Abstract
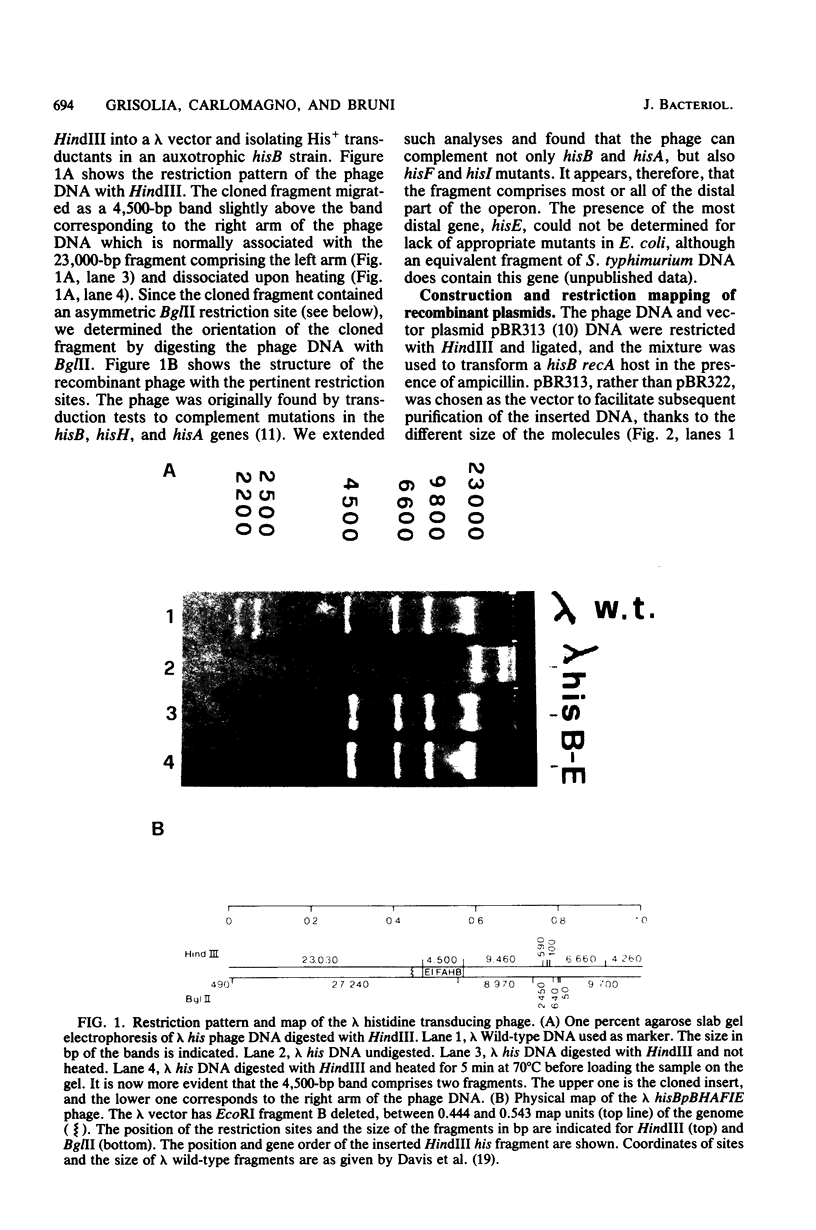
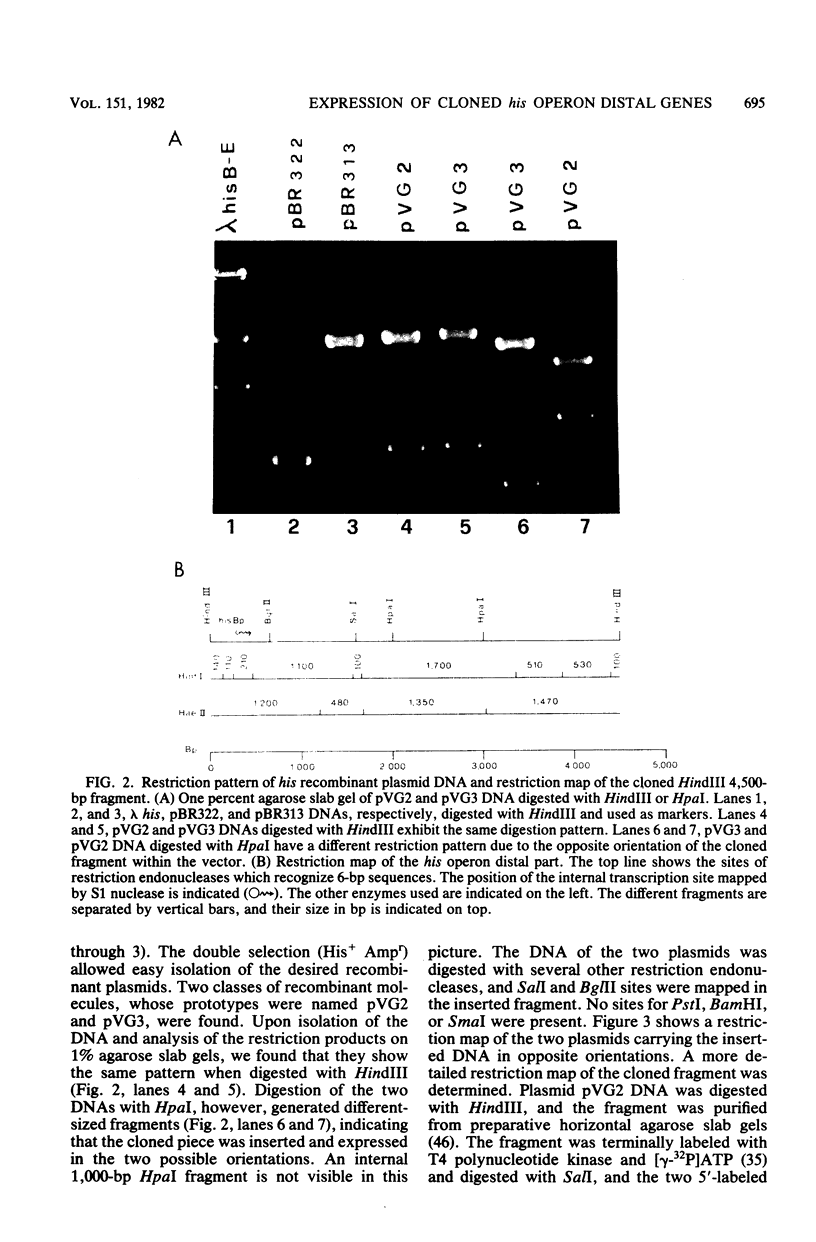
The operator-distal genes hisBHAFI(E) of the Escherichia coli K-12 histidine operon were mapped on a DNA fragment 4,500 base pairs long. This fragment, originally present in a lambda transducing phage, was cloned in the vector plasmid pBR313. A restriction map was determined, allowing identification of the orientation of the genes in the fragment. The cloned genes were expressed in appropriate hosts, independent of the orientation of the DNA fragment, as shown by transformation tests and by enzyme assays of one of the gene products, hisB, histidinol phosphatase. An internal transcription initiation site was identified by isolation of the cellular RNA, hybridization to specific DNA probes, and mapping by S1 nuclease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Loper J. C. Transcription initiation in the histidine operon of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1970 Apr;65(4):925–932. doi: 10.1073/pnas.65.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitabile A., Carlomagno-Cerillo S., Favvre R., Blasi F. Isolation of transducing bacteriophages for the histidine and isoleucine-valine operons in Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):40–47. doi: 10.1128/jb.112.1.40-47.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. Cloning and restriction map of the first part of the histidine operon of Salmonella typhimurium. J Bacteriol. 1981 Jul;147(1):124–134. doi: 10.1128/jb.147.1.124-134.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. Evidence for two sites for initiation of gene expression in the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1967 Jun 28;26(3):423–436. doi: 10.1016/0022-2836(67)90313-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bickle T. A., Pirrotta V., Imber R. A simple, general procedure for purifying restriction endonucleases. Nucleic Acids Res. 1977 Aug;4(8):2561–2572. doi: 10.1093/nar/4.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F., Bruni C. B. Regulation of the histidine operon: translation-controlled transcription termination (a mechanism common to several biosynthetic operons). Curr Top Cell Regul. 1981;19:1–45. doi: 10.1016/b978-0-12-152819-5.50018-x. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Bruni C. B., Musti A. M., Frunzio R., Blasi F. Structural and physiological studies of the Escherichia coli histidine operon inserted into plasmid vectors. J Bacteriol. 1980 Apr;142(1):32–42. doi: 10.1128/jb.142.1.32-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Chessin H., Summers W. C. Initiation by RNA polymerase on UV or x-ray damaged T7 DNA. Biochem Biophys Res Commun. 1970 Jan 6;38(1):40–45. doi: 10.1016/0006-291x(70)91080-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Di Nocera P. P., Blasi F., Di Lauro R., Frunzio R., Bruni C. B. Nucleotide sequence of the attenuator region of the histidine operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4276–4280. doi: 10.1073/pnas.75.9.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Ciesla Z. Internal promoter P2 of the histidine operon of Salmonella typhimurium. J Bacteriol. 1974 Nov;120(2):984–986. doi: 10.1128/jb.120.2.984-986.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Frunzio R., Bruni C. B., Blasi F. In vivo and in vitro detection of the leader RNA of the histidine operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 May;78(5):2767–2771. doi: 10.1073/pnas.78.5.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick-Silversmith L., Hartman P. E. Histidine-requiring mutants of Escherichia coli K12. Genetics. 1970 Oct;66(2):231–244. doi: 10.1093/genetics/66.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt E. P., Cater M. S., Matney T. S., Butler M. A., Greene A. Genetic analysis of the histidine operon in Escherichia coli K12. Genetics. 1970 Oct;66(2):219–229. doi: 10.1093/genetics/66.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Internal promoter of the tryptophan operon of Escherichia coli is located in a structural gene. J Mol Biol. 1972 Aug 21;69(2):307–313. doi: 10.1016/0022-2836(72)90232-x. [DOI] [PubMed] [Google Scholar]
- Johnston H. M., Roth J. R. DNA sequence changes of mutations altering attenuation control of the histidine operon of Salmonella typhimurium. J Mol Biol. 1981 Feb 5;145(4):735–756. doi: 10.1016/0022-2836(81)90312-0. [DOI] [PubMed] [Google Scholar]
- Johnston H. M., Roth J. R. Genetic analysis of the histidine operon control region of Salmonella typhimurium. J Mol Biol. 1981 Feb 5;145(4):713–734. doi: 10.1016/0022-2836(81)90311-9. [DOI] [PubMed] [Google Scholar]
- Kasai T. Regulation of the expression of the histidine operon in Salmonella typhimurium. Nature. 1974 Jun 7;249(457):523–527. doi: 10.1038/249523a0. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Effects of altered rho gene product on the expression of the Escherichia coli histidine operon. J Bacteriol. 1978 Dec;136(3):1201–1204. doi: 10.1128/jb.136.3.1201-1204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. The internal low-efficiency promoter of the tryptophan operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):447–451. doi: 10.1016/0022-2836(68)90401-4. [DOI] [PubMed] [Google Scholar]
- Murray M. L., Hartman P. E. Overproduction of hisH and hisF gene products leads to inhibition of cell cell division in Salmonella. Can J Microbiol. 1972 May;18(5):671–681. doi: 10.1139/m72-105. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Bruni C. B., Martin R. G., Terry W. An intercistronic region in the histidine operon of Salmonella typhimurium. J Mol Biol. 1972 Aug 28;69(3):427–452. doi: 10.1016/0022-2836(72)90256-2. [DOI] [PubMed] [Google Scholar]
- Staples M. A., Houston L. L. Proteolytic degradation of imidazoleglycerolphosphate dehydratase-histidinol phosphatase from Salmonella typhimurium and the isolation of a resistant bifunctional core enzyme. J Biol Chem. 1979 Feb 25;254(4):1395–1401. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Verde P., Frunzio R., di Nocera P. P., Blasi F., Bruni C. B. Identification, nucleotide sequence and expression of the regulatory region of the histidine operon of Escherichia coli K-12. Nucleic Acids Res. 1981 May 11;9(9):2075–2086. doi: 10.1093/nar/9.9.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M. E., Roth D. J., Hartman P. E. Promoter- and attenuator-related metabolic regulation of the Salmonella typhimurium histidine operon. J Bacteriol. 1978 Feb;133(2):830–843. doi: 10.1128/jb.133.2.830-843.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]