Abstract
Adenovirus (Ad)-based vectors are useful gene delivery vehicles for a variety of applications. Despite their attractive properties, many in vivo applications require modulation of the viral tropism. Targeting approaches applied to adenoviral vectors included genetic modification of the viral capsid, controlled expression of the transgene and combinatorial approaches that combine two or more targeting elements in single vectors. Most of these studies confirmed successful retargeting in cell cultures, however, in vivo gains of targeted adenoviral vectors have not been widely demonstrated. We have developed a combinatorial retargeting approach utilizing metabolically biotinylated Ad, where the biotin acceptor peptide was incorporated in one of the fibers in a dual fiber viral particle resulting in metabolically biotinylated fiber-mosaic Ad (mBfMAd). We have utilized this vector in complex with epidermal growth factor (EGF)-Streptavidin to retarget fiber-mosaic virus to EGF receptor (EGFR) expressing cells in vitro and confirmed an increased infectivity of the retargeting complex. Most importantly, the utility of this strategy was demonstrated in vivo in two distinct animal models. In both models tested, retargeted mBfMAd demonstrated an increased ratio of gene expression in target tissues compared to the liver expression profile. Thus, metabolically biotinylated fiber-mosaic virus in combination with appropriate adapters can be successfully exploited for adenoviral retargeting strategies.
Keywords: targeted delivery, EGFR, adenoviral vector, fiber-mosaic, metabolic biotinylation
Introduction
Adenovirus (Ad)-based vectors remain one of the most useful gene delivery vehicles for a variety of clinical contexts and a preferable vector for cancer gene therapy. However, several limitations preclude safe and efficient Ad-based gene transfer, specifically for in vivo settings. One limitation is the broad tropism of Ad, due to the ubiquitous expression pattern of the primary cell receptor CAR and secondary integrin receptors, which leads to undesired virus uptake and gene expression in non-target tissues. Furthermore, low and inconsistent expression of CAR on tumor cells raises concerns about the efficacy of Ad-mediated gene therapy in cancer applications. Thus, for efficient and versatile use of Ad as an in vivo gene therapy vector, modulation of the viral tropism is highly desirable.
Various targeting approaches have been designed to change viral tropism. One approach includes genetic modification of the viral capsid proteins via addition of foreign targeting ligands such as short peptides or polypeptide binding domains or the substitution of the fiber with other types of Ad fiber. In adapter-mediated approaches, the tropism of the virus is modified by a targeting moiety utilizing a ligand, which associates with the Ad virion. Adapter molecules successfully used for Ad targeting include bispecific antibody (Ab) conjugates,1 genetic fusions of single-chain Ab (scFv) with soluble CAR,2 or scFv–scFv diabodies.3
The combined use of two or more targeting components in single vector has also been reported to significantly enhance the utility of individual targeting approaches. The immunoglobulin (Ig)-binding domain of the Staphylococcus aureus protein A has been genetically-incorporated into Ad fiber, allowing antigen-specific Ig to serve as bifunctional adapter molecules. Another complex targeting system designed by Barry, Campos and co-workers utilizes the high affinity biotin-avidin interaction.4,5 This system exploits the incorporation of a biotin acceptor peptide (BAP) into the structural proteins of Ad, which allowed metabolic biotinylation of these vectors during propagation in 293 cells. All of these studies have demonstrated successful retargeting of Ads in vitro through alternative receptors. However, several limitations could be envisioned for the translation of these targeting approaches for in vivo applications. For instance, viruses with incorporated IgG-binding domains may face a competition with IgGs abundantly present in serum and other biological fluids. On the other hand, drastic modifications of adenoviral structural proteins usually hamper infection and subsequent steps in the viral life cycle, limiting the ability to scale up viral preparations. This could explain why no targeting gains have been reported to date using this strategy in vivo.
In this study, we developed a variation of the combined retargeting approach utilizing metabolically biotinylated fibers in the context of a fiber-mosaic viral capsid. The fiber mosaic construct allows expression of two fibers: fiber-fibritin (FF) and the wild-type (wt) fiber, both of which are incorporated into viral particles (vp). We have previously demonstrated that inclusion of a second fiber with distinct binding properties can provide virus binding and infection.6 In contrast to studies by Barry et al., the BAP was incorporated into one of the fibers resulting in metabolically biotinylated fiber-mosaic Ad (mBfMAd). The wt fiber facilitates the viral life cycle and allows the fiber-mosaic virus to be propagated to levels near that of the wt Ad. In our study, we have exploited biotinylated FF in complex with adapter epidermal growth factor (EGF)-Streptavidin to retarget the fiber-mosaic virus to EGF receptor (EGFR) expressing cells both in vitro and in vivo.
Results
Design and characterization of mBfMAd
The genome design of the mBfMAd Ad5FF.PSTCD.F5luc (mBfMAd) was similar to that previously described for AdFF6H.F5luc.6 A minimal domain of the 1.3S subunit of Propionibacterium shermanii transcarboxylase (PSTCD) was added to the C-terminus of chimeric protein FF. This domain is naturally biotinylated at lysine 89, when expressed in Escherichia coli (E. coli) and Saccharomyces cerevisiae by each organism’s cellular biotin ligase enzyme7 and is also metabolically biotinylated in mammalian cells.8 Thus, the mBfMAd genome encodes two fibers in the L5 region: a chimeric FF containing a C-terminal 6His tag and the PSTCD domain (FF.PSTCD.6H) and the Ad5 wt fiber (Figure 1a). Both fibers contain the tail portion of Ad5 fiber, which anchors them to the penton of the virion and also allows both fibers to be detected with anti-fiber tail AB. The coding sequences of both fibers were spanned by untranslated 5′ and 3′ sequences of the wt fiber thereby providing equal transcription conditions (splicing, polyadenylation as well as regulation by the Major Late Promoter) for both fibers. The fiber-mosaic vector carries the firefly luciferase gene under the control of the cytomegalovirus (CMV) promoter in the E1 region of the Ad5 genome (Figure 1a). The mBfMAd was rescued in 293 cells expressing the complementary E1 region for Ad5 growth. The titer of fiber-mosaic virus used for this study was 4.83 × 1012 vp/ml. 293 cell culture infected by these viruses at the same multiplicity of infection (MOI) exhibited similar rates of CPE development, indicating a comparable time course of infection. Furthermore, the total yield of mBfMad and physical titers calculated in vp were comparable to the preps of wt virus, indicating that viral yield was not affected. Several lots of the virus have been obtained in the standard lab preparations with the titers ranged from 1.5 × 1012 to 1.3 × 1013 vp/ml. Thus, the yield of mBfMAd was similar to the yield routinely observed for the recombinant Ad5 preparations with unmodified capsid, suggesting that the additional fiber did not hamper virus propagation and packaging and allowed efficient production of the fiber-mosaic virus.
Figure 1.

Design and characterization of mBfMAd (a) Diagram of the fiber-mosaic adenovirus Ad.FF.PSTCD.6H.F5luc (mBfMAd) genome. Modification of L5 region of Ad genome includes tandem of fiber genes: FF with BAP PSTCD and 6His tag and wt Ad5 fiber. (b) Fiber content in fiber-mosaic AdFF6H.F5luc virions. Equal amounts of Ad5luc AdFF6 H.F5luc and Ad.FF.PSTCD.6H.F5luc (5 × 109 vp) in SDS sample buffer were loaded on gradient (4–20%) SDS gel either boiled or unboiled. Electrophoretically separated viral proteins were transferred to PVDF membrane and developed with monoclonal antibodies specific to fiber tail (4D2), fiber trimer (2A6), 6His-Tag and Streptavidin-HRP. (c, d) Binding characteristics of mBfMAd. Binding of mBfMAd to streptavidin (c) and recombinant CAR (d) was tested in ELISA. The number of virus particles used for binding experiments was within the range of 1 × 107–2 × 1010, which corresponded to 0.01–5 μg of viral protein per well. Control lane shown represents nonspecific binding of mBfMAd to the plastic surface in the absence of CAR.
Fiber content in fiber-mosaic virions
To examine fiber incorporation into mosaic virions, we performed a series of Western blots where an equal number of vp (2 × 109) of Ad5luc, Ad5.FF6H. F5luc and Ad5FF.PSTCD.F5luc were loaded on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Figure 1b). Incorporation of FF.PSTCD.6H into vp was confirmed by the interaction with antibodies specific to fiber tail (4D2), trimeric fiber (2A6) and 6His. Western blots with the 4D2 and 2A6 antibodies revealed fiber monomers and timers of the correct size. The anti-His Ab only reacted with chimeric fibers from mosaic virions: FF.6H and FF.PSTCD.6H. Attachment of biotin on FF.PSTCD.6H fiber was confirmed by interaction with streptavidin both in Western blot (Figure 1b) and enzyme-linked immunosorbent assays (ELISA), where mBfMAd was captured either by streptavidin or EGF-Streptavidin coated on plates (Figure 1c). In addition, the mBfMAd retained CAR binding through wt fiber as demonstrated in Figure 1d. Thus, the presence of the FF.PSTCD.6H in the capsid of the mosaic virus was detected by antibodies directed to all of the functional domains of the protein – fiber tail, fiber trimer and 6His, thereby demonstrating that the additional fiber was incorporated into the virion and correctly displays all of its functional motifs. The presence of the biotin on this fiber has also been confirmed, thus, enabling utilization of our vector in combination with bioltinylated adapters for Ad retargeting strategies.
The fiber content in the mosaic virions was semi-quantitatively estimated by the protein band intensity after staining with 4D2 AB employing Image Tool software. This analysis showed that the average ratio of FF.PSTCD.6H to wt fiber in mBFmAd was similar to the average ratio of FF.6H to wt fiber in AdFF.6H and has been estimated as 1:5 (ranging from 1:3 to 1:6, where higher ratios were obtained analyzing fiber monomers and lower ratios were obtained based on fiber trimers). Therefore, it could be expected that fiber-mosaic has 1–3 vertices of the viral capsid that contained biotinylated fiber, which can be utilized in retargeting strategy.
Targeting metabolically biotinylated fiber-mosaic vectors to EGFR using the bi-functional adapter EGF-Streptavidin in vitro
Theoretically, any molecule equipped with bispecific binding affinities, one to biotin and another to a target cell-specific receptor, can function as an adapter to redirect biotinylated virus to specific cell types. The bispecific adapter contained human EGF fused to the core-streptavidin9 has been previously expressed in the bacterial system and its design and production was described in detail by Ponnazhagan et al.10 This adapter has been thoroughly characterized and used to retarget AAV to EGFR expressing cell lines. Thus, the availability of this adapter enabled us to test the utility of our vector in an EGFR retargeting strategy. We first wanted to determine the infectivity of our vector when applied with the EGF-Streptavidin adapter in vitro.
To analyze EGFR targeting in vitro, we evaluated adapter-mediated infection by mBfMAd of several cell lines with different EGFR expression status. Cancer cell lines with low (MDA-MB-453) and high EGFR expression (A431, SKOV3ip1, A549) were transduced with Ad5luc or mBfMAd at 100 vp/cell. Viruses were pre-incubated with EGF-Streptavidin at concentration of 10 μg/ml and purified from excess of adapter using Microcon filter tubes. The results are shown in Figure 2. On the cell line with low EGFR expression (MDA-MB-453), both viruses showed comparable infectivity as measured by luciferase transgene activity. However, EGF-Streptavidin targeted transduction of mBfMAd resulted in significantly enhanced luciferase activity on EGFR-positive cells compared to that obtained with Ad5luc. The increase in transduction varied from more than 10-fold in A431 to more than 30-fold in SKOV3ip1 cells (Figure 2a). Additional evidence of efficient in vitro retargeting with the bispecific adapter was also obtained using stable cell lines that express the extracelular domain of human EGFR.11 Murine fibroblasts NR6 (EGFR-deficient) or NR6wt (EGFR-expressing) were preincubated with either phosphate-buffered saline (PBS) (no adapter) or with increasing concentration of EGF-Streptavidin 1–25 μg/ml, washed, and then transduced with Ad5luc or mBfMAd. Addition of the adapter enhanced the transduction of EGFR-positive cells in a dose-dependent manner, but had no effect on cells lacking EGFR receptor expression. The highest concentration of adapter used for retargeting mBfMAd to EGFR-positive NR6wt cells resulted in an eightfold increase in transgene expression, whereas it only increased transduction of wt Ad by 3.2-folds at the same experimental conditions (Figure 2b). Thus, mBfMAd can achieve enhanced transduction via bispecific adapter in cell lines expressing target receptor.
Figure 2.
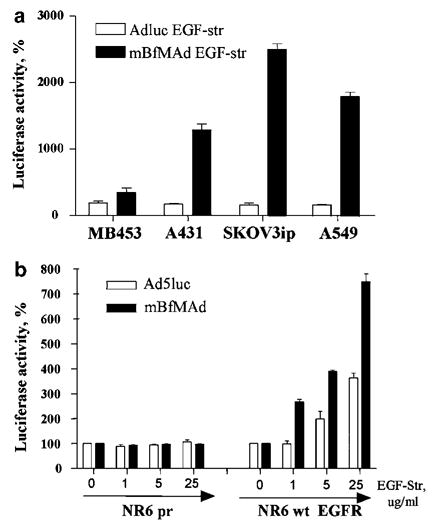
Increased gene transfer to EGFR-expressing cell lines. (a) Cancer cell lines with low (MDA-MB-453) and high (A431, SKOV3ip1, A549) EGFR expression were transduced with Ad5luc or mBfMAd at 100 vp/cell. Viruses were preincubated with EGF-Streptavidin at concentration of 10 μg/ml, purified from excess of adapter using centricon tubes and applied on cells. Results are presented as % of increase of luciferase expression with adapter. Mean luciferase expression of the same virus without adapter was designated as 100%. (b) Murine fibroblasts NR6 or NR6wt EGFR, expressing extracellular domain of human EGFR, were preincubated with either PBS (no adapter) or with increasing concentration of EGF-Streptavidin 1–25 μg/ml. Cells has been were transduced with Ad5luc or mBfMAd at 50 PFU/cell. Results are presented as increase of luciferase expression with adapter. Mean luciferase expression of the same virus without adapter was designated as 100%.
Targeting metabolically biotinylated fiber-mosaic vectors to EGFR in vitro after blocking fiber-CAR interaction
Our EGFR retargeting strategy relies upon the incorporation of modified FF, which is one of the two fibers present in the capsid of the fiber-mosaic virus. Retaining the wt fiber gene in the viral genome (i.e. the wt fiber protein in the vp) gives the potential advantage of facilitation of viral life cycle and virus propagation. A possible disadvantage for retargeting is the presence of the wt fiber, which can direct the infection of fiber-mosaic via the CAR-mediated pathway on CAR-positive cell lines. To confirm that biotinylated fibers, which represent only a fraction in the context of viral capsid, are able to mediate virus infectivity and to confirm the dependence of transgene expression on EGF–EGFR interaction, we performed retargeting gene transfer experiments in the presence of the Ad5knob. Virus infectivity in conditions when the binding ability of the wt fiber is blocked would indicate the relative contribution of the second fiber to the overall virus infectivity.
The low EGFR-expressing cell line, MDA-MB-453, and cell lines overexpressing EGFR (A431, A549, SKOV3) were transduced with Ad5luc or mBfMAd at 50 PFU/cell preincubated either with PBS (no adapter) or with EGF-Streptavidin at concentration of 10 μg/ml. To block the infection via wt fiber, cells were preincubated with 50 μg/ml of recombinant Ad5 knob protein. We have previously shown that this concentration of Ad5knob blocked infectivity of wt virus more than 95% on high CAR-expressing cell lines. The results are shown in Figure 3. In line with previous experiments, an addition of the EGF-Streptavidin retargeting adapter augmented infectivity of the fiber-mosaic virus only, whereas it did not influence the infectivity of Ad5luc in any cell line tested. In general, mBfMAd demonstrated similar patterns of EGFR-dependent transduction as in the previous in vitro experiment with significant gene transfer increase in EGFR-overexpressing cells (A431, SKOV3) and no effect in cells with low EGFR expression (MDA-MB-453). In the presence of Ad5 knob, Ad5luc had predicted pattern of infection block for all cell lines tested. The results of CAR blocking was particularly clear in the highly CAR-positive cell line A549, where gene transfer was blocked by 99% in the presence of Ad5knob. Wt Ad infectivity remained at 20–50% of normal levels in other cell lines that express lower levels of CAR, presumably via infection through cellular integrins (A451, SKOV3). Ad5 knob-mediated blocking of mBfMAd infection in the absence of adapter did not reduce infectivity as significantly as that for the Ad5luc, and was either similar to the infectivity levels without blocking or only decreased to 37–45% of the initial infectivity levels. Most importantly, the fiber-mosaic virus demonstrated efficient adapter-based retargeting in EGFR-positive cells in Ad5 knob blocking conditions. Infectivity was increased by approximately 300% in A431 cells and 200% in SKOV3 cells in blocking conditions, where wt fiber presumably did not participate in infection. The level of EGFR-based infectivity was higher on cell lines with high EGFR expression and moderate CAR expression, indicating that in the situation where CAR-mediated infection is negligible, transduction via a second fiber dominates and is sufficient to mediate infection. Thus, biotinylated fiber can mediate adapter-driven transduction in the context of mosaic virions.
Figure 3.
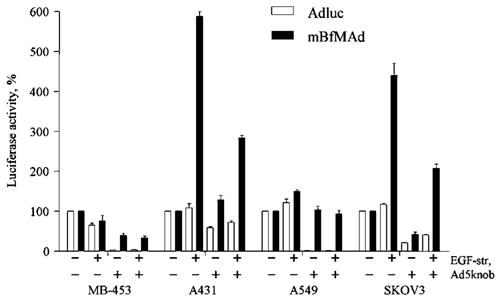
Infectivity of mBfMAd Ad after blocking fiber-CAR interaction. Cell lines with low EGFR expression (MDA-MB-453) and cell lines overexpressing EGFR (A431, A549, SKOV3) were transduced with Ad5luc or mBfMAd at 50 PFU/cell preincubated either with PBS (no adapter) or with EGF-Streptavidin at concentration of 10 μg/ml. To block infection via wt fiber cells were preincubated with Ad5 knob recombinant protein at 50 μg/ml. Twenty-four hours later the cells were processed for luciferase assay. Results are presented as % of luciferase expression compared to infection of this virus without adapter or knob blocking. Data presented are mean average of triplicates plus sample standard deviation.
Analysis of EGFR retargeting with mBfMAd in vivo using a murine model of ovarian carcinoma
Next, we wanted to test whether our retargeting strategy gives any benefits when applied in vivo. The SKOV3ip1 cell line has been previously shown to have high expression of EGFR.12 This cell line also demonstrated high levels of EGFR-mediated retargeting in our in vitro experiments. Thus, we utilized an intraperitoneal (i.p.) xenograft model of human ovarian cancer based on i.p. injection of SKOV3ip1 cells into CB17 severely combined-immunodeficient (SCID) mice for analysis of in vivo targeting by mBFmAd. After tumors have been developed, the animals were injected i.p. with Ad5luc or mBfMAd viruses with or without preincubation with the EGF-Streptavidin adapter. Expression of luciferase was measured 48 h later in tumor and liver tissue lysates. As shown in Figure 4a, EGFR-mediated infection with mBFmAd resulted in a statistically significant increase in luciferase expression in tumors. Addition of the adapter augmented mBfMAd-mediated gene transfer in EGFR-expressing tumors by over sevenfold, with average of relative light units (RLU)/mg protein values of 1668 000±530 100 and 223 900±38 910 for groups with and without adapter, respectively. Liver gene transfer was not influenced by addition of the adapter in animals receiving mBfMAd with or without the adapter. Ad5luc showed higher levels of the gene transfer both in tumors and in livers (Figure 4b) compared to mBfMAd. However, the pattern of retargeting with adapter for Ad5luc was different from mBfMAd. Preincubation of Ad5luc with EGF-Streptavidin resulted in only a slight increase of gene transfer noted for both tumors and livers in all groups, but this enhancement was not statistically significant. Despite of potential of adenoviral vectors for cancer gene therapy, a major impediment of Ad-based delivery is liver tropism of the vector. Vector modifications that could minimize liver uptake and maximize tumor transduction would enhance vector applicability. In this context, we were also interested how our retargeting efforts were reflected in changing the tumor-to-liver gene transfer ratio. The tumor-to-liver ratio was calculated for each individual animal and presented as individual dots on a graph in Figure 4c. The mean values of these ratios for the two groups receiving Ad5luc with or without adapter were similar and corresponded to 9.4 and 8.9. The mean ratio calculated for the group receiving mBfMAd increased from 39 for the group receiving virus without adapter to 69 for the group receiving EGFR-retargeted virus. Large variations between the values calculated for individual animals within each group were noted, and rendered the differences between these two groups statistically insignificant. However, the EGF-mediated targeting capacities of mBfMAd and Ad5luc as measured by the tumor-to-liver luciferase activity ratio, were statistically significant (P = 0.01). Thus, these data demonstrate the gains of adapter-based retargeting to EGFR-expressing tumor xenografts in the model of localized tumor.
Figure 4.
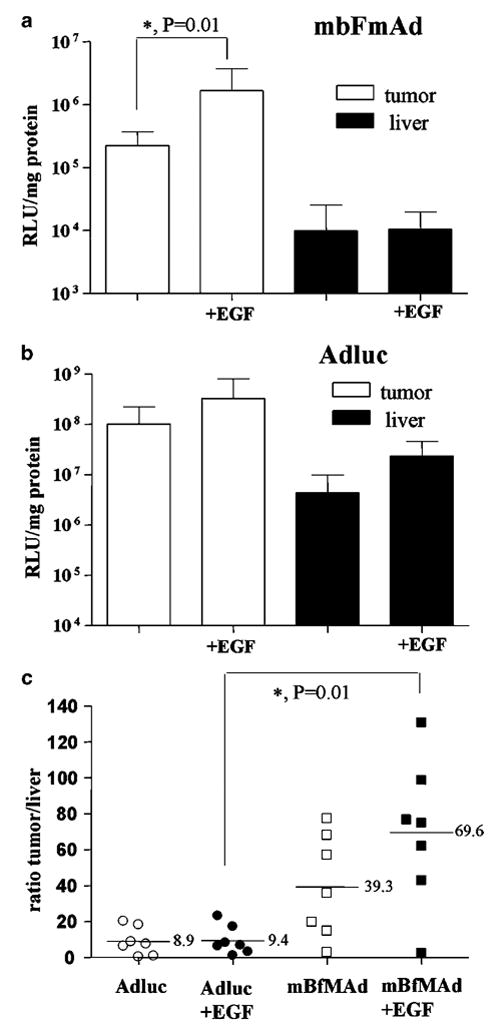
In vivo retargeting of mBfMAd to EGFR using animal model of ovarian carcinoma. CB17 SCID mice were injected i.p. with 10 × 106 SKOV3ip1 cells to establish i.p. ovarian tumors. At day 21 after SKOV3ip injection, four groups of mice (n = 7) were injected with Ad5luc or mBfMAd viruses either without adapter or preincubated with EGF-streptavidin. Viruses were injected i.p. at 1 × 1010 vp/animal. Mice were killed 48 h later, tumors and livers were excised and luciferase assay was performed in tissue lysates. Results are presented as luciferase expression (RLU) normalized for protein content (mg protein) for mBfMAd (a) and Ad5luc (b), and as tumor-to-liver ratio of luciferase expression (c). Tumor-to-liver ratio was calculated for individual animals, each represented as one dot on the graph. Bars represent averages of values in each group (n = 7), P = 0.01.
In vivo retargeting of mBfMAd to EGFR-expressing lung endothelium in EGFR transient transgenic human CAR (hCAR) mice
We also explored the feasibility of targeting mBFmAd to hEGFR expressed in the pulmonary vasculature of mice. For these experiments we have utilized the model recently developed in our group that is based on transient induction of the target molecule in the pulmonary endothelium. This is accomplished using a combination of hCAR transgenic mice and Ad-based vectors that allowed endothelial cell-specific antigen expression via placing the transgene under control of the endothelial specific flt-1 promoter.
The hCAR transgenic mice express a truncated hCAR under control of the ubiquitin promoter, resulting in hCAR protein expression in all organs including the lungs and therefore sensitizing the animal to adenoviral infection.13 The major benefit of this model for testing vector targeting gains is the accessibility of the antigen to systemically introduced targeted vectors. To transiently induce human EGFR in the pulmonary endothelium of hCAR transgenic mice, we used the AdfltEGFR adenovirus. We have previously confirmed the functionality of this virus in vitro. Further, we confirmed the expression of hEGFR in the pulmonary vasculature of hCAR mice upon tail vein injection of AdflthEGFR (1 × 1011 vp) by staining for EGFR in lung sections (data not shown). To validate EGFR targeting in vivo, hCAR mice preconditioned with AdflthEGFR injection were reinjected 48 h later with 5.0 × 1010 vp of mBfMAd preincubated with either PBS or EGF-Streptavidin. The animals were killed 48 h subsequent to the second injection, and luciferase activities in the lungs and livers were quantified. As shown in Figure 5a, EGF-mediated luciferase activity of mBfMAd in the lungs of pre-treated animals was increased by almost fivefold compared to that in animals receiving mBfMAd without the adapter (341 587 vs 70 625 mean RLU/mg protein, respectively). This indicates that mBfBAd complexed with molecular adapter was able to efficiently target cells expressing antigen in the lung of mice following intravenous administration. Furthermore, liver transduction by the targeted virus did not increase. This outcome provided a significantly higher lung-to-liver ratio for the EGFR-retargeted group vs the control virus infected group (6.5 vs 1.3 mean ratios, respectively; P = 0.01) (Figure 5b). Therefore, the accessibility of EGFR expressed in the lung vasculature enables to augment EGFR-based transduction of targeted cells by systemically introduced mBfBAd. In aggregate, these results demonstrate the targeting capacities of our strategy in vivo.
Figure 5.
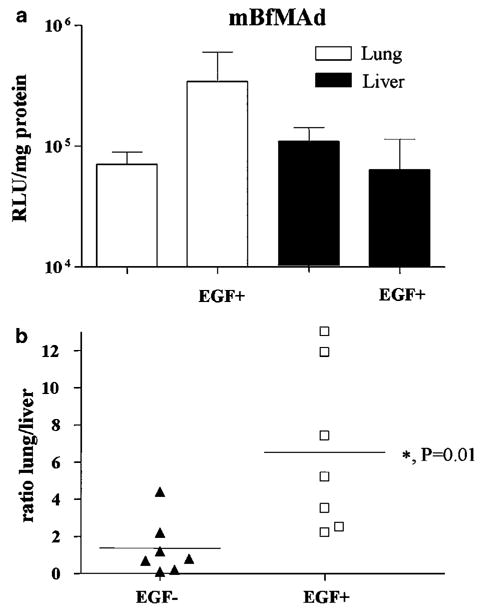
In vivo retargeting of mBfMAd to EGFR-expressing lung endothelium in EGFR transient transgenic hCAR mice. hCAR transgenic mice were injected with AdfltEGFR at 1011 vp/mice via tail vein for expression of EGFR in lung endothelium followed by a second injection of mBfMAd with or without adapter at 5 × 1010 vp/animal via tail vein 48 h later. Forty-eight hours after mBfMAd all animals were killed. (a) Lung and liver luciferase activities 48 h after intravenous administration of mBfMAd with or without the EGF-Streptavidin adapter. Results are presented as lung and liver luciferase expression (RLU) normalized for protein content (mg protein). (b) Lung-to-liver ratios of luciferase activities in individual animals (n = 7). Bars represent averages of values in each group (P = 0.01).
Discussion
In this study, we evaluated a targeting approach, utilizing a genetically modified Ad fiber and adapter-mediated retargeting, both combined in the context of a fiber-mosaic viral capsid. We applied this approach for EGFR targeting, to demonstrate the functionality of our strategy in vitro and to establish the potential of this system for in vivo targeting applications.
Bifunctional adapter molecules have been engineered with specificity for both the Ad capsid protein (mainly Ad fiber) and to alternative cellular receptors distinct from CAR. The main advantage of this system is in its flexibility for using high affinity binders, regardless of their nature and size. However, the necessity for producing complex adapter molecules, such as whole antibodies, Ab fragments or recombinant fusion proteins, has always been regarded as an inherent drawback of this approach. Nevertheless, efficient adenovirus targeting in vivo has only been reported using adapter-based approaches.14–16
Genetically modified vectors have a theoretical advantage in that they do not require additional soluble components for infectivity. A number of viruses with fibers constructed in this manner have been reported ranging from minor modifications, including single amino-acid substitutions17 and addition of small peptides18,19 to more substantial alterations, such as switching entire protein functional domains (knob, shaft)20–22 and generation of artificial fiber-like scaffolds that retain the essential functional properties of the whole Ad fiber.23,24 These vectors generally fulfill the basic targeting requirements on cells in vitro, but the expected targeting efficacy has not yet been translated to in vivo experiments. In fact, the genetically modified single component vectors most often require careful optimization of vector design by trial and error. In view of these obstacles, we hypothesized that the fiber mosaic platform, where two fibers are included in the viral genome, presents a more flexible mode for Ad vector modification and may simplify optimization of targeting efforts.
Here, we engineered a novel fiber-mosaic Ad vector, which comprises two fiber types: the wt fiber and FF chimera fused to the BAP. This strategy has previously been used to metabolically conjugate biotin to viral proteins in mammalian cells, which allows them to be coupled to retargeting adapter molecules that contain streptavidin.4,5,25 However, the utility of biotin incorporation for virus retargeting has thus far only been demonstrated in vitro and, among all of the structural proteins tested, only fiber-incorporated biotin was capable of efficient retargeting.5 These studies established that biotin attachment is determined by the locale for BAP incorporation and provided the rationale for our fiber-based retargeting strategy.
Although our fiber-mosaic vector does provide an alternative tropism, the native tropism is not completely ablated due to the retention of the wt fiber in the viral capsid. The targeting function is delegated to a recombinant fiber, while keeping the structural and functional integrity of the wt fiber during all stages of the viral life cycle. The presence of wt fiber served to amplify the fiber-mosaic Ad to titers as high as 1012–1013 vp/ml in regular laboratory-scale preparations. The presence of wt fiber may undermine the efficiency of retargeting, as just a fraction of fibers present on the viral capsid will serve for retargeting purposes. However, efficient redirection of our vector towards cells expressing EGFR was validated in vitro. The adapter-driven mBfMAd increased transduction of EGFR-positive cell lines 10- to 23-fold, compared to transduction without adapter. This level of enhancement is comparable with previously reported values obtained utilizing a complex of the wt virus with different bispecific molecular adapters.12,26 Similarly, adenovirus retargeted through an ‘adenobody’ strategy demonstrated a 10-fold enhancement of infectivity on A431 cell line.27 Efficient utilization of the retargeting fiber was demonstrated using blocking experiments. The mBfMAd vector complexed with EGF-Streptavidin maintained a high level of gene transfer in the presence of Ad5 fiber knob concentrations that blocked infectivity of the Ad5luc control virus in the presence or absence of the adapter. These data additionally support the ability of mBfMAd to redirect infection through the EGFR pathway. Thus, we believe that the proposed mosaic virus can efficiently utilize one of the mosaic fibers to redirect virus to alternative receptor in vitro.
Retargeting of different viruses through cell growth factor receptors, such as EGFR, which is highly expressed on tumors of different origin, has been validated in several adapter-based studies.12,26–29 All reported adapter constructs were effective at coupling Ad to EGFR and resulted in increased gene transfer to EGFR expressing cell lines. This proved that EGFR pathway is compatible with the adenoviral infection cycle. EGF exhibits high affinity binding to EGFR, which leads to rapid internalization via the receptor-mediated endocytic pathway but no recycling of the receptor-ligand complex.30 Thus, the EGFR pathway is one of the best studied and proven pathways for adenoviral retargeting in vitro. However, in vivo studies involving such experiments are scarce and primarily utilize intratumoral administration of retargeted viruses.28,31 There is often a disconnect between the virus targeting efficacy in vitro and that in vivo, whereby the retargeting results obtained in cell culture does not translated into in vivo gains. Thus, we next wanted to test if our retargeting strategy using fiber-mosaic virus can be effective in vivo and retarget mBFmAd to EGFR expressing cells.
Initial in vivo studies for retargeting adenoviral vectors were carried out using adapter-mediated approaches in i.p. models of human cancer in mice.32,33 This approach allows evaluation of targeting without the major hurdle of systemic virus administration, sequestration of the injected virus by the liver. The SKOV3ip ovarian cancer cell line expresses a very high level of EGFR and thus presents a good model to test EGFR targeting. Depending on the experimental conditions, mBfmAd demonstrated a 5-to 20–fold increase in gene transfer on these cells in vitro. Thus, it seemed logical to test whether the targeting gains would be paralleled in vivo. When injected in mice with pre-established i.p. SKOV3ip xenografts, EGFR-retargeted mBfmAd increased tumor luciferase expression sevenfold, whereas gene expression in the liver was not affected. Gene transfer efficiency with the Ad5luc vector was also slightly enhanced by the presence of the adapter. We have noted the similar effect of adapter on Ad5luc infectivity in vitro, but the adapter-based gene transfer enhancement of Ad5luc was lower than that of the mBFmAd. Moreover, the addition of the adapter to Ad5luc increased gene transfer in both tumor and liver to the same extent, which finally resulted in similar tumor-to-liver ratios for Ad5luc with or without adapter (9.4 vs 8.9), whereas the mean tumor-to-liver ratio calculated for the group receiving mBfMAd increased from 39 to 69 for virus without adapter and EGFR-retargeted virus, respectively. Of note, previous publications on adapter-mediated Ad retargeting, under similar experimental conditions, report only qualitative data on targeting gains32,34 or targeting gains of at most two- to fivefold. Thus, the retargeting strategy applied to mBFmAd allowed to achieve comparable increase in tumor gene transfer in the context of ovarian cancer xenografts.
Another approach to test retargeting properties of adenoviral vectors in an efficient manner was recently developed in our group.2 As systemically introduced vectors retargeted to tumors are to overcome multiple physiological barriers before reaching their targets, it has been proposed that the display of targeting molecules at accessible sites would facilitate testing of targeting gains of systemically administered adenoviral vectors. An hCAR-transgenic mouse model that is sensitized to Ad infection and is used to transiently express tumor antigens in the lung vasculature was recently reported to be efficient for evaluating vectors targeted to CD40 and carcinoembryonic antigen (CEA).2,35 Thus, the hCAR mice were used to confirm whether our targeting strategy using the combination of two targeting modes in a single vector could efficiently target hEGFR expressed in the lungs. Expression of hEGFR was transiently induced in the mice pulmonary endothelium by systemic injection of recombinant adenovirus AdfltEGFR. In this model, mBFmAd retargeted to EGFR expressed in mouse lungs showed a fivefold enhancement in the lung gene transfer, which resulted in increased the lung-to-liver retargeting ratio from 1.3 for the virus without adapter to 6.3 for the retargeted virus. The lung-to-liver ratios calculated in our experiment for the retargeted fiber-mosaic vector correlated well with the values obtained in a previous study utilizing this model for CD40 retargeting (lung-to-liver ratio of 5.2 for CD40-retargeted virus and 1.2 for irrelevant virus),35 thus providing a good estimate of the level of transductional retargeting gains achievable in this in vivo model. Overall, this transient transgenic model system allowed our targeting strategy to be evaluated.
Despite the fact that statistically significant differences in gene transfer was obtained by targeted vs untargeted virus in both models tested, a considerable variation of gene transfer values were obtained for individual animals, particularly for the calculated tumor-to-liver or lung-to-liver ratios. Specifically, this was observed in the groups receiving mBFmAd with adapter. These variations could be associated with the individual in vivo conditions, experimental errors or factors related to the retargeting system itself in its current design, such as uniformity of virus prep and virus-adapter formulation.
All experimental work included in this study was carried out using single mBFmAd and Ad5luc preps, which were characterized for vp and PFU content, as well as the degree of fiber incorporation and biotinylation. However, we would like to stress that the uniformity of viral preparation in terms of the fiber content of each individual vp remains unknown. It is likely that the preparation of fiber-mosaic virus could contain (i) truly fiber-mosaic particles having both fibers in one viral capsid, (ii) a mixture of viral capsids displaying just one of the fibers and (iii) a combination of both variants. This factor may affect the overall performance of the fiber-mosaic virus. Of note, native fiber-mosaic viruses of serotype 40 and 41 have equal presentation of both fibers and apparently display both fibers in a single vp.
Another possible cause for inconsistence of viral preps is potential recombination. Although we were trying to minimize homologous sequences in mosaic genome, the rearrangement at low level still exists. DNA isolated from several viral preps of mBFmAd was tested for rearrangement and reversion to the single fiber genome by PCR. A low level of PCR product with that size corresponded to the recombination event was detected in viral preps, and in plasmid preps of mBFmAd Shuttle vector and Ad genome, which are the standard steps of designing adenoviral vector. We believe that recombination at some low level occurred in the plasmid DNA, whereas it is being propagated in bacteria, thus mBFmAd viral prep also may carry the low level of contamination with viruses with one fiber. To minimize recombination in improved generations of fiber-mosaics, silent point mutations can be introduced into fiber tail sequences. In addition, we also cannot exclude possible batch to batch variation of mBFmAd preps in terms of biotinylated fiber incorporation. For this experiment virus was preincubated with the adapter without any additional purification. However, further optimization for obtaining the virus-adapter complex can be considered. The crude viral prep obtained after the first virus CsCl banding can be further purified on avidin columns to eliminate virions lacking the recombinant fibers fibers. We are currently testing the consistency of fiber-mosaic virus preparations. Several lots of virus preparation did show a similar ratio of wt fiber to biotinylated fiber incorporation as described previously. However, different experimental and viral amplification conditions may bias or favor incorporation of the retargeting fiber and influence the overall efficiency of the retargeting strategy.
In this study, we have demonstrated enhanced gene transfer based on transductional retargeting of our vector. It would be of a considerable interest to investigate the extent to which it will translate to therapeutic end points. Several publications indicated the feasibility of conversion of the vector targeting gains to the treatment benefits in experimental animal models.28,33 Thus, our future goal will include the introduction of an anticancer therapeutic gene in the context of the proposed fiber-mosaic platform to test therapeutic efficacy of our retargeting strategy.
In summary, we have confirmed that mBfMAd complexed with EGF-Streptavidin could successfully retarget virus to EGFR-expressing cancer cell lines. Most importantly, the evidence of utility of this strategy was demonstrated in vivo. The targeting potential of mBfMAd complexed with EGF-Streptavidin was tested on two in vivo models that overexpress EGFR in different tissues. First, we utilized a mouse model of locoregional EGFR-positive ovarian xenografts. Secondly, the retargeting capacity of mBfMAd/EGF was tested on a ‘transient transgenic’ mouse model with transient expression of hEGFR in the mouse lung vasculature. mBfMAd/EGF achieved a higher transduction level in the lung compared to mBfMAd without adapter. Moreover, tumor-to-liver or lung-to-liver gene expression ratios for retargeted mBfMAd increased in both models tested. Thus, we demonstrated that an additional fiber in fiber-mosaic virions could be used for biotinylation and this modification, in combination with appropriate adapters, can be successfully exploited for adenoviral retargeting strategies. Importantly, our study demonstrated the proof of preclinical utility of our targeting strategy in animal models.
Materials and methods
Cells
The 293 human kidney cell line was purchased from Microbix (Toronto, Ontario, Canada). The human ovarian carcinoma cell line SKOV3.ip1 was obtained from Janet Price (MD Anderson Cancer Center, Houston, TX, USA). The human epidermoid carcinoma (A-431), human ovarian carcinoma (SKOV3), lung carcinoma (A549) and human breast cancer (MDA-MB-453) cell lines were from the American Type Culture Collection (Manassas, VA, USA). NR6 and NR6wt cell lines were obtained from Dr Alan Wells (University of Pittsburgh, Pittsburgh, PA, USA). These cell lines represent 3T3-derived fibroblasts lacking endogenous EGFRs (NR6) or the same cell line retrovirally transduced with the complete human EGFR (NR6wt).11
Antibodies
Monoclonal antibodies against the Ad5 fiber tail (4D2) and the fiber trimer (2A6) were purchased from Lab Vision (Fremont, CA, USA). Anti-His Ab was obtained from Qiagen (Valencia, CA, USA). Secondary goat anti-mouse IgG horseradish peroxidase (HRP)-conjugated Ab was obtained from DAKO (Carpinteria, CA, USA). Streptavidin and biotinylated HRP for Western blot were from Vector Labs (components of Vectastain ABC kit). Streptavidin for ELISA was from Southern Biotechnology Association (Birmingham, AL, USA).
Recombinant proteins
Recombinant soluble CAR protein was provided by Dr Igor Dmitriev, The Gene Therapy Center, University of Alabama at Birmingham.12 The fiber-knob domain of Ad5 fibers was produced in E. coli with N-terminal tags of six consecutive histidine residues (6His), using the pQE30 expression vector (Qiagen, Valencia, CA, USA). Recombinant EGF-Streptavidin was obtained as described by Ponnazhagan et al.10 Briefly, this adapter was obtained by cloning the coding sequences of EGF in frame with core-streptavidin in the vector pSTE2-215 Yol. For large-scale production of the fusion protein, 1 l of bacterial culture was induced with 20 μM IPTG (iso-propyl-β-D-thiogalactopyranoside) for 5 h at 30°C. Protein was purified from both soluble periplastic extract and remaining inclusion bodies. To obtain soluble periplasmic extract of recombinant protein the cell pellet was resuspended in 1/100 of the original culture volume in a buffer containing 50 mM Tris-HCl and 20% sucrose (pH 8.0). To purify protein from inclusion bodies, the pellet remaining after previous step was resuspended in 1/50 volume (to that of the original culture) of a buffer containing 6 M guanidine-HCl and 100 mM Tris (pH 7.0) and left rotating overnight. Following centrifugation at 13 000 r.p.m. for 30 min, the supernatant containing the fusion protein was affinity purified with a Ni-nitrilo-triacetic acid (Ni-NTA) column (Qiagen) according the manufacturers instruction. The eluted fusion protein was dialyzed against a buffer containing 100 mM Tris and 400 mM L-arginine and stored as frozen in aliquots at −20°C.
Viruses
Adenoviral vector having wt fiber (Ad5luc) was used as a control for infectivity assessment of fiber-mosaic virus. Fiber mosaic virus AdFF/F56 genetically encoding two fibers: FF containing 6His at C-teminus23 and wt fiber was used in virus binding experiments. Both viral genomes are isogenic to mBfMAd except the fiber region and contain the firefly luciferase gene under CMV promoter in E1 region. AdfltEGFR was used for transient expression of human EGFR in mouse lung vasculature in experiments testing systemic targeting of mBfMAd. The design of this virus is similar to the virus AdfltCEA described by Everts et al.,2 where EGFR cDNA replaced CEA. EGFR cDNA was amplified from plasmid pcDNA3-EGFR.36 All viruses were CsCl purified and vp titer was determined according to standard procedure.
Generation of mBfmAd
The design of the genome of mBfMAd Ad5-FF.PSTCD.F5luc (mBfMAd) was similar to that described previously for AdFF6H.F5luc,6 where the virus genome encodes two fibers in the L5 region: chimeric FF containing C-terminal 6His (FF6H)37 and Ad5 wt fiber. The coding sequences of both fibers are spanned by untranslated 5′and 3′ sequences of wt fiber, with intent to provide equal transcription conditions for both fibers (splicing, polyadenylation and regulation by the Major Late Promoter). To create Ad5FF.PSTCD.F5luc we added a minimal domain of the 1.3S subunit of PSTCD to the chimeric protein FF. This domain is naturally biotinylated at lysine 89, when expressed in E. coli and Saccharomyces cerevisiae by each organism’s cellular biotin ligase enzyme7 and also can be metabolically biotinylated in mammalian cells.8 DNA of PSTCD domain was amplified by PCR from the PinPoint-Xa2 plasmid (Promega, Madison, WI, USA) using primers 5′-GGCT CTAGAGCCGGTAAGGCCGGAGAG and 5′-CCGTCTA GAGAGATCCCCGATCTTGATG. The amplified fragment was then inserted into the XbaI site of plasmid pZeroFF6H, to obtain FF.PSTCD.6H. The cDNA of the resulting fiber FF.PSTCD.6H was amplified and cloned into a fiber mosaic shuttle vector pTGbx using ClaI and SwaI sites, therefore the shuttle plasmid finally has tandem fibers: FF.PSTCD.6H and Ad5 wt fiber flanked by Ad sequences. The Ad5 fiber-mosaic genome was obtained by homologous recombination of the mosaic shuttle vector (pTGbx FF.PSTCD.6H) with SwaI-linearized Ad5 genome backbone plasmid pVK70038 in E. coli BJ5183. The pVK700 plasmid contains CMV promoter-driven firefly luciferase gene in the E1 region as a reporter gene. The plasmid obtained was designated as pAdFF.PSTCD.6 H.F5luc. The fiber-mosaic virus AdFF.PSTCD.6H.F5luc was rescued in 293 cells and purified by a standard CsCl gradient protocol. Titers of viral preps were determined in physical (vp) units, and infectious (PFU) units by TCID50 method according to the AdEasy protocol (Strategene, La Jolla, CA, USA). Single virus preps of both Ad5luc and mBfMAd were used throughout the entire study. The physical titers obtained were 4.15 × 1012 and 4.83 × 1012 vp/ml for Ad5luc and the mBfMAd vector, whereas the infectious titers were 4.50 × 1011 and 8.97 × 109 PFU/ml, respectively. Ratio of vp/PFU for Ad5luc and mBfMAd was 1:9 and 1:530, respectively.
Western blot analysis
Western blot to detect virus fibers and confirm fiber biotinylation was performed as described.6 Briefly, aliquots of Ad vectors equal to 5 × 109 vp were loaded on sodium dodecyl sulfate 4–20% gradient PAGE (BioRad, Hercules, CA, USA) either boiled or unboiled. After separation, viral proteins were electroblotted onto a polyvinylidene difluoride (PVDF) membrane and detected with 4D2, 2A6, anti-His monoclonal AB followed by secondary Ab conjugated with HRP or strepavidin/biotinylated HRP for detection of fiber biotinylation. The blots were developed with 3,3′-diaminobenzidine.
Enzyme-linked immunosorbent assay
To test the binding properties of fiber-mosaic Ad, solid-phase binding ELISA was performed. Binding attributed to wt Ad fiber was confirmed by interaction with CAR as described.6 Binding properties of the mosaic virus attributed to the biotinylated fiber were tested in interaction with streptavidin and EGF-streptavidin, which were coated on plastic at 50 and 5 μg/ml correspondingly in 100 μl of 100 mM carbonate buffer (pH 9.5) during overnight incubation at 4°C. After washing and blocking, the wells were incubated with the virus followed the secondary antibodies.
Ad-mediated gene transfer assay
Infectivity of the mBfMAd in tumor cell lines was determined by gene transfer assay. The amount of mBfMAd or Ad5luc was calculated for each gene transfer experiment, depending on cell numbers in the experimental protocol, to correspond to the desired MOI. Viruses were preincubated with EGF-Streptavidin at the concentration of 10 μg/ml in 300 μl of PBS for 1 h at room temperature (RT) and purified from excess of adapter using Microcon Centrifugal Filter Devices YM-100 (molecular weight cutoff 100 kDa) (Amicon Bioseparation, Millipore, Bed-ford, MA, USA). Viruses used as no adapter control underwent the same procedure as above except PBS was added instead of the adapter. Aliquots of the prepared viruses in DMEM-F12 containing 2% fetal bovine serum (FBS) were added to the cells and infection was carried out for 2 h at 37°C. The cells were incubated in complete media with 10% FBS at 37°C to allow expression of the luciferase gene for 24 h. Luciferase activity in the cell lysates was analyzed by using the Promega (Madison, WI, USA) luciferase assay system and a Berthold (Gaithersburg, MD, USA) luminometer.
In the system with artificial expression of human EGFR, mouse fibroblast cells NR6 or NR6wt stably transduced with human EGFR were first preincubated for 10 min at RT with either PBS (no adapter) or with increasing concentration of EGF-Streptavidin (1–25 μg/ml). After washing with PBS cells were transduced with Ad5luc or mBfMAd at 50 PFU/cell. Luciferase assay was performed as described above.
Competitive inhibition assay
Cell lines with low (MDA-MB-453) or high EGFR expression (A431, A549, SKOV3) were plated in 24-well plates at a density of 1 × 106 cells/well. On the following day the cells were preincubated with recombinant Ad5 knob at 50 μg/ml for 10 min at RT to block infection via the wt fiber. Viruses were prepared as described in previous section and applied to cells, after the blocking step was completed, at MOI 50 PFU/cell for 2 h at 37°C. Unbound virus was washed away with PBS, and medium with 10% FBS was added to each well. Forty hours later, the cells were processed for luciferase assay as described above.
In vivo retargeting of mBfMAd to EGFR using animal model of ovarian carcinoma
CB17 SCID female mice 6–8 weeks of age (Charles River) were injected i.p. with 10 × 106 SKOV3ip1 cells to establish i.p. ovarian tumors. At day 21 after SKOV3ip injection, mice (n = 7 per group) were followed with i.p. injection of Ad5luc or mBfMAd viruses at 1 × 1010 vp/animal preincubated either with adapter EGF-Streptavidin (10 μg) or PBS. Mice were killed 48 h later, all visible tumor nodules (combined and treated as one tumor sample from each animal) and livers were excised and luciferase assay was performed in tissue lysates. Same lysates were used to determine protein concentration by Bio-Rad assay.
In vivo retargeting of mBfMAd to EGFR-expressing lung endothelium in EGFR transient transgenic hCAR mice
The transgenic hCAR mice were a generous gift from Dr Sven Pettersson (Karolinska Institute, Sweden). These mice express truncated hCAR under the control of the human ubiquitin-C promoter, thus allowing hCAR expression in a variety of tissues, including the lungs, kidneys, liver, heart, brain and muscle.13 In this study, 8-to 12-week-old hCAR mice screened for presence of hCAR using PCR and flow cytometry were used. First, hCAR transgenic mice were preconditioned to achieve transient EGFR expression in lung endothelium. For that mice were injected via tail vein with 1 × 1011 vp of AdfltEGFR per animal. Transient expression of Ad transgene in lung endothelium under these conditions has been first demonstrated by Everts et al.2 Forty-eight hours after the first injection, each mouse was injected iv with 5 × 1010 vp of mBfMAd preincubated either with EGF-Streptavidin (100 ng) or PBS. All animals were killed 48 h after the last viral injection. Lung and liver luciferase activities were measured and normalized for protein content determined by Bio-Rad assay.
Animal experiments and protocols were reviewed and approved by the Institutional Animal Care and Use Committee of University of Alabama at Birmingham.
Statistics
Student’s t-test was employed for statistical analysis where P<0.05 was considered to be statistically significant.
Acknowledgments
We thank Joel Glasgow and Alex Pereboev for proofing the manuscript and helpful critique. This study was supported, in part, by NIH grants RO1CA083821-06, 1P01HL076540, 1P01CA104177-01A2, CA075930-07 and DOD grant W81XWH-05-1-0035 and Muscular Dystrophy Association.
References
- 1.Reynolds PN, Zinn KR, Gavrilyuk VD, Balyasnikova IV, Rogers BE, Buchsbaum DJ, et al. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol Ther. 2000;2:562–578. doi: 10.1006/mthe.2000.0205. [DOI] [PubMed] [Google Scholar]
- 2.Everts M, Kim-Park SA, Preuss MA, Passineau MJ, Glasgow JN, Pereboev AV, et al. Selective induction of tumor-associated antigens in murine pulmonary vasculature using double-targeted adenoviral vectors. Gene Therapy. 2005;12:1042–1048. doi: 10.1038/sj.gt.3302491. [DOI] [PubMed] [Google Scholar]
- 3.Nettelbeck DM, Miller DW, Jerome V, Zuzarte M, Watkins SJ, Hawkins RE, et al. Targeting of adenovirus to endothelial cells by a bispecific single-chain diabody directed against the adenovirus fiber knob domain and human endoglin (CD105) Mol Ther. 2001;3:882–891. doi: 10.1006/mthe.2001.0342. [DOI] [PubMed] [Google Scholar]
- 4.Campos SK, Parrott MB, Barry MA. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol Ther. 2004;9:942–954. doi: 10.1016/j.ymthe.2004.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos SK, Barry MA. Comparison of adenovirus fiber, protein IX, and hexon capsomeres as scaffolds for vector purification and cell targeting. Virology. 2006;349:453–462. doi: 10.1016/j.virol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Pereboeva L, Komarova S, Mahasreshti PJ, Curiel DT. Fiber-mosaic adenovirus as a novel approach to design genetically modified adenoviral vectors. Virus Res. 2004;105:35–46. doi: 10.1016/j.virusres.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Cronan JE., Jr Biotination of proteins in vivo. A post-translational modification to label, purify, and study proteins. J Biol Chem. 1990;265:10327–10333. [PubMed] [Google Scholar]
- 8.Parrott MB, Barry MA. Metabolic biotinylation of recombinant proteins in mammalian cells and in mice. Mol Ther. 2000;1:96–104. doi: 10.1006/mthe.1999.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano T, Pandori MW, Chen X, Smith CL, Cantor CR. Recombinant core streptavidins. A minimum-sized core streptavidin has enhanced structural stability and higher accessibility to biotinylated macromolecules. J Biol Chem. 1995;270:28204–28209. doi: 10.1074/jbc.270.47.28204. [DOI] [PubMed] [Google Scholar]
- 10.Ponnazhagan S, Mahendra G, Kumar S, Thompson JA, Castillas M., Jr Conjugate-based targeting of recombinant adeno-associated virus type 2 vectors by using avidin-linked ligands. J Virol. 2002;76:12900–12907. doi: 10.1128/JVI.76.24.12900-12907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen P, Murphy-Ullrich JE, Wells A. A role for gelsolin in actuating epidermal growth factor receptor-mediated cell motility. J Cell Biol. 1996;134:689–698. doi: 10.1083/jcb.134.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V, Curiel DT. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J Virol. 2000;74:6875–6884. doi: 10.1128/jvi.74.15.6875-6884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tallone T, Malin S, Samuelsson A, Wilbertz J, Miyahara M, Okamoto K, et al. A mouse model for adenovirus gene delivery. Proc Natl Acad Sci USA. 2001;98:7910–7915. doi: 10.1073/pnas.141223398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu DL, Gonzalez AM, Printz MA, Doukas J, Ying W, D’Andrea M, et al. Fibroblast growth factor 2 retargeted adenovirus has redirected cellular tropism: evidence for reduced toxicity and enhanced antitumor activity in mice. Cancer Res. 1999;59:2608–2614. [PubMed] [Google Scholar]
- 15.Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V, Seymour LW. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Therapy. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 16.Lanciotti J, Song A, Doukas J, Sosnowski B, Pierce G, Gregory R, et al. Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol Ther. 2003;8:99–107. doi: 10.1016/s1525-0016(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 17.Roelvink PW, Mi Lee G, Einfeld DA, Kovesdi I, Wickham TJ. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- 18.Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wickham TJ, Tzeng E, Shears IILL, Roelvink PW, Li Y, Lee GM, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Beusechem VW, van Rijswijk AL, van Es HH, Haisma HJ, Pinedo HM, Gerritsen WR. Recombinant adenovirus vectors with knobless fibers for targeted gene transfer. Gene Therapy. 2000;7:1940–1946. doi: 10.1038/sj.gt.3301323. [DOI] [PubMed] [Google Scholar]
- 21.Magnusson MK, Hong SS, Boulanger P, Lindholm L. Genetic retargeting of adenovirus: novel strategy employing ‘deknobbing’ of the fiber. J Virol. 2001;75:7280–7289. doi: 10.1128/JVI.75.16.7280-7289.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercier GT, Campbell JA, Chappell JD, Stehle T, Dermody TS, Barry MA. A chimeric adenovirus vector encoding reovirus attachment protein sigma1 targets cells expressing junctional adhesion molecule 1. Proc Natl Acad Sci USA. 2004;101:6188–6193. doi: 10.1073/pnas.0400542101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krasnykh V, Belousova N, Korokhov N, Mikheeva G, Curiel DT. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J Virol. 2001;75:4176–4183. doi: 10.1128/JVI.75.9.4176-4183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belousova N, Korokhov N, Krendelshchikova V, Simonenko V, Mikheeva G, Triozzi PL, et al. Genetically targeted adenovirus vector directed to CD40-expressing cells. J Virol. 2003;77:11367–11377. doi: 10.1128/JVI.77.21.11367-11377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parrott MB, Adams KE, Mercier GT, Mok H, Campos SK, Barry MA. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol Ther. 2003;8:688–700. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- 26.Haisma HJ, Grill J, Curiel DT, Hoogeland S, van Beusechem VW, Pinedo HM, et al. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther. 2000;7:901–904. doi: 10.1038/sj.cgt.7700198. [DOI] [PubMed] [Google Scholar]
- 27.Watkins SJ, Mesyanzhinov VV, Kurochkina LP, Hawkins RE. The ‘adenobody’ approach to viral targeting: specific and enhanced adenoviral gene delivery. Gene Therapy. 1997;4:1004–1012. doi: 10.1038/sj.gt.3300511. [DOI] [PubMed] [Google Scholar]
- 28.Hemminki A, Dmitriev I, Liu B, Desmond RA, Alemany R, Curiel DT. Targeting oncolytic adenoviral agents to the epidermal growth factor pathway with a secretory fusion molecule. Cancer Res. 2001;61:6377–6381. [PubMed] [Google Scholar]
- 29.van Beusechem VW, Grill J, Mastenbroek DC, Wickham TJ, Roelvink PW, Haisma HJ, et al. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenoviral vectors with native tropism abolished. J Virol. 2002;76:2753–2762. doi: 10.1128/JVI.76.6.2753-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolibaba KS, Druker BJ. Protein tyrosine kinases and cancer. Biochim Biophys Acta. 1997;1333:F217–F248. doi: 10.1016/s0304-419x(97)00022-x. [DOI] [PubMed] [Google Scholar]
- 31.Liang Q, Dmitriev I, Kashentseva E, Curiel DT, Herschman HR. Noninvasive of adenovirus tumor retargeting in living subjects by a soluble adenovirus receptor-epidermal growth factor (sCAR-EGF) fusion protein. Mol Imag Biol. 2004;6:385–394. doi: 10.1016/j.mibio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Printz MA, Gonzalez AM, Cunningham M, Gu DL, Ong M, Pierce GF, et al. Fibroblast growth factor 2-retargeted adenoviral vectors exhibit a modified biolocalization pattern and display reduced toxicity relative to native adenoviral vectors. Hum Gene Ther. 2000;11:191–204. doi: 10.1089/10430340050016265. [DOI] [PubMed] [Google Scholar]
- 33.Rancourt C, Rogers BE, Sosnowski BA, Wang M, Piche A, Pierce GF, et al. Basic fibroblast growth factor enhancement of adenovirus-mediated delivery of the herpes simplex virus thymidine kinase gene results in augmented therapeutic benefit in a murine model of ovarian cancer. Clin Cancer Res. 1998;4:2455–2461. [PubMed] [Google Scholar]
- 34.Tanaka T, Huang J, Hirai S, Kuroki M, Watanabe N, Tomihara K, et al. Carcinoembryonic antigen-targeted selective gene therapy for gastric cancer through FZ33 fiber-modified adenovirus vectors. Clin Cancer Res. 2006;12:3803–3813. doi: 10.1158/1078-0432.CCR-06-0024. [DOI] [PubMed] [Google Scholar]
- 35.Izumi M, Kawakami Y, Glasgow JN, Belousova N, Everts M, Kim-Park S, et al. In vivo analysis of a genetically modified adenoviral vector targeted to human CD40 using a novel transient transgenic model. J Gene Med. 2005;7:1517–1525. doi: 10.1002/jgm.806. [DOI] [PubMed] [Google Scholar]
- 36.Bonner JA, Buchsbaum DJ, Russo SM, Fiveash JB, Trummell HQ, Curiel DT, et al. Anti-EGFR-mediated radiosensitization as a result of augmented EGFR expression. Int J Radiat Oncol Biol Phys. 2004;59:2–10. doi: 10.1016/j.ijrobp.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 37.Krasnykh V, Belousova N, Korokhov N, Mikheeva G, Curiel DT. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J Virol. 2001;75:4176–4183. doi: 10.1128/JVI.75.9.4176-4183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J Virol. 2002;76:8621–8631. doi: 10.1128/JVI.76.17.8621-8631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


