Abstract
Methodology which allows consistent shotgun cloning of streptomycete genes is presented. Parameters that increase transformation efficiency of Streptomyces lividans 66 were adjusted to generate reproducibly a population of cloned genes likely to represent the entire genome. Factors which influence the recovery of viable transformants include: growth phase of the mycelium, ionic and osmotic characteristics of the medium during protoplast formation and transformation, and moisture content and protoplast density during regeneration. A modified transformation procedure was devised which increased transformation frequency more than 20-fold (allowing up to 10(7) primary transformants per microgram of SLP1.2 covalently closed circular DNA) and greatly facilitated the cloning of drug resistance genes and biosynthetic genes, using one of two plasmid vectors. Viomycin resistance genes on BamHI or PstI fragments were cloned from S. vinaceus genomic DNA into S. lividans, using the SLP1.2 vector. At least three different S. vinaceus BamHI fragments (1.9, 5.8, or 8.5 kilobases) confer viomycin resistance; only one PstI fragment (4.3 kilobases) was found. Recombinant plasmids were all able to produce lethal zygosis and to be transferred by conjugation within S. lividans. SCP2 was used to clone S. coelicolor A3(2) genes that "complemented" the auxotrophic mutation hisD3, argA1, or guaA1. Recombinant DNA technology can now be applied to economically and academically interesting problems unique to streptomycete molecular biology.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benigni R., Petrov P. A., Carere A. Estimate of the genome size by renaturation studies in Streptomyces. Appl Microbiol. 1975 Aug;30(2):324–326. doi: 10.1128/am.30.2.324-326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Kieser T., Cohen S. N., Hopwood D. A. Excision of chromosomal DNA sequences from Streptomyces coelicolor forms a novel family of plasmids detectable in Streptomyces lividans. Mol Gen Genet. 1981;184(2):230–240. doi: 10.1007/BF00272910. [DOI] [PubMed] [Google Scholar]
- Bibb M., Schottel J. L., Cohen S. N. A DNA cloning system for interspecies gene transfer in antibiotic-producing Streptomyces. Nature. 1980 Apr 10;284(5756):526–531. doi: 10.1038/284526a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Daniels D. L., de Wet J. R., Blattner F. R. New map of bacteriophage lambda DNA. J Virol. 1980 Jan;33(1):390–400. doi: 10.1128/jvi.33.1.390-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T., Contente S., Dubnau D. Molecular cloning of heterologous chromosomal DNA by recombination between a plasmid vector and a homologous resident plasmid in Bacillus subtilis. Mol Gen Genet. 1980 Feb;177(3):459–467. doi: 10.1007/BF00271485. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Chater K. F., Dowding J. E., Vivian A. Advances in Streptomyces coelicolor genetics. Bacteriol Rev. 1973 Sep;37(3):371–405. doi: 10.1128/br.37.3.371-405.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967 Dec;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft J., Hughes C. Cloning vectors derived from plasmids and phage of Bacillus. Curr Top Microbiol Immunol. 1982;96:1–17. doi: 10.1007/978-3-642-68315-2_1. [DOI] [PubMed] [Google Scholar]
- Lomovskaya N. D., Chater K. F., Mkrtumian N. M. Genetics and molecular biology of Streptomyces bacteriophages. Microbiol Rev. 1980 Jun;44(2):206–229. doi: 10.1128/mr.44.2.206-229.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya N. D., Mkrtumian N. M., Gostimskaya N. L., Danilenko V. N. Characterization of temperate actinophage phi C31 isolated from Streptomyces coelicolor A3(2). J Virol. 1972 Feb;9(2):258–262. doi: 10.1128/jvi.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B., Palla E., Niaudet B., Ehrlich S. D. DNA cloning in Bacillus subtilis. III. Efficiency of random-segment cloning and insertional inactivation vectors. Gene. 1980 Dec;12(1-2):147–154. doi: 10.1016/0378-1119(80)90025-6. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Bruce S. A., Murray K. Molecular cloning of the DNA ligase gene from bacteriophage T4. II. Amplification and preparation of the gene product. J Mol Biol. 1979 Aug 15;132(3):493–505. doi: 10.1016/0022-2836(79)90271-7. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Suzuki K., Umezawa H. Formation and reversion of Streptomycete protoplasts: cultural condition and morphological study. J Gen Microbiol. 1974 Feb;80(2):389–400. doi: 10.1099/00221287-80-2-389. [DOI] [PubMed] [Google Scholar]
- Schrempf H., Bujard H., Hopwood D. A., Goebel W. Isolation of covalently closed circular deoxyribonucleic acid from Streptomyces coelicolor A3(2). J Bacteriol. 1975 Feb;121(2):416–421. doi: 10.1128/jb.121.2.416-421.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf H., Goebel W. Characterization of a plasmid from Streptomyces coelicolor A3(2). J Bacteriol. 1977 Jul;131(1):251–258. doi: 10.1128/jb.131.1.251-258.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
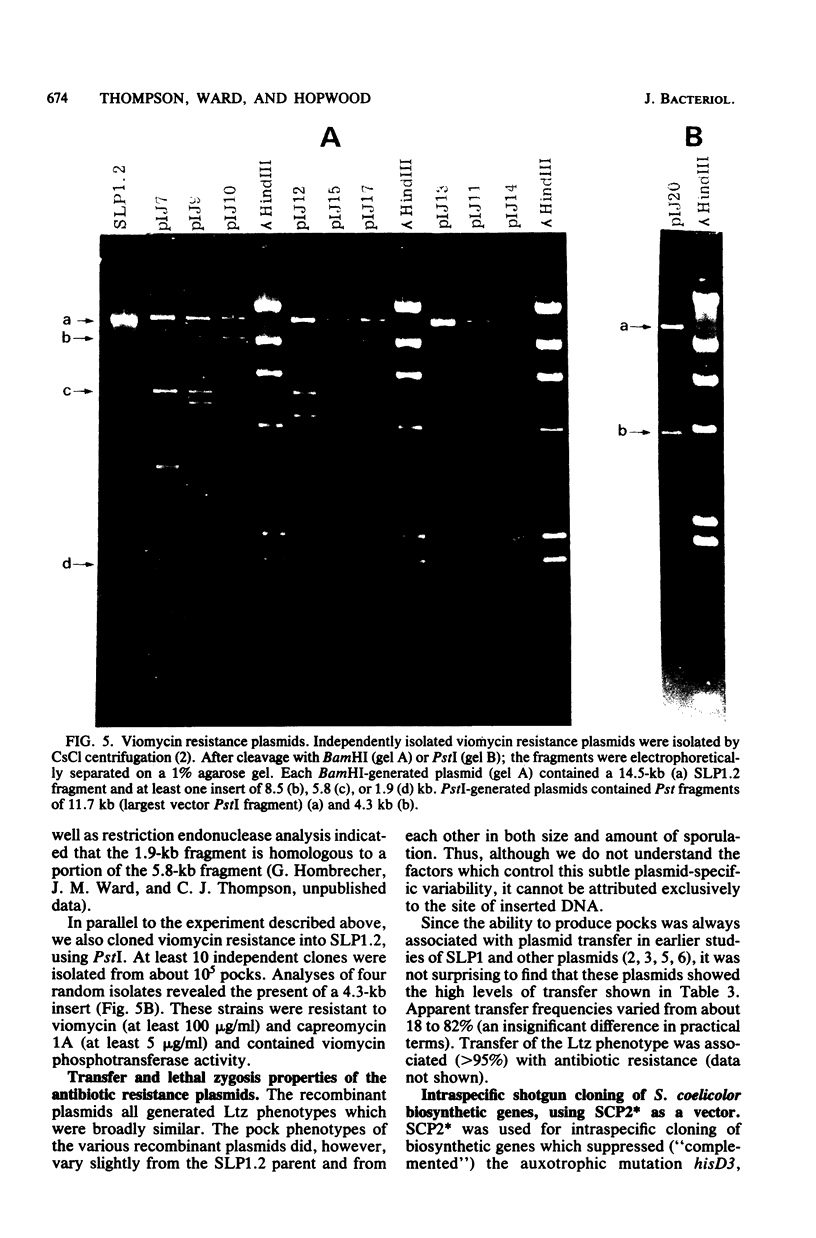
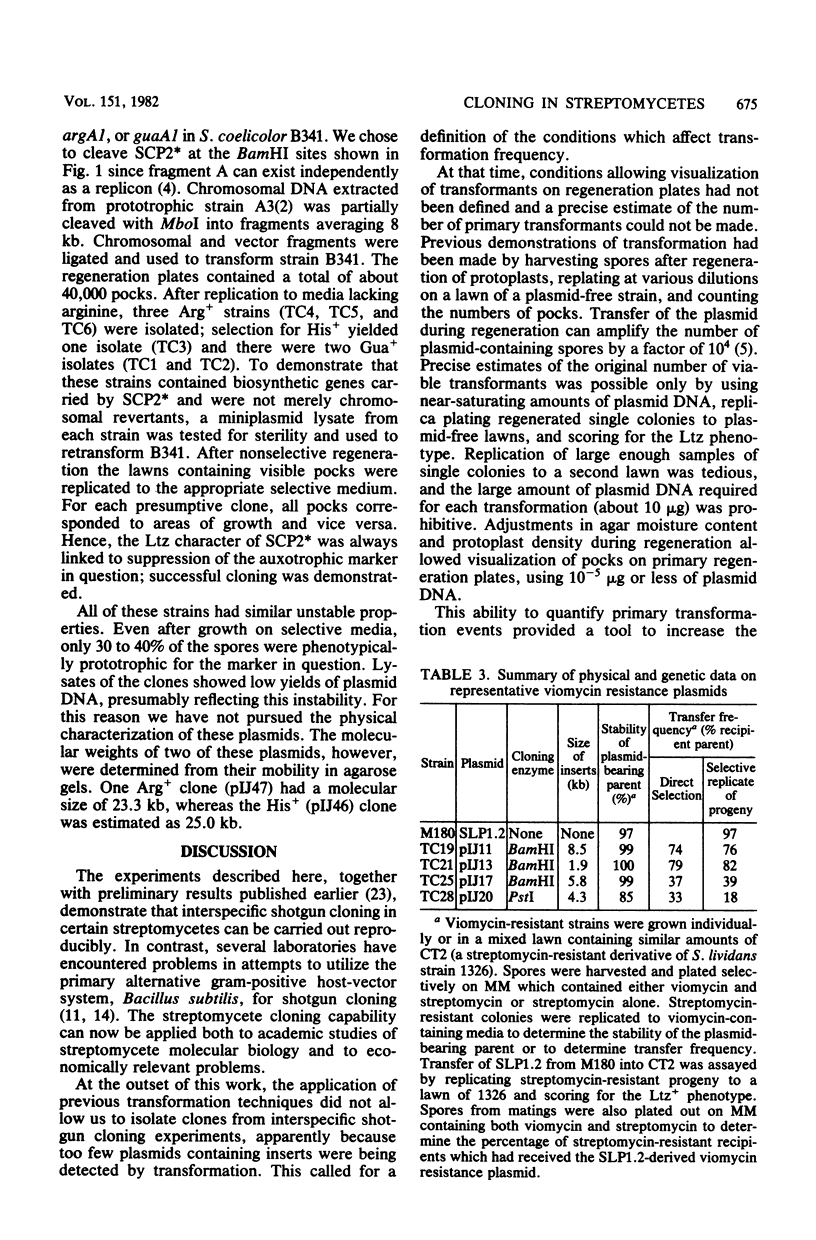
- Thompson C. J., Skinner R. H., Thompson J., Ward J. M., Hopwood D. A., Cundliffe E. Biochemical characterization of resistance determinants cloned from antibiotic-producing streptomycetes. J Bacteriol. 1982 Aug;151(2):678–685. doi: 10.1128/jb.151.2.678-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. DNA cloning in Streptomyces: resistance genes from antibiotic-producing species. Nature. 1980 Jul 31;286(5772):525–527. doi: 10.1038/286525a0. [DOI] [PubMed] [Google Scholar]