Abstract
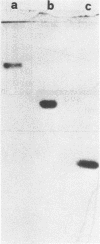
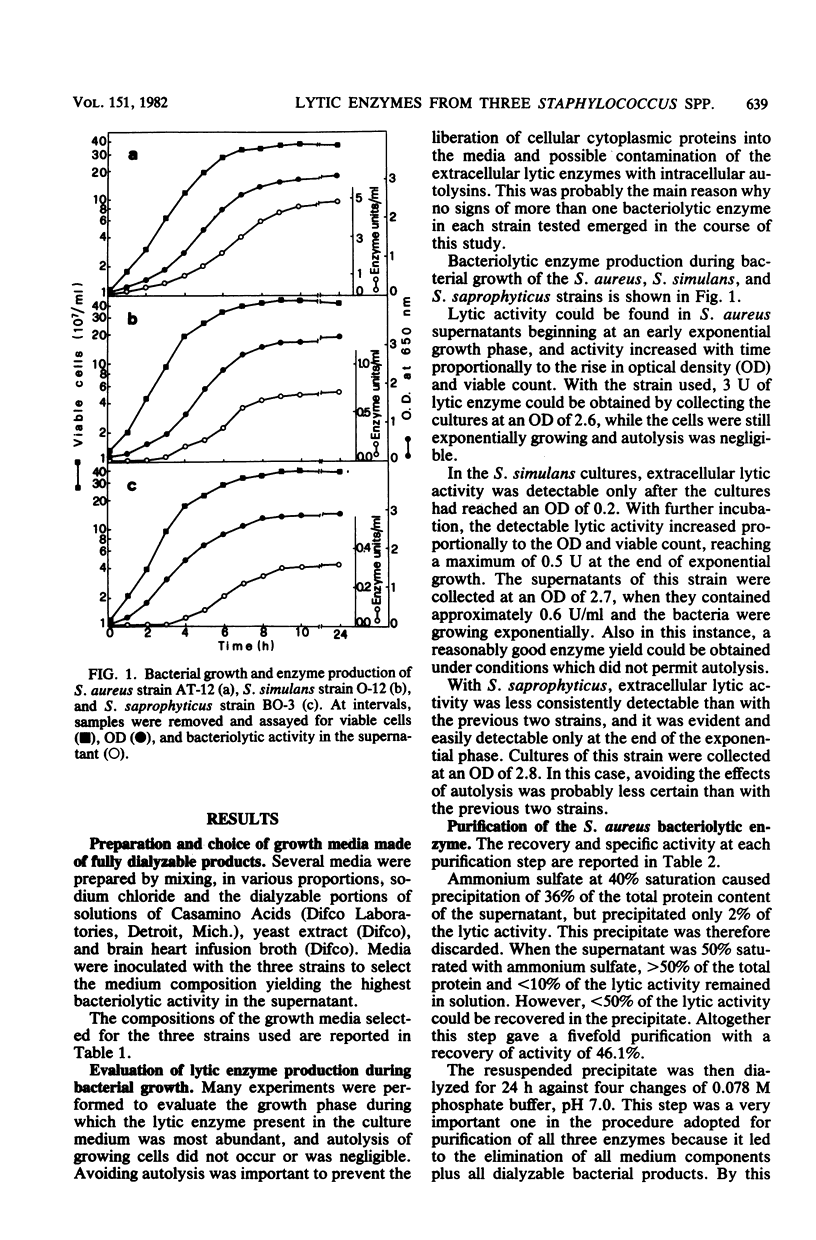
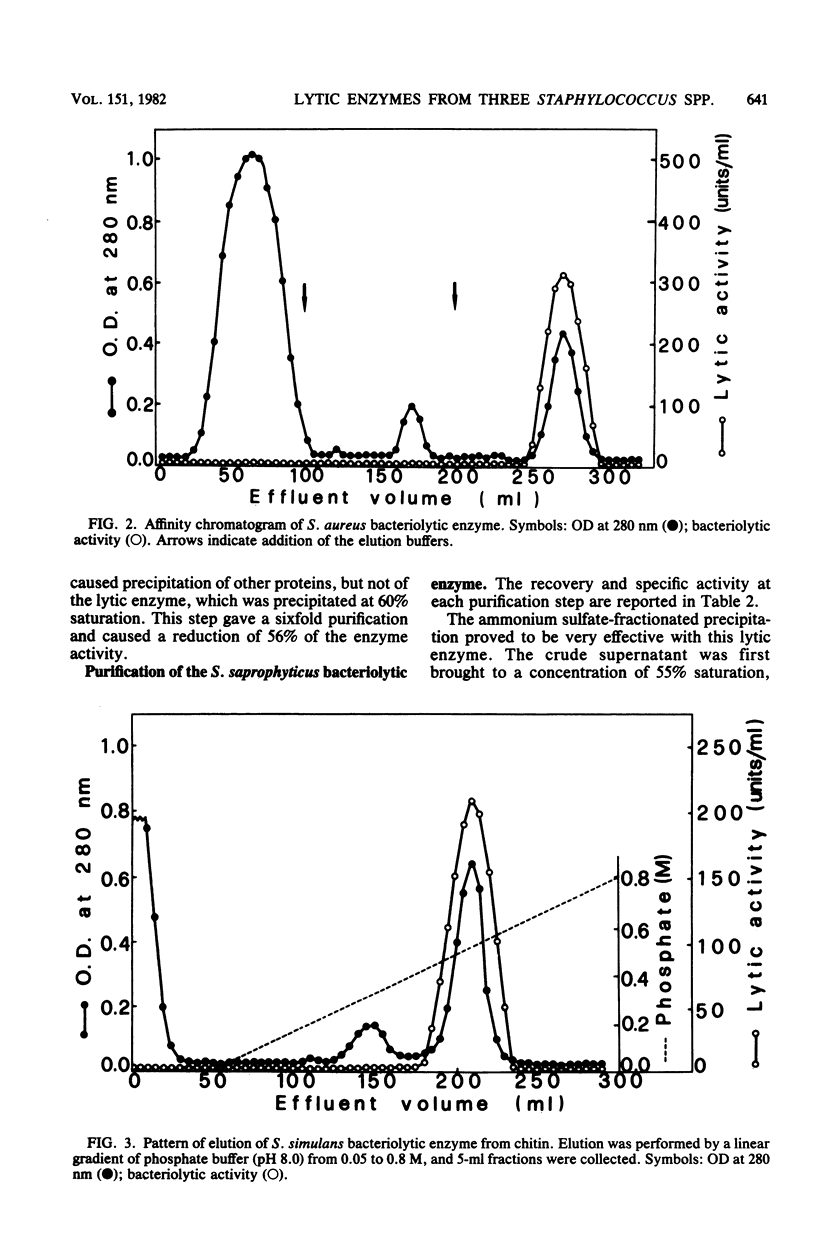
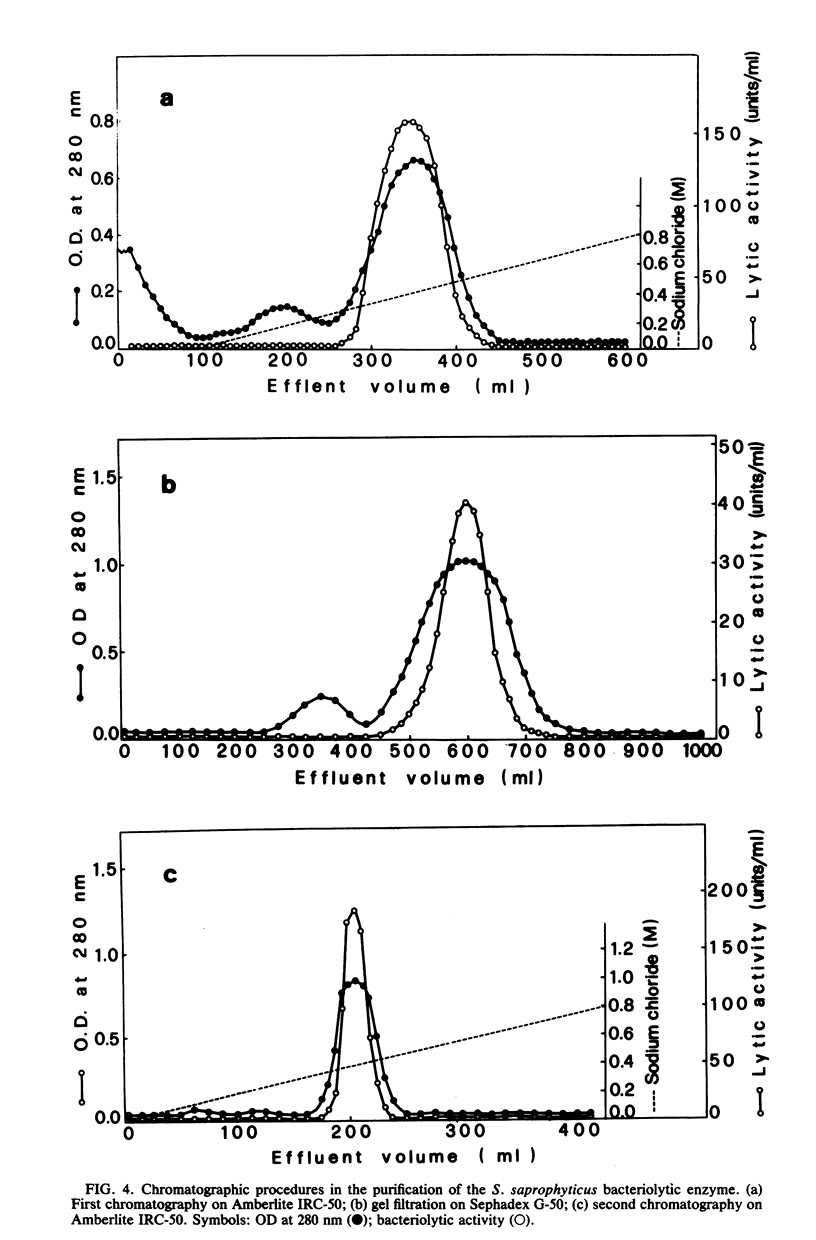
As a further development of previous investigations showing that different staphylococcal species display different bacteriolytic activity patterns (lyogroups), the bacteriolytic enzymes excreted by three different Staphylococcus species, Staphylococcus aureus (lyogroup I), S. simulans (lyogroup II), and S. saprophyticus (lyogroup IV); have been purified and characterized. A representative strain from each species was grown in a preselected medium made of fully dialyzable products. Culture supernatants were collected in the appropriate growth phase. Two different affinity adsorbents were used for enzyme purification. One was obtained by coupling lysozyme-digested pure peptidoglycan from Micrococcus luteus to cyanogen bromide-activated Sepharose 4B. The second affinity adsorbent used was chitin. The S. aureus bacteriolytic enzyme bound to the solubilized peptidoglycan but not to chitin, whereas the opposite was true for the S. simulans enzyme. The bacteriolytic enzyme from S. saprophyticus did not bind to either the Sepharose 4B-peptidoglycan resin or to chitin, and its purification was achieved by two ion-exchange chromatography steps combined with gel filtration. All three enzymes were purified to apparent homogeneity. Their subsequent characterization indicated that all acted as endo-beta-N-acetylglucosaminidases. However, the three glucosaminidases differed significantly in their kinetics of activity and bacteriolytic spectrum against heat-killed cells of a variety of microorganisms. Very different values also resulted from molecular weight determinations: 80,000 for the S. aureus enzyme, 45,000 for the S. simulans enzyme, and 31,000 for the S. saprophyticus enzyme. Other important differences were observed in their stability, optimal pH and ionic strength for their activity, and their responses to temperature and divalent cations. These results confirmed the previous proposal that different staphylococcal species excrete different lytic enzymes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Fontana R., Canepari P., Satta G. Alterations in peptidoglycan chemical composition associated with rod-to-sphere transition in a conditional mutant of Klebsiella pneumoniae. J Bacteriol. 1979 Sep;139(3):1028–1038. doi: 10.1128/jb.139.3.1028-1038.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossgebauer K., Schmidt B., Langmaack H. Lysozyme production as an aid for identification of potentially pathogenic strains of staphylococci. Appl Microbiol. 1968 Nov;16(11):1745–1747. doi: 10.1128/am.16.11.1745-1747.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J. Purification and properties of lysozyme produced by Staphylococcus aureus. J Bacteriol. 1968 Feb;95(2):376–384. doi: 10.1128/jb.95.2.376-384.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen H. B., Kleppe K. Studies on T4 lysozyme. Affinity for chitin and the use of chitin in the purification of the enzyme. Eur J Biochem. 1972 Apr 11;26(3):305–312. doi: 10.1111/j.1432-1033.1972.tb01769.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leduc M., van Heijenoort J., Kaminski M., Szulmajster J. An unusual type of bacterial cell-wall in a mutant of Bacillus subtilis 168. Eur J Biochem. 1973 Aug 17;37(2):389–400. doi: 10.1111/j.1432-1033.1973.tb02998.x. [DOI] [PubMed] [Google Scholar]
- Neuberger A., Wilson B. M. Inhibition of lysozyme by derivatives of D-glucosamine. I. Biochim Biophys Acta. 1967 Dec 12;147(3):473–486. doi: 10.1016/0005-2795(67)90007-4. [DOI] [PubMed] [Google Scholar]
- PARRY R. M., Jr, CHANDAN R. C., SHAHANI K. M. A RAPID AND SENSITIVE ASSAY OF MURAMIDASE. Proc Soc Exp Biol Med. 1965 Jun;119:384–386. doi: 10.3181/00379727-119-30188. [DOI] [PubMed] [Google Scholar]
- Satta G., Azzarone B., Varaldo P. E., Fontana R., Valisena S. Stimulation of spreading of trypsinized human fibroblasts by lysozymes from Staphylococcus aureus, hen egg white, and human urine. In Vitro. 1980 Sep;16(9):738–750. doi: 10.1007/BF02619307. [DOI] [PubMed] [Google Scholar]
- Satta G., Varaldo P. E., Azzarone B., Romanzi C. A. Effects of Staphylococcus aureus lysozyme on human fibroblasts. Cell Biol Int Rep. 1979 Sep;3(6):525–533. doi: 10.1016/0309-1651(79)90088-2. [DOI] [PubMed] [Google Scholar]
- Satta G., Varaldo P. E., Grazi G., Fontana R. Bacteriolytic activity in staphylococci. Infect Immun. 1977 Apr;16(1):37–42. doi: 10.1128/iai.16.1.37-42.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Varaldo P. E., Tenca M., Radin L. The relevance of bacterial lytic activity in the taxonomy of the micrococcaceae: failure of its production by Micrococcus and Planococcus as opposed to Staphylococcus. J Gen Microbiol. 1978 Dec;109(2):385–388. doi: 10.1099/00221287-109-2-385. [DOI] [PubMed] [Google Scholar]
- Schindler M., Mirelman D., Sharon N. Substrate-induced evolution of lysozymes. Biochim Biophys Acta. 1977 Jun 10;482(2):386–392. doi: 10.1016/0005-2744(77)90252-2. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Varaldo P. E., Grazi G., Cisani G., Satta G. Routine separation of staphylococci from micrococci based on bacteriolytic activity production. J Clin Microbiol. 1979 Jan;9(1):147–148. doi: 10.1128/jcm.9.1.147-148.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaldo P. E., Grazi G., Soro O., Cisani G., Satta G. Simplified lyogroup system, a new method for routine identification of staphylococci: description and comparison with three other methods. J Clin Microbiol. 1980 Jul;12(1):63–68. doi: 10.1128/jcm.12.1.63-68.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadström T., Hisatsune K. Bacteriolytic enzymes from Staphylococcus aureus. Purification of an endo-beta-N-acetylglucosaminidase. Biochem J. 1970 Dec;120(4):725–734. doi: 10.1042/bj1200725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadström T., Hisatsune K. Bacteriolytic enzymes from Staphylococcus aureus. Specificity of ction of endo-beta-N-acetylglucosaminidase. Biochem J. 1970 Dec;120(4):735–744. doi: 10.1042/bj1200735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Hayashida S., Tobiishi M., Kado K., Tsuru D. Purification of several bacteriolytic enzymes by affinity chromatography on lysozyme-lysate of Micrococcus lysodeikticus cell wall coupled with sepharose. J Biochem. 1975 Aug;78(2):253–259. doi: 10.1093/oxfordjournals.jbchem.a130902. [DOI] [PubMed] [Google Scholar]
- ZILLIKEN F., SMITH P. N., TOMARELLI R. M., GYORGY P. 4-O-beta-D-Galactopyranosyl-N-acetyl-D-Glucosamine in hog mucin. Arch Biochem Biophys. 1955 Feb;54(2):398–405. doi: 10.1016/0003-9861(55)90053-9. [DOI] [PubMed] [Google Scholar]