Abstract
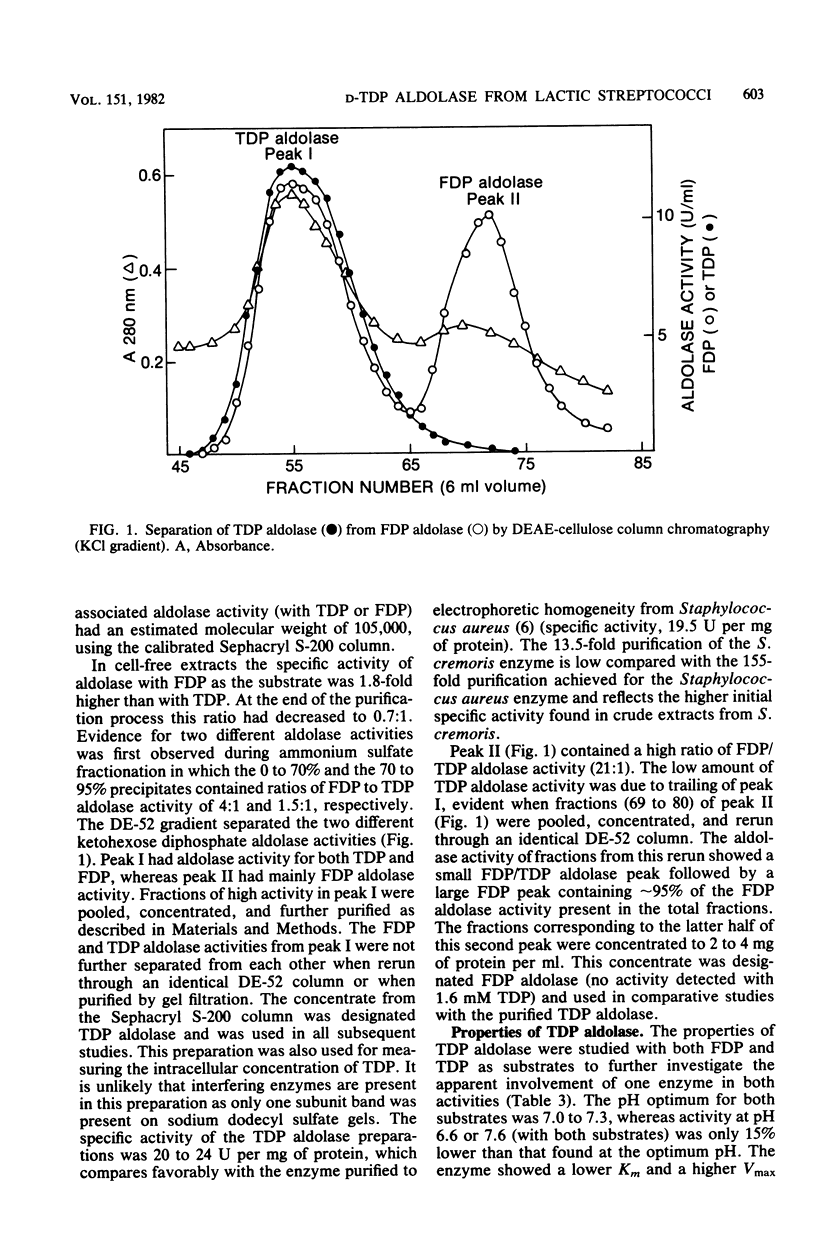
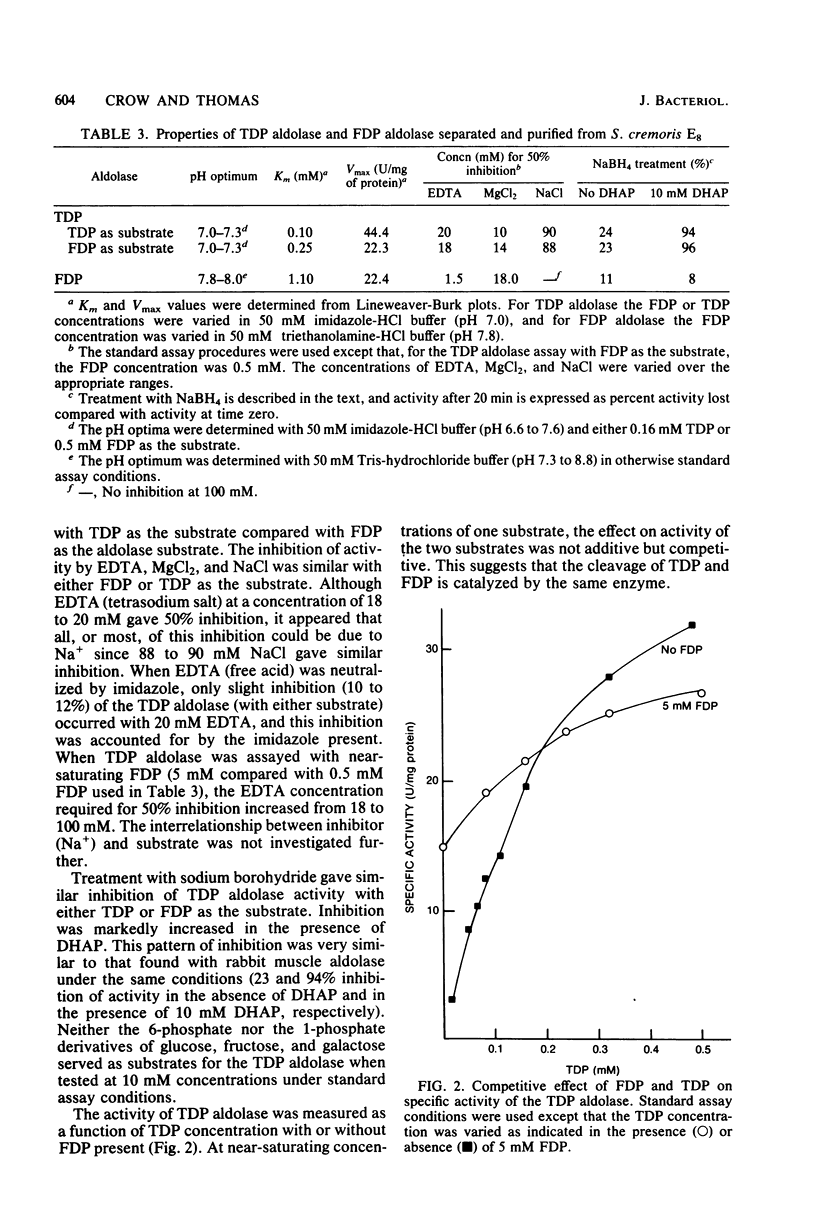
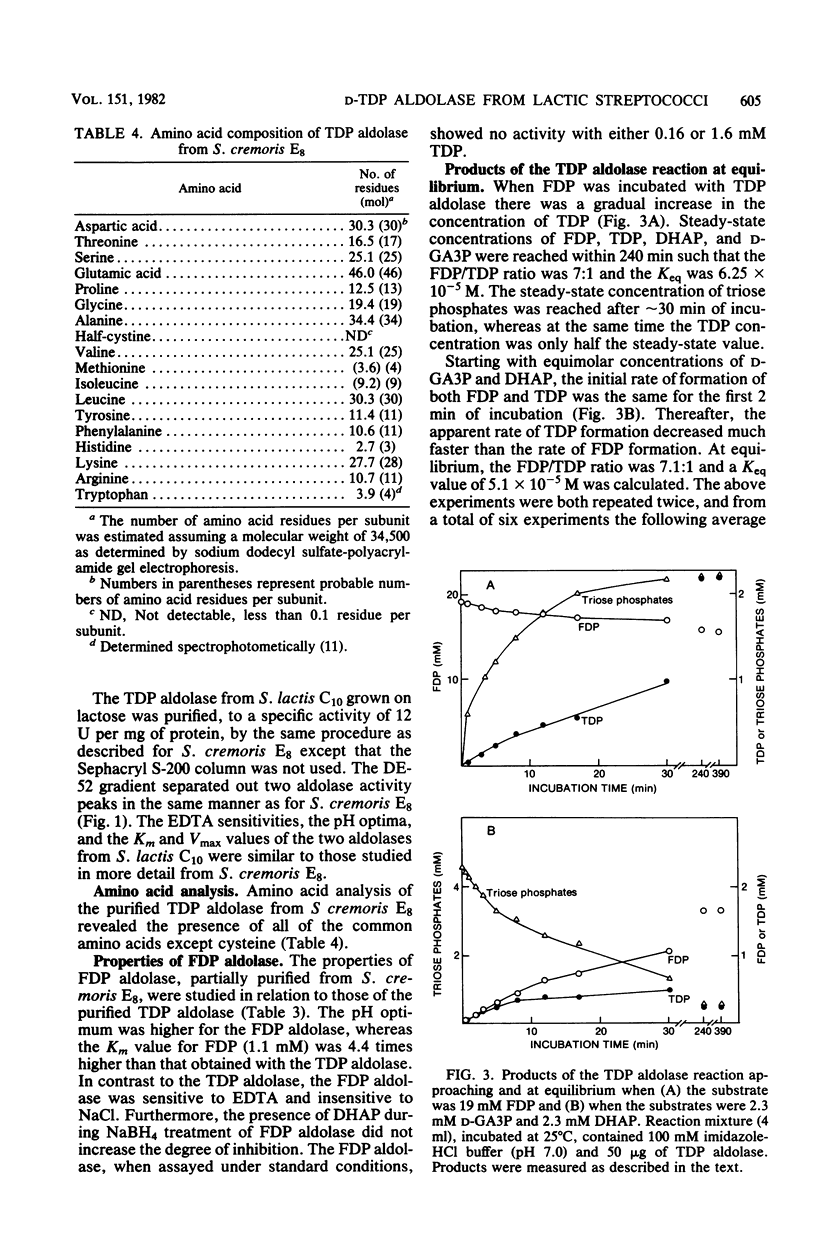
Two D-ketohexose 1,6-diphosphate aldolases are present in Streptococcus cremoris E8 and S. lactis C10. One aldolase, which was induced by growth on either lactose or galactose, was active with both tagatose 1,6-diphosphate (TDP) and fructose 1,6-diphosphate (FDP), having a lower Km and a higher Vmax with TDP as the substrate. This enzyme, named TDP aldolase, had properties typical of a class I aldolase, being insensitive to EDTA and showing substrate-dependent inactivation by sodium borohydride. Sodium dodecyl sulfate-gel electrophoresis indicated a subunit molecular weight of 34,500. The amino acid composition of TDP aldolase is reported. When the enzyme was incubated with either triose phosphates or FDP, the equilibrium mixture contained an FDP/TDP ratio of 6.9:1. The other aldolase, which had properties typical of a class II aldolase, showed activity with FDP but not with TDP. The intracellular TDP concentration, measured with the purified TDP aldolase, was 0.4 to 4.0 mM in cells growing on lactose or galactose and was lower (0 to 1.0 mM) in cells growing on glucose. The intracellular concentration of FDP was always higher than that of TDP. The role of ketohexose diphosphates in the regulation of end product fermentation by lactic streptococci is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissett D. L., Anderson R. L. Genetic evidence for the physiological significance of the D-tagatose 6-phosphate pathway of lactose and D-galactose degradation in staphylococcus aureus. J Bacteriol. 1974 Sep;119(3):698–704. doi: 10.1128/jb.119.3.698-704.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in Staphylococcus aureus. III. Purification and properties of D-tagatose-6-phosphate kinase. J Biol Chem. 1980 Sep 25;255(18):8745–8749. [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in Staphylococcus aureus. IV. Isolation and properties of a class I D-ketohexose-1,6-diphosphate aldolase that catalyzes the cleavage of D-tagatose 1,6-diphosphate. J Biol Chem. 1980 Sep 25;255(18):8750–8755. [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in group N streptococci: presence of enzymes for both the D-galactose 1-phosphate and D-tagatose 6-phosphate pathways. J Bacteriol. 1974 Jan;117(1):318–320. doi: 10.1128/jb.117.1.318-320.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D0galactose metabolism in Staphylococcus aureus: pathway of D-galactose 6-phosphate degradation. Biochem Biophys Res Commun. 1973 May 15;52(2):641–647. doi: 10.1016/0006-291x(73)90761-4. [DOI] [PubMed] [Google Scholar]
- Bissett D. L., Wenger W. C., Anderson R. L. Lactose and D-galactose metabolism in Staphylococcus aureus. II. Isomerization of D-galactose 6-phosphate to D-tagatose 6-phosphate by a specific D-galactose-6-phosphate isomerase. J Biol Chem. 1980 Sep 25;255(18):8740–8744. [PubMed] [Google Scholar]
- Crow V. L., Pritchard G. G. Fructose 1,6-diphosphate-activated L-lactate dehydrogenase from Streptococcus lactis: kinetic properties and factors affecting activation. J Bacteriol. 1977 Jul;131(1):82–91. doi: 10.1128/jb.131.1.82-91.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Wittenberger C. L. Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from Streptococcus mutans. J Biol Chem. 1979 Feb 25;254(4):1134–1142. [PubMed] [Google Scholar]
- DeVries G. H., Binkley S. B. N-acetylneuraminic acid aldolase of Clostridium perfringens: purification, properties and mechanism of action. Arch Biochem Biophys. 1972 Jul;151(1):234–242. doi: 10.1016/0003-9861(72)90493-6. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- GRAZI E., CHENG T., HORECKER B. L. The formation of a stable aldolase-dihydroxyacetone phosphate complex. Biochem Biophys Res Commun. 1962 Apr 20;7:250–253. doi: 10.1016/0006-291x(62)90184-5. [DOI] [PubMed] [Google Scholar]
- GRAZI E., MELOCHE H., MARTINEZ G., WOOD W. A., HORECKER B. L. Evidence for Schiff base formation in enzymatic aldol condensations. Biochem Biophys Res Commun. 1963 Jan 18;10:4–10. doi: 10.1016/0006-291x(63)90257-2. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Lebtag H. Lactose metabolism by Streptococcus mutans: evidence for induction of the tagatose 6-phosphate pathway. J Bacteriol. 1979 Dec;140(3):1102–1104. doi: 10.1128/jb.140.3.1102-1104.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Morse M. L. Purification of the staphylococcal 6-phospho-beta-D-- galactosidase. Eur J Biochem. 1970 May 1;14(1):27–32. doi: 10.1111/j.1432-1033.1970.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Jago G. R., Nichol L. W., O'Dea K., Sawyer W. H. Physicochemical studies on the lactate dehydrogenase of Streptococcus cremoris US3: the effects of modifiers. Biochim Biophys Acta. 1971 Nov 13;250(2):271–285. doi: 10.1016/0005-2744(71)90184-7. [DOI] [PubMed] [Google Scholar]
- Jayanthi Bia N., Ramachandra Pai M., Suryanarayana Murthy P., Venkitasubramanian T. A. Fructose diphosphate aldolase from Mycobacterium smegmatis. Purification and properties. Arch Biochem Biophys. 1975 May;168(1):230–234. doi: 10.1016/0003-9861(75)90245-3. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., McDonald I. J. Beta-D-phosphogalactoside galactohydrolase from Streptococcus cremoris HP: purification and enzyme properties. J Bacteriol. 1974 Feb;117(2):667–674. doi: 10.1128/jb.117.2.667-674.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas H. A., Anders R. F., Jago G. R. Factors affecting the activity of the lactate dehydrognease of Streptococcus cremoris. J Bacteriol. 1972 Aug;111(2):397–403. doi: 10.1128/jb.111.2.397-403.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebherz H. G., Bradshaw R. A., Rutter W. J. Structural comparisons between the class I fructose diphosphate aldolases from Micrococcus aerogenes and rabbit. J Biol Chem. 1973 Mar 10;248(5):1660–1665. [PubMed] [Google Scholar]
- Lebherz H. G., Rutter W. J. A class I (Schiff base) fructose diphosphate aldolase of prokaryotic origin. Purification and properties of Micrococcus aerogenes aldolase. J Biol Chem. 1973 Mar 10;248(5):1650–1659. [PubMed] [Google Scholar]
- Lengeler J. Nature and properties of hexitol transport systems in Escherichia coli. J Bacteriol. 1975 Oct;124(1):39–47. doi: 10.1128/jb.124.1.39-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J. Variations in the quaternary structure of three lactic acid bacteria aldolases. Evidence for the existence of a class I and class II aldolase in Lactobacillus casei. J Biol Chem. 1974 Dec 25;249(24):7977–7983. [PubMed] [Google Scholar]
- Markwell J., Shimamoto G. T., Bissett D. L., Anderson R. L. Pathway of galactitol catabolism in Klebsiella pneumoniae. Biochem Biophys Res Commun. 1976 Jul 12;71(1):221–227. doi: 10.1016/0006-291x(76)90271-0. [DOI] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Horecker B. L. The mechanism of action of aldolases. Adv Enzymol Relat Areas Mol Biol. 1968;31:125–181. doi: 10.1002/9780470122761.ch4. [DOI] [PubMed] [Google Scholar]
- RUTTER W. J. EVOLUTION OF ALDOLASE. Fed Proc. 1964 Nov-Dec;23:1248–1257. [PubMed] [Google Scholar]
- Stribling D., Perham R. N. Purification and characterization of two fructose diphosphate aldolases from Escherichia coli (Crookes' strain). Biochem J. 1973 Apr;131(4):833–841. doi: 10.1042/bj1310833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumiło T. An alternative route for D-galactose catabolism shared with that for dulcitol degradation route in mycobacteria. FEBS Lett. 1981 Feb 23;124(2):270–272. doi: 10.1016/0014-5793(81)80153-6. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Jarvis B. D., Skipper N. A. Localization of proteinase(s) near the cell surface of Streptococcus lactis. J Bacteriol. 1974 May;118(2):329–333. doi: 10.1128/jb.118.2.329-333.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Regulation of lactose fermentation in group N streptococci. Appl Environ Microbiol. 1976 Oct;32(4):474–478. doi: 10.1128/aem.32.4.474-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Tagatose-1, 6-diphosphate activation of lactate dehydrogenase from Streptococcus cremoris. Biochem Biophys Res Commun. 1975 Apr 21;63(4):1035–1042. doi: 10.1016/0006-291x(75)90673-7. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Turner K. W., Crow V. L. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J Bacteriol. 1980 Nov;144(2):672–682. doi: 10.1128/jb.144.2.672-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Lactose metabolism in Streptococcus lactis: phosphorylation of galactose and glucose moieties in vivo. J Bacteriol. 1979 Dec;140(3):774–785. doi: 10.1128/jb.140.3.774-785.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



