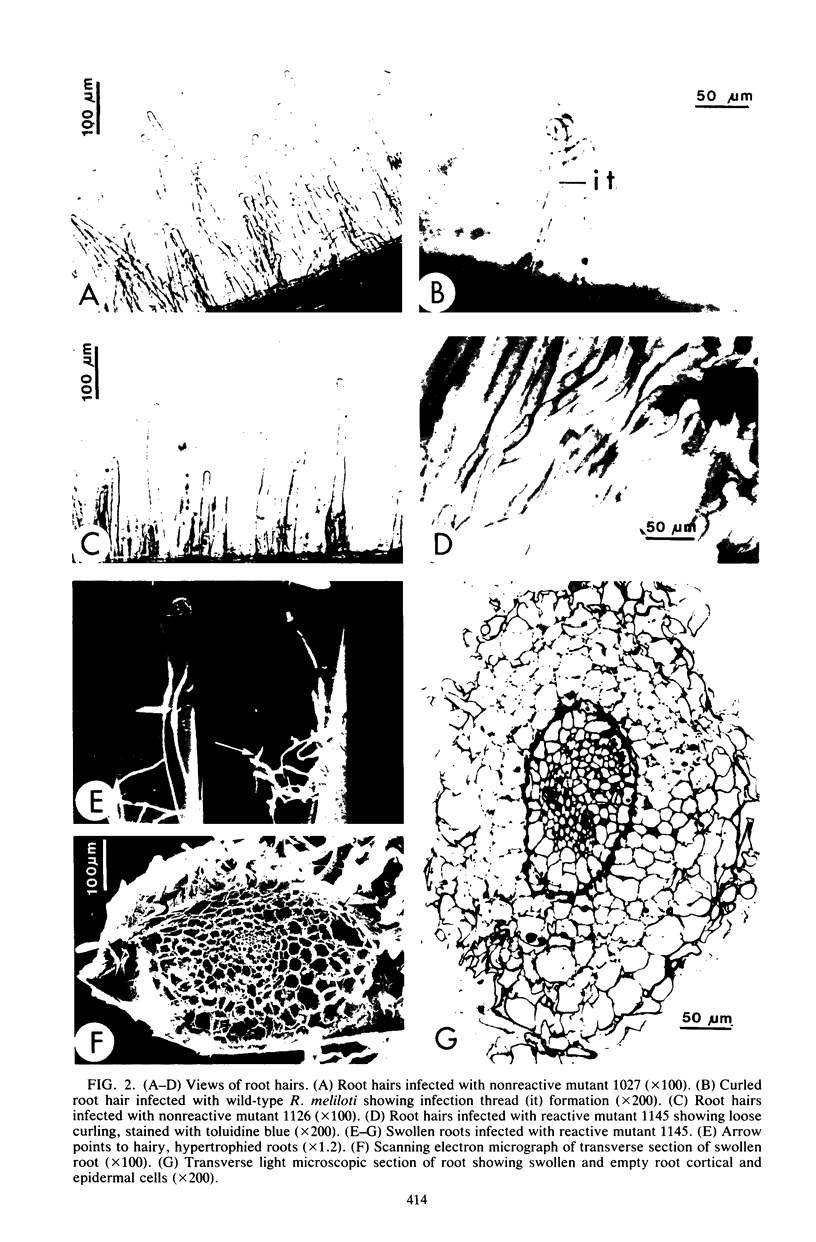
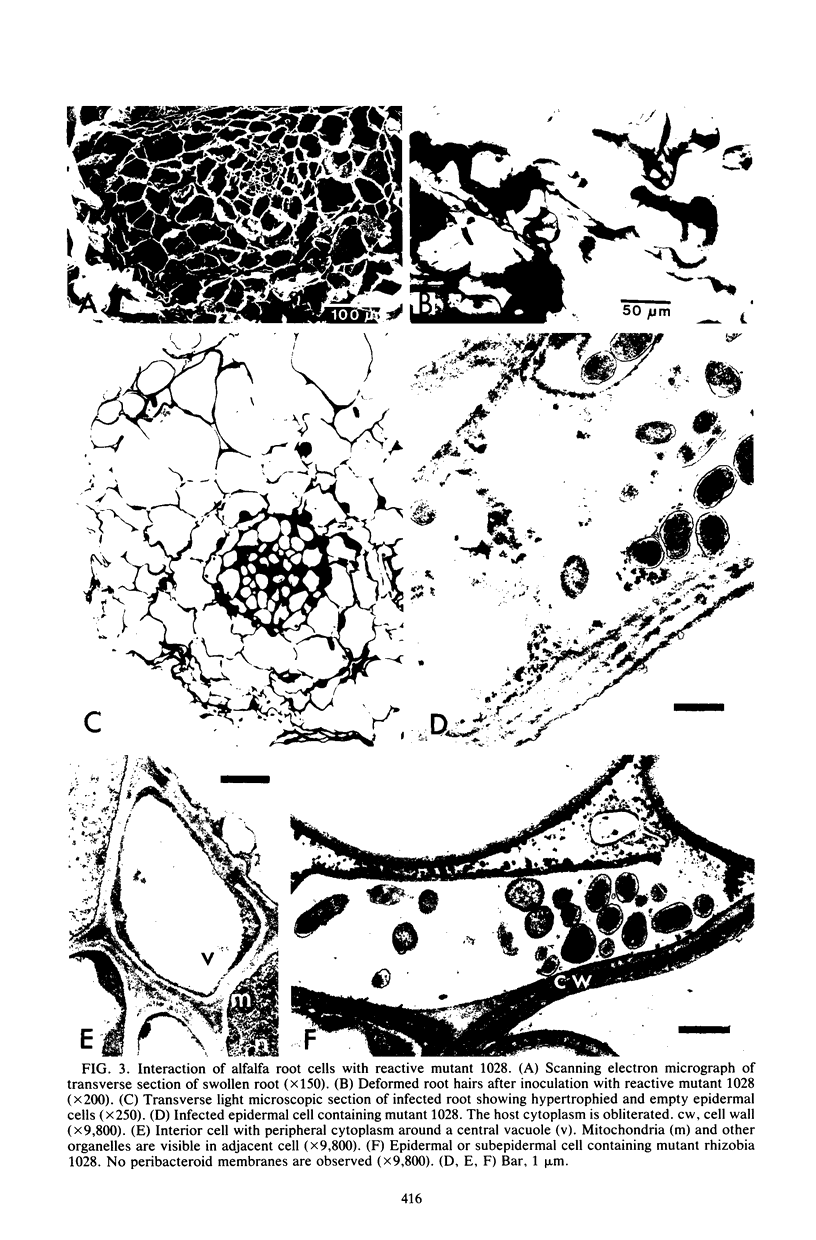
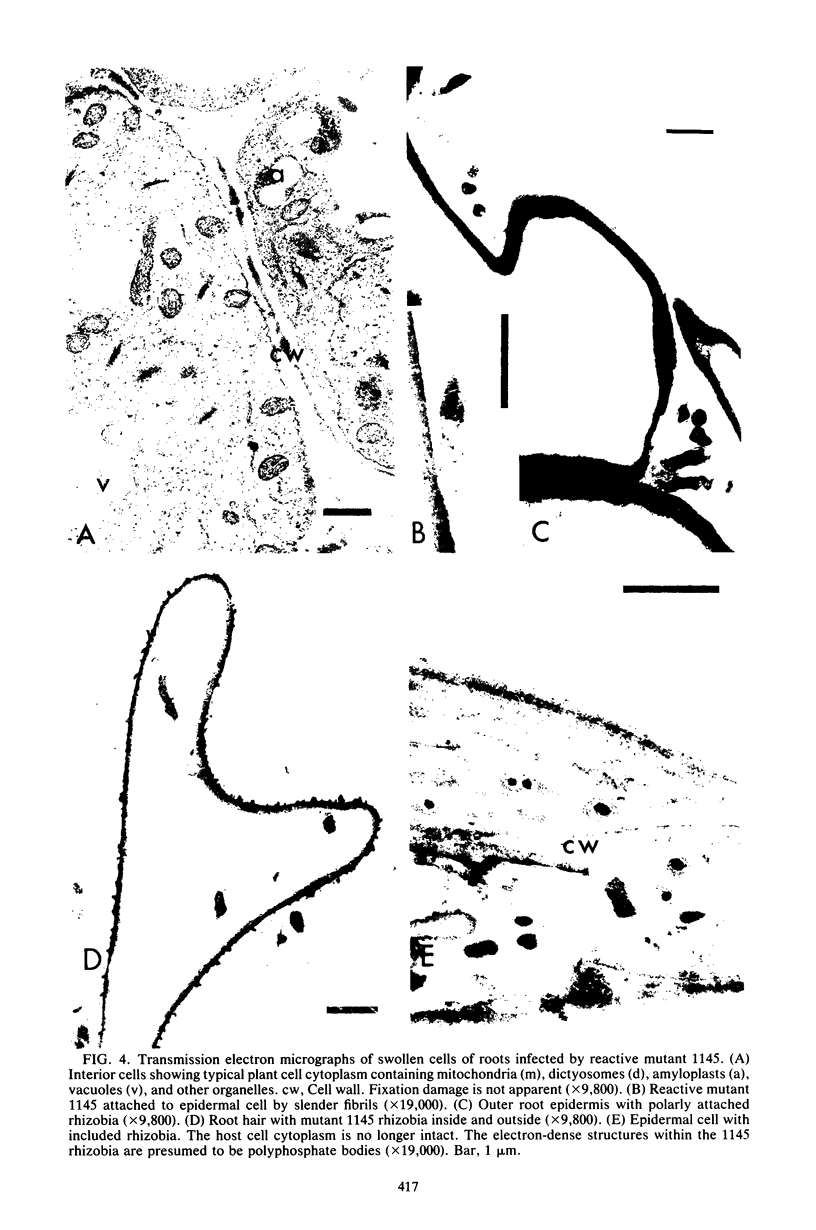
Alfalfa roots infected with four nodulation defective (Nod-) mutants of Rhizobium meliloti which were generated by transposon Tn5 mutagenesis were examined by light and electron microscopy. In one class of Nod- mutants, which we can nonreactive, the bacteria did not induce root hair curling or penetrate host cells. In a second class of Nod- mutants, which we call reactive, the bacteria induced some root hair curling and entered root epidermal cells, although no infection threads were formed. In addition, reactive Nod- mutants induced extensive root hair proliferation and hypertrophied roots. This study presents the details of the phenotype of the association between each mutant strain and alfalfa roots.
Full text
PDF

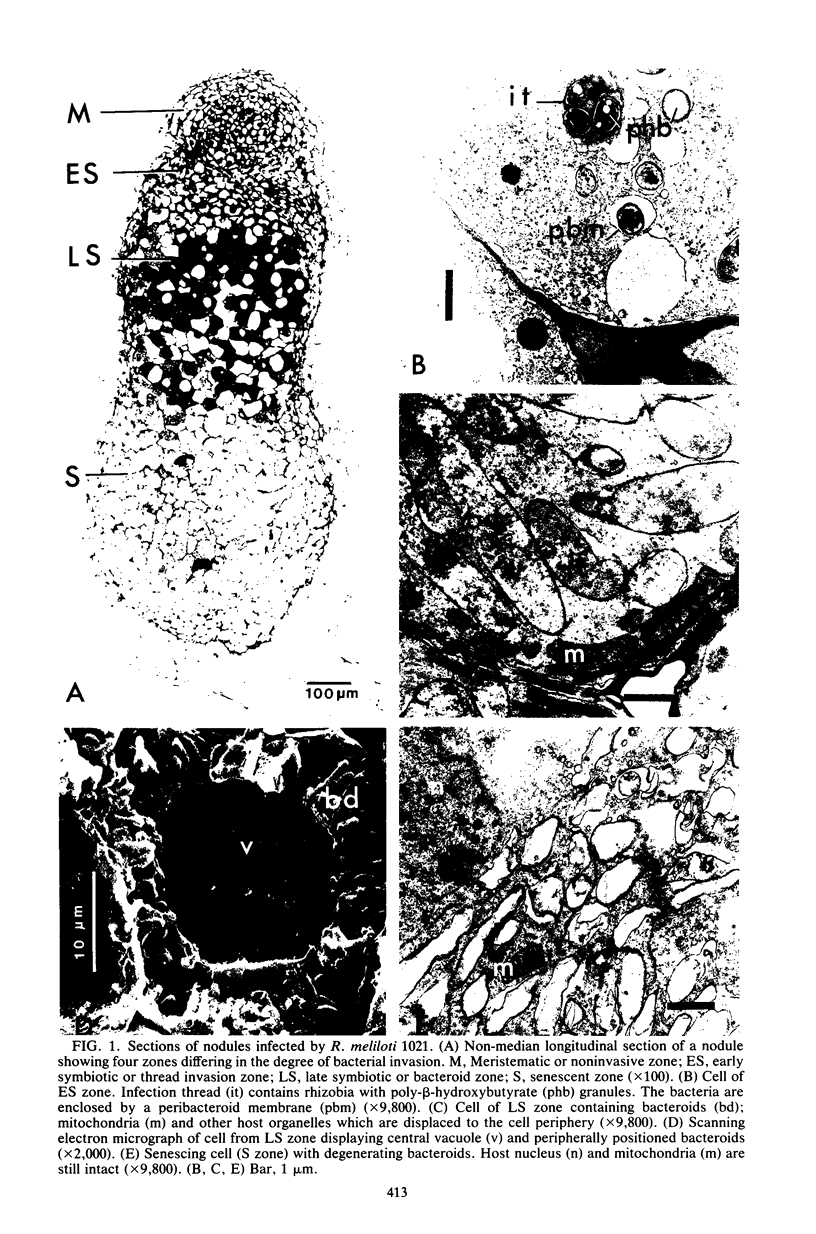



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dazzo F. B., Brill W. J. Receptor site on clover and alfalfa roots for Rhizobium. Appl Environ Microbiol. 1977 Jan;33(1):132–136. doi: 10.1128/aem.33.1.132-136.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- JORDAN D. C., GRINYER I., COULTER W. H. ELECTRON MICROSCOPY OF INFECTION THREADS AND BACTERIA IN YOUNG ROOT NODULES OF MEDICAGO SATIVA. J Bacteriol. 1963 Jul;86:125–137. doi: 10.1128/jb.86.1.125-137.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Marshall K. C., Cruickshank R. H., Bushby H. V. The orientation of certain root-nodule bacteria at interfaces, including legume root-hair surfaces. J Gen Microbiol. 1975 Nov;91(1):198–200. doi: 10.1099/00221287-91-1-198. [DOI] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C. A., Hubbell D. H. Ultrastructure of Rhizobium-induced infection threads in clover root hairs. Appl Microbiol. 1975 Dec;30(6):1003–1009. doi: 10.1128/am.30.6.1003-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Dazzo F., Hubbell D. Production of cellulose microfibrils by Rhizobium. Appl Microbiol. 1975 Jul;30(1):123–131. doi: 10.1128/am.30.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S., Leps W. T., Brill W. J. Agglutinin from Alfalfa Necessary for Binding and Nodulation by Rhizobium meliloti. Science. 1981 Sep 25;213(4515):1513–1515. doi: 10.1126/science.213.4515.1513. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. G., Lyttleton P., Bullivant S., Grayston G. F. Membranes in lupin root nodules. I. The role of Golgi bodies in the biogenesis of infection threads and peribacteroid membranes. J Cell Sci. 1978 Apr;30:129–149. doi: 10.1242/jcs.30.1.129. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- SAHLMAN K. AN ELECTRON MICROSCOPE STUDY OF ROOT-HAIR INFECTION BY RHIZOBIUM. J Gen Microbiol. 1963 Dec;33:425–427. doi: 10.1099/00221287-33-3-425. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- van Brussel A. A., Costerton J. W., Child J. J. Nitrogen fixation by Rhizobium sp. 32H1. A morphological and ultrastructural comparison of asymbiotic and symbiotic nitrogen-fixing forms. Can J Microbiol. 1979 Mar;25(3):352–361. doi: 10.1139/m79-055. [DOI] [PubMed] [Google Scholar]