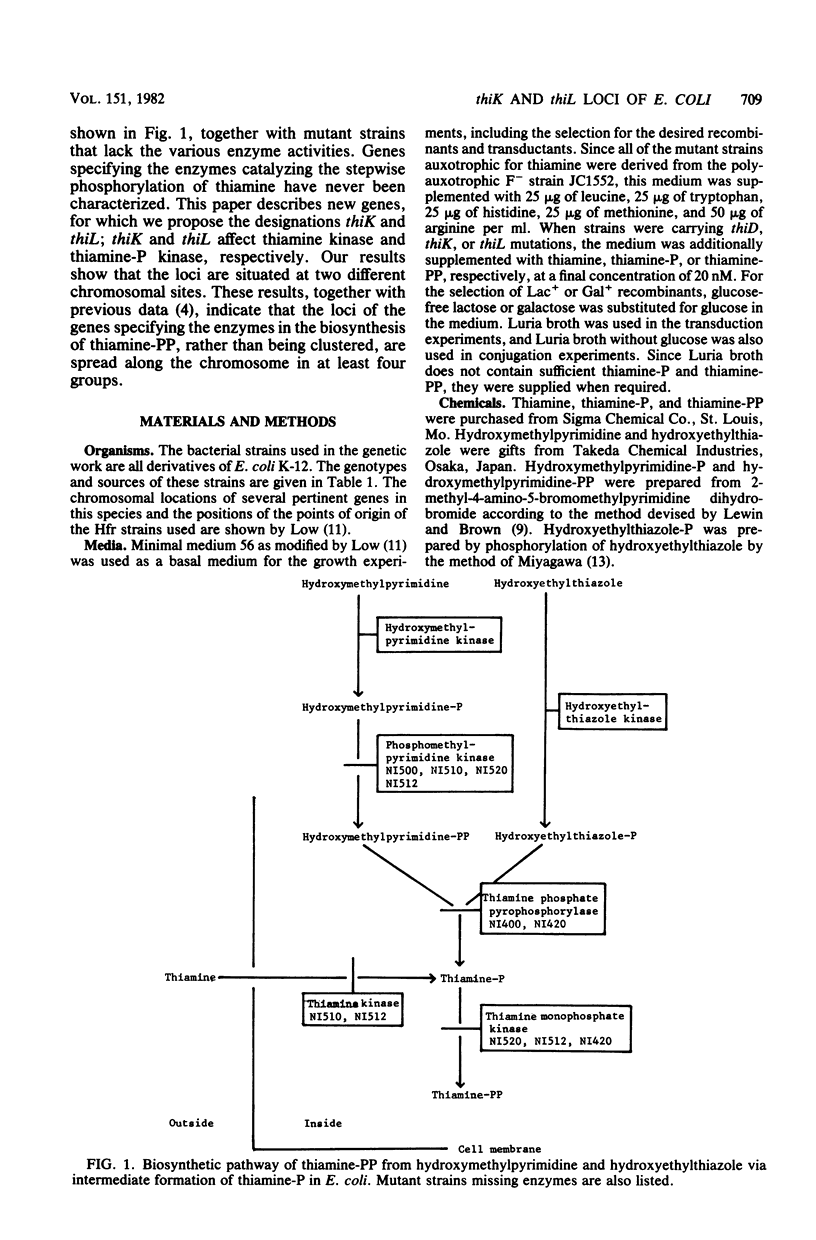
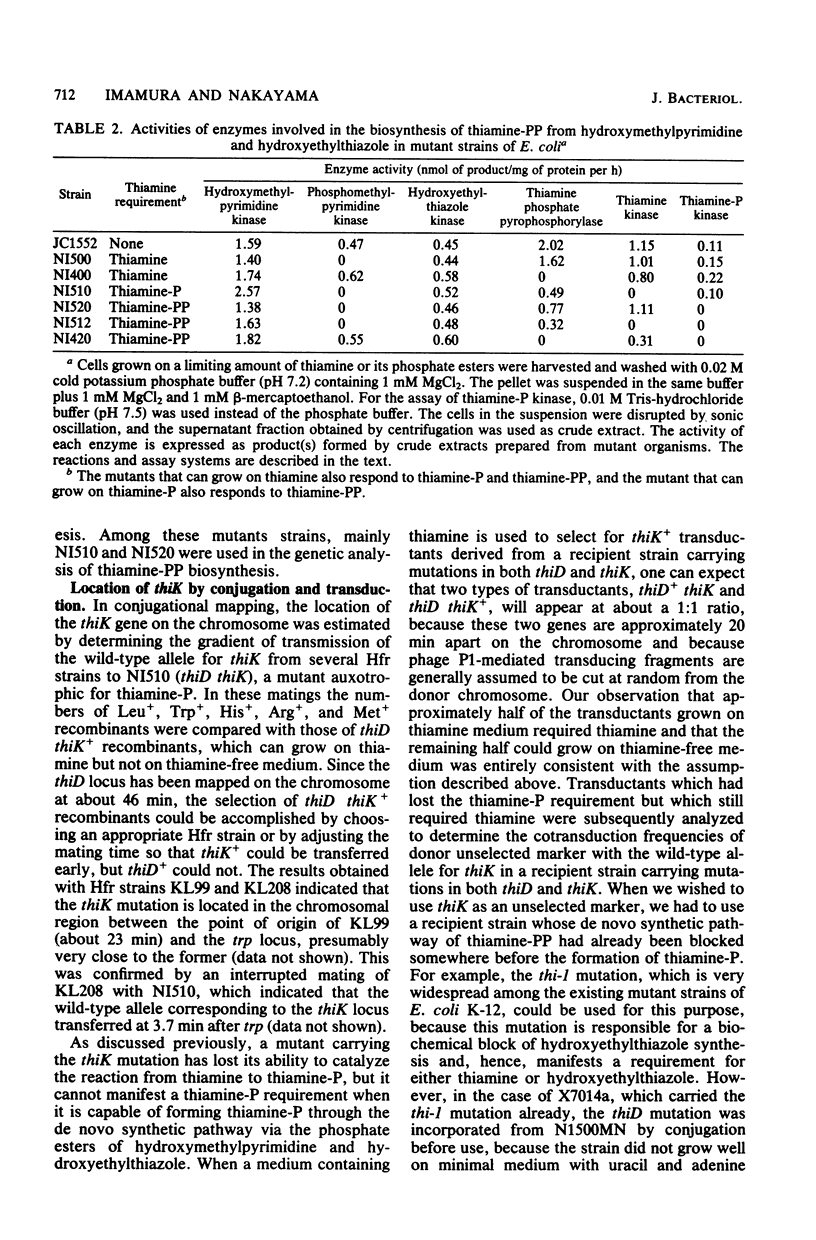
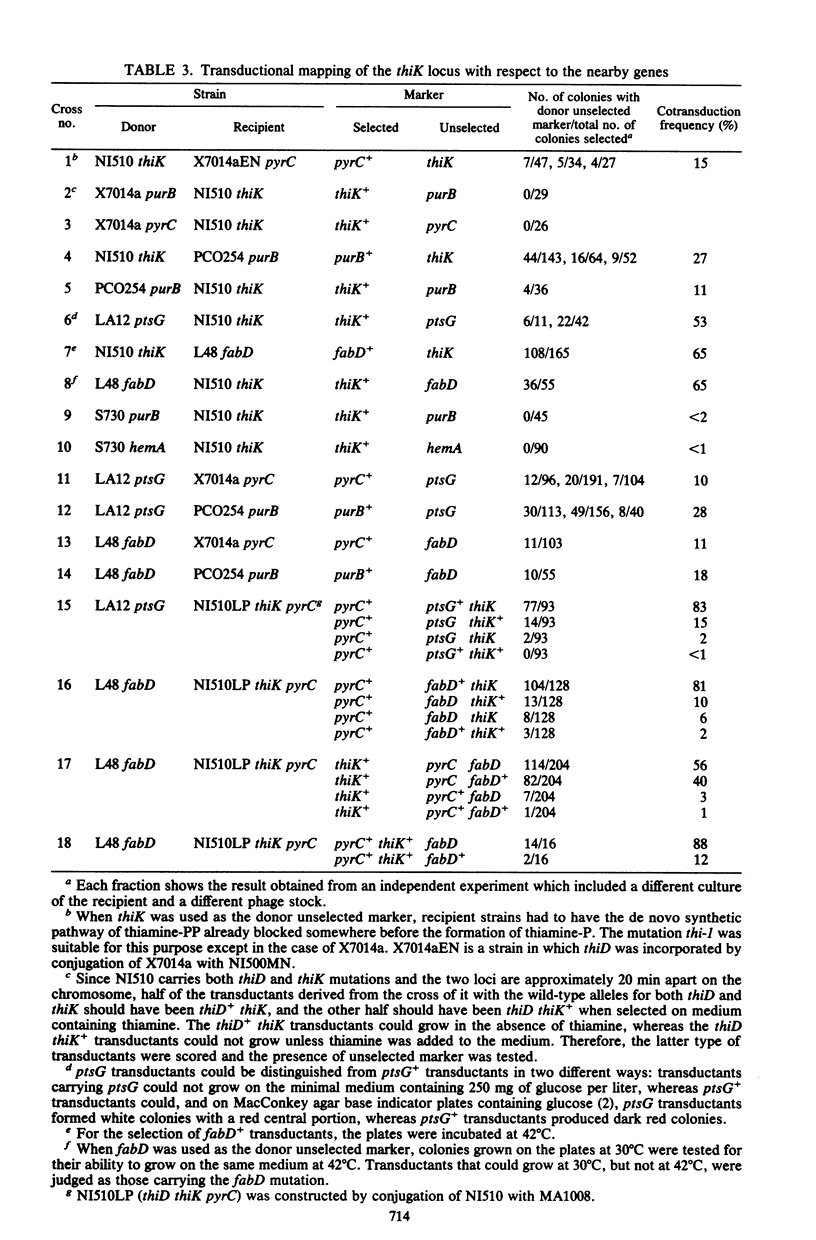
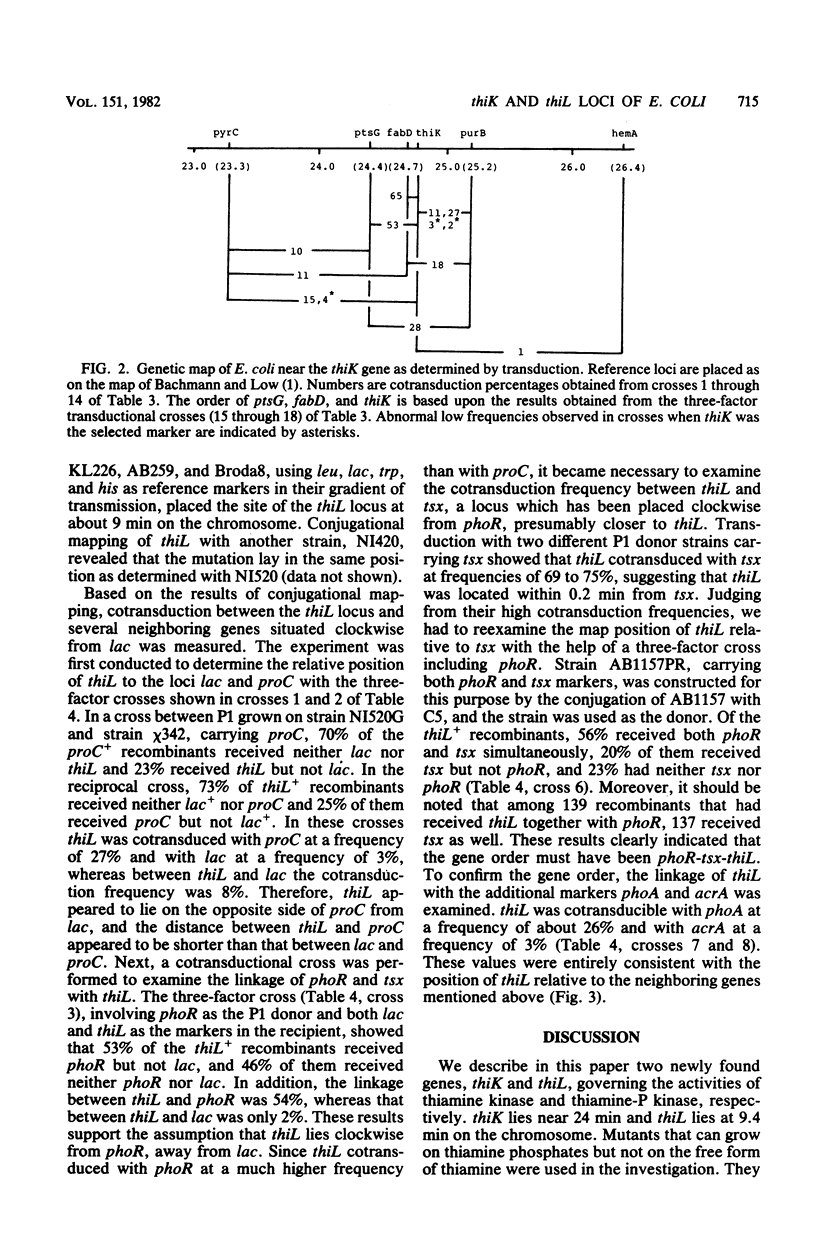
Abstract
Mutants of Escherichia coli K-12 auxotrophic for thiamine phosphates were produced in stepwise fashion from the polyauxotrophic F- strain JC1552, via intermediate production of thiamine auxotrophs that had lost the enzymatic activity of either phosphomethylpyrimidine kinase or thiamine phosphate pyrophosphorylase. They include two types: one responds to thiamine monophosphate or thiamine pyrophosphate, and the other responds to thiamine pyrophosphate only; the former lacks thiamine kinase activity, and the latter lacks thiamine monophosphate kinase activity, in addition to the enzymatic defects caused by the first mutations. We found two genes, for which we propose the designations thiK and thiL, which govern the activities of thiamine kinase and thiamine monophosphate kinase, respectively. By conjugation and P1 transduction, the thiK locus was mapped at about 25 min, between pyrC and purB and close to fabD. The relative order of thiK with respect to nearby genes was tentatively established as pyrC-ptsG-fabD-thiK-purB. In the case of thiL, the locus was situated at about 9 min, between tsx and acrA and probably 0.2 min clockwise from the former.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- Imamura N., Nakayama H. thiD locus of Escherichia coli. Experientia. 1981 Dec 15;37(12):1265–1266. doi: 10.1007/BF01948350. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Nakata T., Nose Y. Genetic mapping with a thiamine-requiring auxotroph of Escherichia coli K-12 defective in thiamine phosphate pyrophosphorylase. J Bacteriol. 1968 Apr;95(4):1483–1485. doi: 10.1128/jb.95.4.1483-1485.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Nose Y. Thiamine regulatory mutants in Escherichia coli. J Biochem. 1969 Mar;65(3):417–425. doi: 10.1093/oxfordjournals.jbchem.a129029. [DOI] [PubMed] [Google Scholar]
- Kawasi T., Iwashima A., Nose Y. Regulation of thiamine biosynthesis in Escherichia coli. J Biochem. 1969 Mar;65(3):407–416. doi: 10.1093/oxfordjournals.jbchem.a129028. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LEWIN L. M., BROWN G. M. The biosynthesis of thiamine. IV. Inhibition by vitamin B6 compounds. Arch Biochem Biophys. 1963 May;101:197–203. doi: 10.1016/s0003-9861(63)80002-8. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Tojo T., Greenberg J. Interaction of the expression of two membrane genes, acrA and plsA, in Escherichia coli K-12. J Bacteriol. 1975 Jun;122(3):874–879. doi: 10.1128/jb.122.3.874-879.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata A., Peterson G. R., Brooks E. L., Rothman F. G. Location and orientation of the phoA locus on the Escherichia coli K-12 linkage map. J Bacteriol. 1971 Sep;107(3):683–689. doi: 10.1128/jb.107.3.683-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Hayashi R. Biosynthesis of thiamine pyrophosphate in Escherichia coli. J Bacteriol. 1972 Feb;109(2):936–938. doi: 10.1128/jb.109.2.936-938.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Hayashi R. Biosynthetic pathway of thiamine pyrophosphate: a special reference to the thiamine monophosphate-requiring mutant and the thiamine pyrophosphate-requiring mutant of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1118–1126. doi: 10.1128/jb.112.3.1118-1126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Hayashi R. Inhibition of thiamine pyrophosphate utilization by thiamine or its monophosphate in Escherichia coli. J Bacteriol. 1974 Apr;118(1):32–40. doi: 10.1128/jb.118.1.32-40.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi Y. Genetic analysis of an Escherichia coli mutant with a lesion in stable RNA turnover. Genetics. 1974 Feb;76(2):185–194. doi: 10.1093/genetics/76.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple K. S., Silbert D. F. Mapping of the fabD locus for fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1975 Mar;121(3):1036–1046. doi: 10.1128/jb.121.3.1036-1046.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer E. R., Beckwith J. R., Brenner S. Mapping of suppressor loci in Escherichia coli. J Mol Biol. 1965 Nov;14(1):153–166. doi: 10.1016/s0022-2836(65)80237-6. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., de Haan P. G., Nijkamp H. J. Mapping of purine markers in Escherichia coli K 12. Genet Res. 1965 Nov;6(3):442–453. doi: 10.1017/s0016672300004328. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Favre A. Localisation génétique d'une mutation qui rend la croissance de E. coli K 12 insensible à 365 nm. C R Acad Sci Hebd Seances Acad Sci D. 1977 Jun 13;284(22):2285–2288. [PubMed] [Google Scholar]



