Abstract
Genetically modified keratinocytes and fibroblasts are suitable for delivery of therapeutic genes capable of modifying the wound healing process. However, efficient gene delivery is a prerequisite for successful gene therapy of wounds. Whereas adenoviral vectors (Ads) exhibit superior levels of in vivo gene transfer, their transductional efficiency to cells resident within wounds may nonetheless be suboptimal, due to deficiency of the primary adenovirus receptor, coxsackie-adenovirus receptor (CAR). We explored CAR-independent transduction to fibroblasts and keratinocytes using a panel of CAR-independent fiber-modified Ads to determine enhancement of infectivity. These fiber-modified adenoviral vectors included Ad 3 knob (Ad5/3), canine Ad serotype 2 knob (Ad5CAV-2), RGD (Ad5.RGD), polylysine (Ad5.pK7), or both RGD and polylysine (Ad5.RGD.pK7). To evaluate whether transduction efficiencies of the fiber-modified adenoviral vectors correlated with the expression of their putative receptors on keratinocytes and fibroblasts, we analyzed the mRNA levels of CAR, αυ integrin, syndecan-1, and glypican-1 using quantitative polymerase chain reaction. Analysis of luciferase and green fluorescent protein transgene expression showed superior transduction efficiency of Ad5.pK7 in keratinocytes and Ad5.RGD.pK7 in fibroblasts. mRNA expression of αυ integrin, syndecan-1 and glypican-1 was significantly higher in primary fibroblasts than CAR. In keratinocytes, syndecan-1 expression was significantly higher than all the other receptors tested. Significant infectivity enhancement was achieved in keratinocytes and fibroblasts using fiber-modified adenoviral vectors. These strategies to enhance infectivity may help to achieve higher clinical efficacy of wound gene therapy.
Characterization of the key cellular and molecular components of the processes of wound healing has led to the development of targeted interventions designed to foster the repair process. In this regard, direct delivery of genes in situ represents a desirable approach to achieve wound healing gene therapy. Indeed, a number of described approaches have exploited this delivery paradigm to achieve the high local levels of cytokines and growth factors critical for realizing effective gene-based wound repair.1–3 These genetic approaches to augment the wound healing process have included in vitro-mediated gene transfer to cells with autologous transplantation, and in vivo-mediated gene transfer to cells resident within a wound to allow these cells to produce therapeutic proteins that will modify the wound healing process.4–6 These diverse approaches have validated the basic concept that gene-based methods may be adjunctive to the achievement of effective wound healing.
A unifying aspect of these interventions is the requirement to achieve effective expression of therapeutic genes (such as vascular endothelial growth factor, transforming growth factor-β, platelet-derived growth factor, fibroblast growth factor, and keratinocyte growth factor) in the context of cells involved in the wound healing process.7,8 One candidate vector for this application is the adenovirus, which embodies unparalleled gene delivery efficacy in selected contexts. The advantage of the adenovirus as a gene transfer vector lies in its ability to transduce dividing and nondividing target cells, to induce high transgene expression in vivo, its well characterized biology, and ability to incorporate large DNA inserts. However, cells resident in wounds such as human fibroblasts and keratinocytes, which are targets of wound healing strategies, may not be efficiently infected by adenoviruses.9–11 In these instances, deficiency of the primary adenovirus receptor, coxsackie-adenovirus receptor (CAR) on keratinocytes and fibroblasts has been understood to be the biological basis of this phenomenon.9–11 Hence, to achieve the levels of efficiency required in the context of wound gene therapy, it may be necessary to route the adenovirus via CAR-independent pathways. In this regard, several capsid modification strategies have been endeavored to circumvent CAR deficiency. These include the incorporation of short heterologous peptide motifs like RGD (Arg-Gly-Asp) or polylysine (pK7)12,13 into the fiber knob domain, as well as knob switching strategies.14 In addition, more radical modifications based on xenotype knob switching have been exploited to achieve enhanced infectivity.15,16 These modified vectors have been shown to transduce a variety of cells with greatly enhanced infectivity.15–18 Heretofore, these different targeting strategies have not been explored nor compared in the context of targeting strategies relevant with wound healing gene therapy.
In this study, we systematically evaluated a panel of fiber-modified adenoviral vectors (Ads) to enhance transduction of immortalized cell lines as well as primary fibroblasts and primary keratinocytes. Our results indicate that polylysine incorporation onto the C-terminus of the adenoviral fiber knob provides the best infectivity enhancement for the transduction of keratinocytes, whereas the Ads containing a double modification with an RGD motif in the HI loop and a polylysine motif at the C-terminus of the fiber results in the highest infectivity enhancement for the transduction of fibroblasts. Our study thus provides valuable information for adenoviral-based gene therapy in wounds of the skin with respect to the most efficient targeting strategies and a rational basis for further development of Ads for their clinical application in gene therapy of dermal wound healing.
MATERIALS AND METHODS
Human fibroblast cell lines CRL-1474, CCL-148, Detroit 5510, and CRL-2106 were obtained from the ATCC (Manassas, VA). The 293 human-transformed embryonal kidney cell line was purchased from Microbix (Toronto, Canada). All cell lines were maintained in a humidified 37°C atmosphere containing 5% CO2 and cultured with the recommended media. Infections were performed in medium with 2% fetal calf serum (Hyclone, Logan, UT). Normal adult primary human epidermal keratinocytes (NHEK and NHEK-neo Cambrex, Walkersville, MD) were propagated in complete keratinocyte growth medium (GM; Cambrex).
Primary fibroblasts and keratinocytes and cell culture
Approval was obtained from the Institutional Review Board for all studies on human tissue. Human dermal fibroblasts were derived from adult skin by trypsinization as described.19,20 Human dermal fibroblasts obtained from outgrowth of explant cultures, were grown in Dulbecco’s modified Eagle’s medium (BioWhittaker, Walkersville, MD) supplemented with 10% fetal calf serum, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and grown as monolayers on plastic Petri dishes in a humidified atmosphere of a CO2 incubator at 37 °C. Primary keratinocytes were isolated from skin as described previously.21
RNA preparation and polymerase chain reaction (PCR) analysis
Total cellular RNA of primary fibroblasts and keratinocytes was extracted from 2 × 105cells using the RNeasy mini-prep kit (Qiagen, Santa Clarita, CA) and treated with DNase I (Life Technologies, Rockville, MD) for 30 minutes. The GeneAmp RNA PCR core kit (Applied Biosystems, Foster City, CA) was used for cDNA synthesis and PCR amplification of cDNA products. TaqMan primers and probes were designed by Primer Express 1.0 software (Applied Biosystems) and synthesized by Applied Biosystems.
Oligonucleotide sequences for amplification of the CAR gene were as follows: forward primer, 5′-GGA CTT GGC CAC GTT CAT G-3′, and reverse primer, 5′-ACC TCA GCC ACA GTC AAC GG-3′and probe 6FAM-AGC ACC GCA TCC GTC CGC AG-TAMRA. The sequences to amplify CD80 were: forward primer 5′-AAG TGG CAA CGC TGT CCT G-3′, reverse primer 5′-CCT TTT GCC AGT AGA TGC GAG-3′and probe 6FAM-TTG TGC CAG CTC TTC AAC AGA AAC ATT GTG-TA-MRA; the sequences to amplify CD86 were: forward primer 5′-CAG ACC TGC CAT GCC AAT T-3′, reverse primer 5′-TTT CCT GGT CCT GCC AAA AT-3′and 6FAM-CAA ACT CTC AAA ACC AAA GCC TGA GTG AGC-TAMRA; and the sequences to amplify CD46 were: forward primer 5′-CTT TCC TTC CTG GCG CTT TC-3′, reverse primer 5′-CGG AGA AGG AGT ACA GCA GCA-3′and 6FAM-CAC CAT GGC CGC CAG AAG CAA-TAMRA. Oligonucleotide sequences for amplification of the subunit β3 of the αυβ3 integrin were as follows: forward primer 5′-CGG ACA CAG GAG AAG TCG-3′, reverse primer 5′-CCA CAG CAG TGA CTT TGG CA-3′ and probe 6FAM-CAC ACT CGC AGT ACT TGC CCG TGA TC-TAMRA,22 the sequences to amplify the subunit β5 of the αυβ5 integrin were as follows forward primer 5′-GGA AGT TCG GAA ACA GAG GGT-3′, reverse primer 5′-TGG AGT ACT GCA TCA AAG CCC-3′and probe 6FAM-CCG GAA CCG AGA TGC CCC TGA-TAMRA, the sequences to amplify αυ integrin subunit were as follows: forward primer 5′-GCG GGA CCA TCT CAT CAC TAA-3′, reverse primer 5′-GAG CAA CTC CAC AAC CCA AAG-3′ and probe 6FAM-CCG GAA CCG AGA TGC CCC TGA-TA-MRA22.
Oligonucleotide sequences to amplify syndecan-1 were as follows forward primer 5′-AGG ACG AAG GCA GCT ACT CCT-3′, reverse primer 5′-TTT GGT GGG CTT CTG GTA GG-3′and probe 6FAM-AGG AGC CGA AAC AAG CCA ACG GC-TAMRA, glypican-1 were as follows forward primer 5′-GTC TCT GAA GCC AGG CCC-3′, reverse primer 5′-GCG GTC ATC ACT GGC AGT G-3′and probe 6FAM-CCG CGA CGT CCA GGA CTT CTG G-TAMRA.
The human housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The sequences to amplify the GAPDH gene were: forward primer 5′-GGT TTA CAT GTT CCA ATA TGA TTC CA-3′, reverse primer 5′-ATG GGA TTT CCA TTG ATG ACA AG-3′and probe 6FAM-CGT TCT CGC CTT GAC GGT GCC AT-TAMRA.
With optimized concentration of primers and probe, the components of real-time PCR mixtures were designed to result in a master mix with a final volume of 9 μL per reaction containing 1X TaqMan® EZ RT-PCT kit (Applied Biosystems), 100 nM forward primer, 100 nM reverse primer, 100 nM probe, and 0.025% bovine serum albumin. One microliter of total RNA sample was added to 9 μL of PCR mixture in each reaction capillary. A no template control received 1 μL of water. All capillaries were then sealed and centrifuged using an LC Carousel centrifuge (Roche Molecular Biochemicals, Indianapolis, IN) to facilitate mixing. All PCR reactions were carried out using a LightCycler™ system (Roche Molecular Biochemicals). Thermal cycling conditions were subjected to 2 minutes at 50 °C, 30 minutes at 60 °C, 5 minutes at 95 °C and 40 cycles of 20 seconds at 94 °C, and 1 minute at 60 °C. Data were analyzed with LightCycler software.
Viral preparations
Ad5, Ad5/3, Ad5CK-1, Ad5CK-2 (Figure 1) are replication-defective Ads containing a luciferase reporter gene driven by the cytomegalovirus (CMV) promoter in the E1 region and have been described previously.16,23,24 Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7 are replication-defective Ads containing a luciferase reporter gene and a green fluorescent protein (GFP) and driven by the CMV promoter in the E1 region as described previously12,13,17,25 and were compared with their isogenic control Ad5. The viruses are all isogenic and were propagated on 293 cells and purified by double CsCl density centrifugation. Of note, the firefly luciferase gene incorporated into Ad5CK-1, Ad5CK-2, and Ad5/3 contains the wild-type sequence. The firefly luciferase gene incorporated into Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7 contains a modified coding region for firefly luciferase (pGL3; Promega, Madison, WI) that has been optimized for monitoring transcriptional activity in transfected eukaryotic cells.
Figure 1.
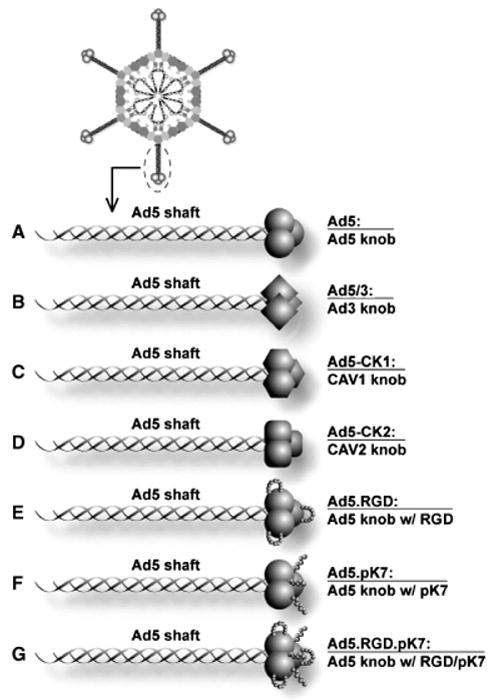
Diagram of the fiber structure of Ad5 vectors used in this study. (A) The knob of Ad5 was substituted (B) by that of Ad3 (Ad5/3), (C) by that of canine adenovirus serotype 1 (Ad5-CK1), or (D) that of canine adenovirus serotype 2 (Ad5-CK1). (E) The RGD motif (CDCRGDCFC) was incorporated into the HI loop (Ad5.RGD), or (F) the pK7 motif (GSGSGSGSGSKKKKKKK) was incorporated at the C-terminus of the fiber (AD5.pK7). (G) The mosaic Ad incorporates both the RGD motif and the pK7 motif (Ad5.RGD.pK7).
Physical viral particle (VP) concentration (VP/mL) was determined by OD260 reading. All experiments were based on VP numbers, although plaque assays were performed to ensure sufficient quality of each virus preparation. We performed crystal violet viability assays26 on fibroblasts and keratinocytes using our adenoviral constructs before performing the lysis and luciferase assays. However, no cell toxicity was observed at the multiplicity of infection (MOI) used in these experiments at 10 and 100 VP/cell, and was only detected at concentrations greater than 1,000 VP/cell (data not shown).
To facilitate comparison of the different vectors, we normalized luciferase activity to relative fold induction compared with the Ad5 counterpart. Thus, to directly compare each of the infectivity-enhanced vectors, luciferase activity of cells infected with Ad5CK-1, Ad5CK-2, and Ad5/3 were normalized to fold activity of cells infected with the isogenic control Ad5Luc1. The ratios of VP:infectious particles were very close, at 4.8, 5.0, 4.2, and 3.9, respectively, for Ad5Luc1, Ad5CK-1, Ad5CK-2, and Ad5/3Luc1.
Likewise, to directly compare each of the infectivity enhanced vectors, luciferase activity of cells infected with Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7 were normalized to fold activity of cells infected with the isogenic control Ad5.GL3. The ratios of VP:infectious particles were very close at 40, 52.2, 45.8, and 51.5, respectively, for Ad5.GL, Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7.
In vitro gene-transfer assays of cells
Cell lines were plated on day 1 at 30,000 cells/well on 24-well plates in 1 mL of 10% GM. On day 2, cells were infected with recombinant Ads at MOI of 10 and 100 for 2 hours in 200 μL of 2% GM on a rocker. Afterward, cells were washed once with 1 mL of phosphate buffered saline solution (PBS), and 1 mL of 10% GM was added per well. After 24 hours, the GM was removed; cells were washed once with PBS, lysed with 200 μL of lysis buffer (Reporter Lysis Buffer; Promega) and freeze-thawed three times. Twenty microliters of these samples were mixed with 100 μL of luciferase assay reagent (Promega) and measured with a Berthold (Wildbad, Germany) Lumat LB 9501. Standardization was accomplished by setting values obtained with Ad5 as 100% for each cell line and primary cells of the skin of each patient.
Visualization of gene transfer via fluorescent microscopy
Cells infected with Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7 were cultured submerged in two- or four-well Lab-Tek® glass chamber slides (Nunc International, Naperville, IL) for 24 hours, washed in PBS and fixed in 4% paraformaldehyde in PBS as per the standard protocol. Fixed slides were mounted with Vectashield® mounting medium containing DAPI (4′,6′-diamidino-2-phenylindole; Vector Laboratories Inc, Burlingame, CA). GFP expression and DAPI staining was analyzed at ×20 objective magnification using an Olympus Provis AX70 fluorescence microscope (Tokyo, Japan) using fluorescein isothiocyanate and DAPI filters. Images were digitally recorded with Axiocam charge-coupled device digital camera (Carl Zeiss, Oberkochen, Germany) using Axiovision image capture software. Images were processed using Photoshop 6.0 software (Adobe Systems, San Jose, CA). The fraction of GFP-positive cells was determined by counting DAPI-positive nuclear signals and GFP-positive cells in five randomly selected high-powered fields (0.031 mm2each) microscopic fields.
Statistics
Data are presented as mean values ± standard deviation. Statistical differences among groups were assessed with a two-tailed Student’s t-test. p(*)<0.05 was considered significant.
RESULTS
Gene delivery efficiency of the fiber-modified non-replicative adenoviruses (Figure 1) was evaluated in two established human keratinocyte cell lines, one adult and one neonatal (NHEK, NHEK neo). Cells were infected with Ad5/3, Ad5CK-1, Ad5-CK2, Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7 at MOI = 10 and 100 (Figure 2). These vectors include serotype knob switching of the Ad5 knob with that of Ad3, “xeno” knob switching of the Ad5 knob with that of canine adenovirus serotype 1 and 2, RGD peptide incorporation into the HI loop of the fiber knob domain, and incorporation of the pK7 motif at the C-terminus of the fiber. The mosaic Ad5.RGD.pK7 incorporates both the RGD motif and the pK7 motif. Infectivity enhancement was assessed by measuring the fold induction of transgene expression compared to an unmodified isogenic Ad5-based vector. Ad5-CK1 and Ad5-CK2 did not significantly increase gene transfer rates compared to Ad5 (p > 0.05). Although Ad5/3 did not show a significant increase of infectivity at MOI of 10 (Figure 2A), significant infectivity enhancement could be shown at a MOI of 100 (Figure 2B). In contrast, Ad5.RGD, Ad5.pK7 and Ad5.RGD.pK7 displayed a statistically significant increased gene transfer compared with Ad5 at both MOIs (p < 0.05). In the aggregate, of all vectors tested Ad5.pK7 showed the highest enhancement in infectivity in both keratinocyte cell lines (66- to 78-fold higher compared with Ad5 at MOI 100; p < 0.05).
Figure 2.
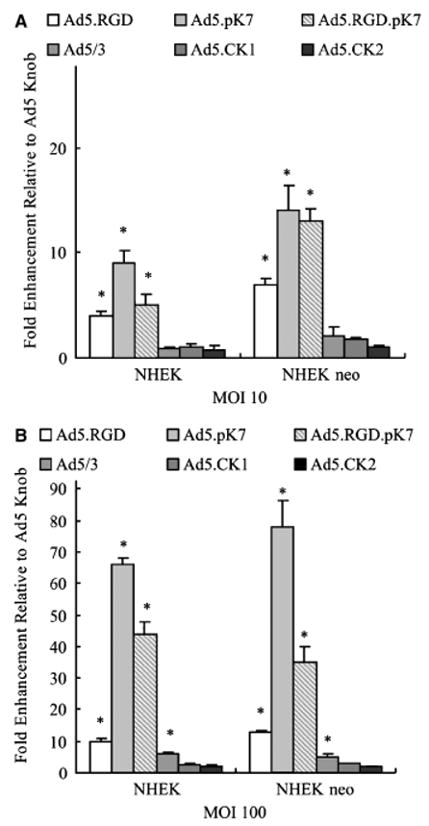
Evaluation of fiber modified vectors for targeting keratinocyte cell lines. Established keratinocyte cell lines (normal human epithelial keratinocytes [NHEK], normal human epithelial keratinocytes-neonatal NHEK-neo) were infected with Ad5, Ad5/3, Ad5CK-1, Ad5CK-2, Ad5.RGD, Ad5.pK7, or Ad5.RGD.pK7 at MOI=10 or 100. Luciferase activity was measured after 24 hours and is expressed as relative light units (RLU) normalized for total protein concentration. Each bar represents the mean of six experiments ± SD. *p<0.05 vs. Ad5.
Transduction efficiency of primary human keratinocytes by Ads in vitro
Cell lines passaged in vitro for years may not reflect the biology of cells in vivo, making the applicability of pre-clinical results to the clinical situation unclear. To more closely model the condition of human keratinocytes found in vivo, gene transfer experiments were performed using human primary keratinocytes that were obtained from five patients (Figure 3). Infection with Ad5-CK1 and Ad5-CK2 resulted in similar transgene expression compared with unmodified Ad5. Ad5/3, Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7 significantly enhanced transduction of primary keratinocytes (p < 0.05 at MOI 10 and 100). Thus, in concordance with the results obtained in keratinocyte cell lines above, Ad5.pK7 showed the highest increase in transgene expression of primary keratinocytes (67- to 134-fold higher in comparison with Ad5 at MOI = 100).
Figure 3.
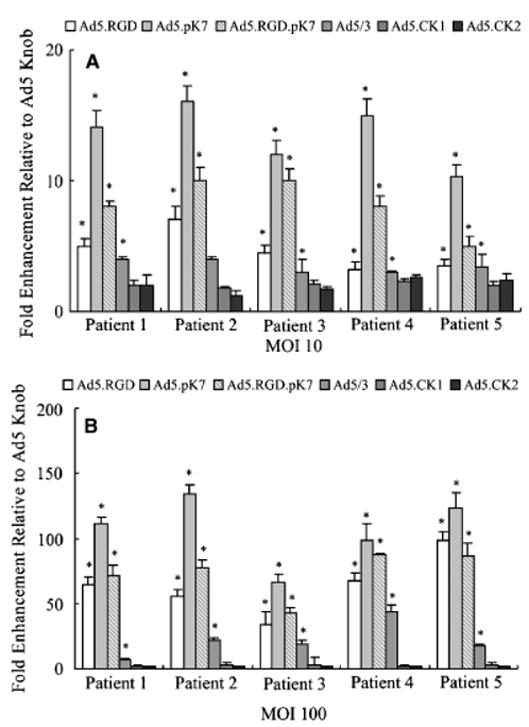
Targeted transduction of primary human keratinocytes. Unpassaged human primary keratinocytes cells obtained from skin explants from five patients were infected with Ad5, Ad5/3, Ad5CK-1, Ad5CK-2, Ad5.RGD, Ad5.pK7, or Ad5.RGD.pK7 at MOI=10 or 100. Luciferase activity was measured after 24 hours and is expressed as relative light units (RLU) normalized for total protein concentration. Each bar represents the mean of six experiments ± SD. *p<0.05 vs. Ad5.
Transduction efficiency of human fibroblast cell lines by Ads in vitro
Fibroblasts are a second major target for gene therapy in wounds. Therefore, four established human fibroblast cell lines were infected to evaluate the gene-delivery efficiency of the fiber-modified vectors (Ad5/3, Ad5CK-1, Ad5CK-2, Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7). As shown in Figure 4, cells were infected with viruses at MOI = 10 and 100, and infectivity enhancement was assessed by measuring the fold induction of transgene expression compared with unmodified Ad5-based vector. In the aggregate, among all the vectors tested Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7 significantly increased (p <0.05) gene transfer levels compared with the Ad5 vector. In the aggregate, among all the vectors tested, Ad5.RGD.pK7 showed the highest infectivity rates of all the viruses tested, with 71- to 123-fold higher luciferase activity compared with Ad5 at MOI = 100.
Figure 4.
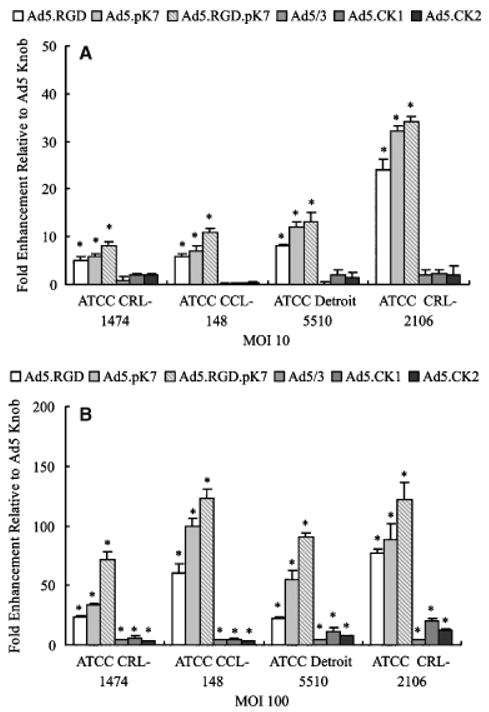
Evaluation of fiber-modified vectors for targeting fib-roblast cell lines. Established fibroblast cell lines (CRL-1474, CCL-148, Detroit 5510 and CRL-2106) were infected with Ad5, Ad5/3, Ad5CK-1, Ad5CK-2, Ad5.RGD, Ad5.pK7, or Ad5.RGD.pK7 at MOI = 10 or 100. Luciferase activity was measured after 24 hours and is expressed as relative light units (RLU) normalized for total protein concentration. Each bar represents the mean of six experiments ± SD. *p<0.05 vs. Ad5.
Transduction efficiency of primary human fibroblasts by Ads vectors
To more closely model the condition of human fibroblasts in vivo, gene transfer experiments were performed using human primary fibroblasts obtained from human skin and infected with our panel of fiber-modified vectors (Figure 5). In primary human fibroblasts, all fiber-modified vectors displayed a significant increase in infectivity in comparison with the unmodified vector Ad5. However, the transduction efficiency among the vectors tested was highest with Ad5RGD.pK7 in all fibroblast samples with 199-to 537-fold higher luciferase expression compared with the unmodified Ad5 vector at MOI = 100.
Figure 5.
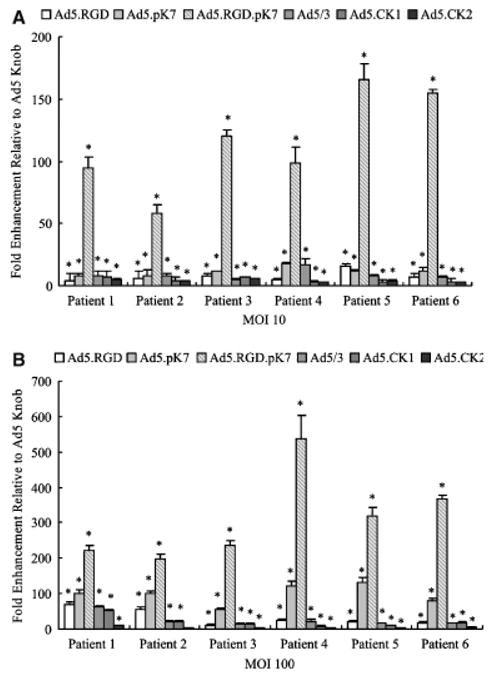
Targeted transduction of primary human fibroblasts. Unpassaged human primary fibroblasts cells obtained from skin explants from six patients were infected with Ad5, Ad5/3, Ad5-CK-1, Ad5CK-2, Ad5.RGD, Ad5.pK7, or Ad5.RGD.pK7 at multiplicity of infection=10 or 100. Luciferase activity was measured after 24 hours and is expressed as relative light units (RLU) normalized for total protein concentration. Each bar represents the mean of six experiments ± SD. *p<0.05 vs. Ad5.
Expression of CAR, CD80, CD86, CD46, integrin subunit αυ, β3, β5, glypican-1, and syndecan-1 in primary keratinocytes and fibroblasts
Quantitative real-time PCR was performed to evaluate the correlation between the transductional efficiency of the fiber-modified adenoviral vectors and the expression levels of their putative receptors27,28 in primary keratinocytes (Figure 6A). All receptor mRNA levels were normalized for expression using expression of the housekeeping gene GAPDH. The mRNA expression levels of the integrin subunits (receptor-ligands of Ad5.RGD, Ad5.RGD. pK7), glypican-1 and syndecan-1 (members of heparin sulfate proteoglycans, receptors for Ad5.pK7 and Ad5. RGD.pK7) were significantly higher than the expression level of CAR (receptor for the unmodified Ad5 vector). Of note, among the putative receptors for Ad5/3 (CD80, CD86, and CD46) only the expression level for CD46 was significantly higher in comparison with CAR. However, syndecan-1 showed the highest expression level in comparison with all other receptors evaluated. These results in receptor expression levels are in concordance with the increased transduction of keratinocytes by Ad5.pK7 compared with the unmodified Ad5.
Figure 6.
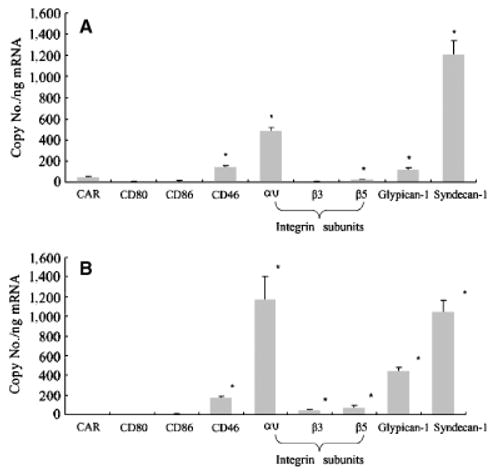
Determination of CAR, CD80, CD86, CD46, integrin subunits αυ, β3, β5, glypican-1 and syndecan-1 in primary keratinocyte and fibroblast mRNA levels. Real-time PCR analysis was performed to quantify the expression of the putative receptors of Ad5 (CAR), Ad5/3 (CD80, CD86, CD46), Ad5.RGD (integrin subunits αυ, β3, β5), Ad5.pK7 (glypican-1 and syndecan-1) in (A) primary keratinocytes from five patients (same patients as in Figure 3) and (B) primary fibroblasts from six patients (same patients as in Figure 5). For each patient, real-time PCR analysis was performed in triplicates. Gene copy numbers are normalized by the GAPDH copy number. Each bar represents the mean copy number per nanogram mRNA of (A) five patients and (B) six patients ± SD. *p < 0.05 vs. CAR.
Next, we evaluated the expression levels of the putative receptors of the fiber-modified vectors in primary fibroblasts (Figure 6B). The mRNA expression levels of the integrin subunits (receptors for Ad5.RGD, Ad5.RGD.pK7), glypican-1 and syndecan-1 (members of the two major gene families of membrane bound heparin sulfate proteoglycans, receptors for Ad5.pK7 and Ad5.RGD.pK7) were significantly higher than the expression level of CAR. Of the putative receptors for Ad5/3 only CD46 was significantly increased. However, among all receptors tested the integrin subunit αυ and syndecan-1 displayed the highest expression levels.
Visualization of enhanced gene transfer of GFP by Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7 in primary keratinocytes and fibroblasts
Finally, we examined transductional efficiency by visualizing and quantifying the gene transfer of the GFP using the fiber-modified Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7 in keratinocytes (Figure 7A) and in fibroblasts (Figure 7B) vs. the unmodified Ad5 vector by fluorescent microscopy in chamber slides. In this analysis, the fraction (%) of GFP+ keratinocytes was 10, 55, and 20 after infection with Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7, respectively. Likewise, the fraction (%) of GFP+ fibroblasts was 5, 15, and 45 after infection with Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7. Thus, the superior transduction efficiency of Ad5.pK7 in keratinocytes and Ad5.RGD.pK7 in fibroblasts was further confirmed by the higher number of cells being infected by these vectors in comparison with Ad5.
Figure 7.
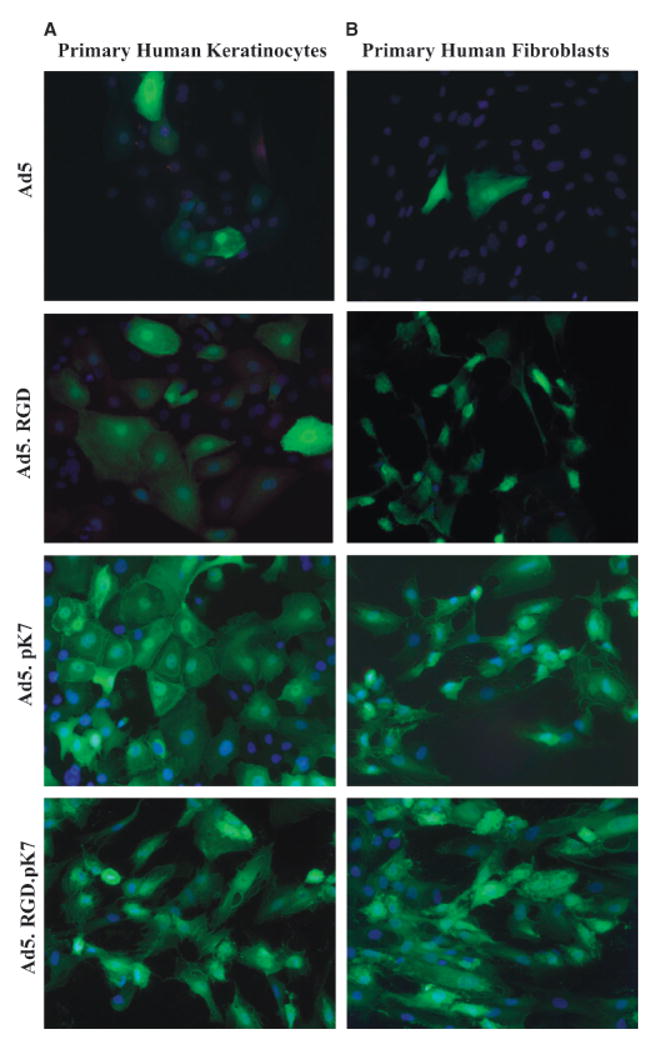
Visualization of enhanced gene transfer by fiber-modified adenoviral vectors via fluorescent microscopy. (A) Primary keratinocytes and (B) fibroblasts were infected with the unmodified vector Ad5 and the fiber-modified vectors Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7 containing GFP driven by the CMV promoter in the E1 region. Infected cells were cultured submerged in 2 or 4 well Lab-Tek® glass chamber slides for 24 hours, washed in PBS and fixed in 4% paraformaldehyde in PBS. GFP expression (green, indicating the reporter gene) and DAPI staining (blue, indicating the nuclei) was analyzed at ×20 objective magnification.
DISCUSSION
Dermal wounds are attractive targets for gene transfer because of their accessibility for manipulation and inspection, as well as the self-renewing capacity of the epidermis.29–31 Gene transfer vectors based on recombinant adenoviruses have gained increasing attention for use in addressing wounds as a potential target site.32–34 However, overall efficacy of adenoviral based gene therapy in cells resident within wounds, such as keratinocytes and fibroblasts, may remain limited by suboptimal vector efficiency. Indeed, the limitation in adenoviral gene transfer has been recently understood to result from a deficiency of the primary adenoviral cellular receptor CAR in fibroblasts35 and keratinocytes.36 Thus, it is clear that augmenting the gene transfer efficiency of Ads of fibroblasts and keratinocytes is of fundamental importance with respect to deriving their full benefit in the context of adenoviral-based wound gene therapy. To circumvent disadvantageous CAR-dependence of Ad5 based vectors for gene therapy, our group previously developed genetically modified Ads with CAR-independent tropism.13,16,17,23,25
In the present study, we explored the possibility of achieving higher transduction in primary fibroblasts and keratinocytes using this repertoire of fiber-modified vectors. These strategies include incorporation of an RGD4C peptide into the HI loop of the fiber knob domain13(Ad5.RGD), to redirect Ad binding to cellular integrins αυβ3 and αυβ5,13 incorporation of a positively charged polylysine pK7 motif at the C-terminus of the fiber (Ad5.pK7) to redirect binding to heparan sulfate proteoglycans,12 a double modification of the fiber with both RGD and pK7 motifs (Ad5.RGD.pK7), serotype knob switching of the Ad5knob with that of Ad3 (Ad5/3)14 to redirect binding to the putative Ad3 receptors CD80, CD86, or CD4637,38 and “xeno” knob switching of the A5knob with that of canine adenovirus serotype 1 (Ad5-CK1)23 and 2 (Ad5-CK2),16 for which the receptors have not been identified yet, but is thought to be different from CAR. These transductionally modified vectors have been shown to increase gene-transfer efficiency in various selected contexts.16,26,28
To evaluate the use of these transductional targeting strategies, we first analyzed gene transfer efficiency in established fibroblast and keratinocyte cell lines. Of all the tested modified vectors, Ad5.pK7 exhibited the highest transductional enhancement in keratinocyte cell lines and Ad5.RGD.pK7 in fibroblast cell lines. These types of cell lines are often used as the standard test system for adenoviral based gene therapy, but often lose essential cell surface proteins and gain gene mutations when grown in culture. In this regard, the adenoviral receptor proteins such as CAR and heparin sulfate glycosamininoglycan expression were previously shown to differ upon culturing the same cell line under various conditions.39–41 Therefore, we next used primary cultures of keratinocytes and fibroblasts. This approach represents a more stringent model for reliable clinical evaluation of efficacy and could help avoid confounding variability, due to genotypic and phenotypic changes involved in the clonal selection process.
In primary keratinocytes, Ad5.pK7, Ad5.RGD, and Ad5.pK7.RGD showed significantly increased transductional efficiency of all fiber-modified vectors tested, with the highest values for Ad5.pK7, corresponding to the results obtained using the keratinocyte cell lines. Ad5.pK7 targets to heparan sulfate proteoglycans via positively charged pK7 motifs.12 In this regard, cell surface heparan sulfate proteoglycans are mostly members of two major gene families of membrane-bound proteoglycans, the syndecans and glypicans.42,43 Syndecans and glypicans bind proteins of the extracellular environment via their heparin sulfate chains, regulating a wide spectrum of biological activities, including cell proliferation and differentiation, morphogenesis, wound repair and host defense. To evaluate whether the observed significantly increased transduction efficiencies of Ad5.RGD, Ad5.RGD.pK7 and, importantly, Ad5.pk7 correlated with the expression of their putative receptors on keratinocytes, we analyzed the mRNA expression levels of CAR, αυ integrins, syndecan-1, and glypican-1 on primary keratinocytes using quantitative PCR. Indeed, mRNA expression of heparan sulfate proteoglycans and αυ integrins was significantly higher than CAR, correlating with the results of the gene transfer. In addition, these results suggest that the high levels of heparan sulfate expression by keratinocytes are rather due to syndecan-1 than to glypican-1.42,44 Importantly, syndecan-1 expression was significantly higher than CAR and all the other putative receptors tested on keratinocytes, and thus correlated with the high transduction efficiency of Ad5.pK7. Of note, syndecan-1 expression is increased during epithelial differentiation and wound healing, underlining the utility of Ad5.pK7 for targeting in gene therapy of wounds.42,45
In primary fibroblasts, Ad5.RGD, Ad5pK7 and Ad5.RGD.pK7 displayed significantly enhanced infectivity among the various vectors evaluated in this study, with Ad5.RGD.pK7, the double modified adenovirus containing an RGD motif in the HI loop and a pK7 motif at the C-terminus of the fiber, revealing the highest efficacy. This suggests that the RGD and pK7 motif may act additively to mediate Ad5.RGD.pK7 infection in fibroblasts. The additive effect of the double-modified Ad5.RGD.pK7 vector has been described in selected contexts.25,28,46 When we compared the two single-modified vectors, Ad5.pK7 resulted in comparable high gene transfer efficacy than Ad5.RGD.35 To evaluate whether observed transduction efficiencies of the vectors achieving the highest infectivity enhancement in fibroblasts (Ad5.RGD, Ad5.pK7, and Ad5.RGD.pK7) correlated with the expression of their putative receptors, we analyzed the mRNA expression levels of CAR, αυ integrins, syndecan-1 and glypican-1 on primary fibroblasts using quantitative PCR. Importantly, mRNA expression of αυ integrins, syndecan-1, and glypican-1 was significantly higher than CAR correlating the results of the gene transfer by the fiber-modified vectors to fibroblasts.10,11,47
At this point, it is clear that the complete characterization of the putative receptors for fiber-modified adenoviruses is still under investigation. For example, receptor identity for Ad serotype 3 remains disputed. Short et al.37 showed that Ad3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors; however, Sirena et al. 38 demonstrated that Ad3 can use CD46 as a receptor. We have demonstrated differences in receptor expression compared to CAR, based on quantitative real-time PCR analysis. Whether or not these differences in RNA levels correlate to receptor levels depends on efficiency of translation, receptor stability, and cell surface exposure. Future studies are in progress to further characterize the putative receptors for fiber-modified adenoviruses.
Finally, the superior transduction efficiency of Ad5.pK7 in keratinocytes and Ad5.RGD.pK7 in fibroblasts assessed by luciferase assay was confirmed by gene transfer of GFP and visualization via fluorescent microscopy. This result underlines that the enhanced transduction of these fiber-modified vectors is due to increased number of cells being infected and not due to the increased luciferase expression per cell.
In conclusion, significant infectivity enhancement could be achieved in primary keratinocytes by the Ad5.pK7 vector and in primary fibroblasts by the Ad5.RGD.pK7. Future studies will be necessary to determine if the enhanced transduction of fibroblasts by Ad5.RGD.pK7 is additive or synergistic compared with Ad5.RGD or Ad5.pK7. This study is the first to provide a systemic evaluation and comparison of CAR-independent Ad transduction strategies in keratinocytes and fibroblasts. These data establish the foundation for rational development of infectivity enhanced Ad-based wound gene therapy and may help to achieve higher clinical efficacy of wound gene therapy in the future.
Acknowledgments
We thank Minghui Wang for his kind technical support. This work was supported by a Grant of the Deutsche Forschungsgemeinschaft Sto 647/1-1 (M. A. Stoff-Khali-li); Department of Defense W81XWH-05-1-0035 and National Institutes of Health RO1CA083821 (D. T. Curiel).
Abbreviations
- Ads
Adenoviral vectors
- CAR
Coxsackie-adenovirus receptor
- CMV
Cytomegalovirus
- DAPI
40,60-diamidino-2-phenylindole
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GFP
Green fluorescent protein
- GM
Growth medium
- MOI
Multiplicity of infection
- NHEK
Normal human epithelial keratinocytes
- PBS
Phosphate buffered saline solution
- PCR
Polymerase chain reaction
- pK7
Polylysine
- RGD
Arginine–glycine–aspartate
- VP
Viral particle
References
- 1.Tepper OM, Mehrara BJ. Gene therapy in plastic surgery. Plast Reconstr Surg. 2002;109:716–34. doi: 10.1097/00006534-200202000-00047. [DOI] [PubMed] [Google Scholar]
- 2.Hoeller D, Petrie N, Yao F, Eriksson E. Gene therapy in soft tissue reconstruction. Cells Tissues Organs. 2002;172:118–25. doi: 10.1159/000065610. [DOI] [PubMed] [Google Scholar]
- 3.Roman S, Lindeman R, O’Toole G, Poole MD. Gene therapy in plastic and reconstructive surgery. Curr Gene Ther. 2005;5:81–99. doi: 10.2174/1566523052997532. [DOI] [PubMed] [Google Scholar]
- 4.Liu W, Chua C, Wu X, Wang D, Ying D, Cui L, Cao Y. Inhibiting scar formation in rat wounds by adenovirus-mediated overexpression of truncated TGF-beta receptor II. Plast Reconstr Surg. 2005;115:860–7. doi: 10.1097/01.prs.0000153037.12900.45. [DOI] [PubMed] [Google Scholar]
- 5.Sumiyoshi K, Nakao A, Setoguchi Y, Okumura K, Ogawa H. Exogenous Smad3 accelerates wound healing in a rabbit dermal ulcer model. J Invest Dermatol. 2004;123:229–36. doi: 10.1111/j.0022-202X.2004.22730.x. [DOI] [PubMed] [Google Scholar]
- 6.Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, Capogrossi MC. Adenovirus-mediated VEGF(165) gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 2002;9:1271–7. doi: 10.1038/sj.gt.3301798. [DOI] [PubMed] [Google Scholar]
- 7.Vranckx JJ, Yao F, Petrie N, Augustinova H, Hoeller D, Visovatti S, Slama J, Eriksson E. In vivo gene delivery of Ad-VEGF121 to full-thickness wounds in aged pigs results in high levels of VEGF expression but not in accelerated healing. Wound Rep Reg. 2005;13:51–60. doi: 10.1111/j.1067-1927.2005.130107.x. [DOI] [PubMed] [Google Scholar]
- 8.Chandler LA, Doukas J, Gonzalez AM, Hoganson DK, Gu DL, Ma C, Nesbit M, Crombleholme TM, Herlyn M, Sosnowski BA, Pierce GF. FGF2-Targeted adenovirus encoding platelet-derived growth factor-B enhances de novo tissue formation. Mol Ther. 2000;2:153–60. doi: 10.1006/mthe.2000.0102. [DOI] [PubMed] [Google Scholar]
- 9.Panetti TS, Wilcox SA, Horzempa C, McKeown-Longo PJ. Alpha v beta 5 integrin receptor-mediated endocytosis of vitronectin is protein kinase C-dependent. J Biol Chem. 1995;270:18593–7. doi: 10.1074/jbc.270.31.18593. [DOI] [PubMed] [Google Scholar]
- 10.Gailit J, Clark RA. Studies in vitro on the role of alpha v and beta 1 integrins in the adhesion of human dermal fibroblasts to provisional matrix proteins fibronectin, vitronectin, and fibrinogen. J Invest Dermatol. 1996;106:102–8. doi: 10.1111/1523-1747.ep12328177. [DOI] [PubMed] [Google Scholar]
- 11.Gailit J, Clarke C, Newman D, Tonnesen MG, Mosesson MW, Clark RA. Human fibroblasts bind directly to fibrinogen at RGD sites through integrin alpha(v)beta3. Exp Cell Res. 1997;232:118–26. doi: 10.1006/excr.1997.3512. [DOI] [PubMed] [Google Scholar]
- 12.Wickham TJ, Roelvink PW, Brough DE, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996;14:1570–3. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 13.Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–13. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–46. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoff-Khalili MA, Rivera AA, Glasgow JN, Le PL, Stoff A, Everts M, Tsuruta Y, Kawakami Y, Bauerschmitz GJ, Siegal GP, Mathis M, Pereboeva L, Dall P, Curiel DT. A human adenoviral vector with a chimeric fiber from canine adenovirus type 1 results in novel expanded tropism for cancer gene therapy. Gene Ther. 2005;12:1696–706. doi: 10.1038/sj.gt.3302588. [DOI] [PubMed] [Google Scholar]
- 16.Glasgow JN, Kremer EJ, Hemminki A, Siegal GP, Douglas JT, Curiel DT. An adenovirus vector with a chimeric fiber derived from canine adenovirus type 2 displays novel tropism. Virology. 2004;324:103–16. doi: 10.1016/j.virol.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM, Barnes MN, Alvarez RD, Siegal GP, Curiel DT, Hemminki A. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther. 2002;5:695–704. doi: 10.1006/mthe.2002.0599. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Han T, Lam JT, Leath CA, Dmitriev I, Kashentseva E, Barnes MN, Alvarez RD, Curiel DT. Preclinical evaluation of a class of infectivity-enhanced adenoviral vectors in ovarian cancer gene therapy. Gene Ther. 2004;11:874–8. doi: 10.1038/sj.gt.3302249. [DOI] [PubMed] [Google Scholar]
- 19.Stark HJ, Baur M, Breitkreutz D, Mirancea N, Fusenig NE. Organotypic keratinocyte cocultures in defined medium with regular epidermal morphogenesis and differentiation. J Invest Dermatol. 1999;112:681–9. doi: 10.1046/j.1523-1747.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- 20.Satish L, Babu M, Tran KT, Hebda PA, Wells A. Keloid fibroblast responsiveness to epidermal growth factor and activation of downstream intracellular signaling pathways. Wound Rep Reg. 2004;12:183–92. doi: 10.1111/j.1067-1927.2004.012111.x. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee NS, Rivera AA, Wang M, Chow LT, Broker TR, Curiel DT, Nettelbeck DM. Analyses of melanoma-targeted oncolytic adenoviruses with tyrosinase enhancer/promoter-driven E1A, E4, or both in submerged cells and organotypic cultures. Mol Cancer Ther. 2004;3:437–49. [PubMed] [Google Scholar]
- 22.Zhou X, Murphy FR, Gehdu N, Zhang J, Iredale JP, Benyon RC. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem. 2004;279:23996–400. doi: 10.1074/jbc.M311668200. [DOI] [PubMed] [Google Scholar]
- 23.Stoff-Khalili MA, Rivera AA, Glasgow JN, Le LP, Stoff A, Everts M, Tsuruta Y, Kawakami Y, Bauerschmitz GJ, Mathis JM, Pereboeva L, Seigal GP, Dall P, Curiel DT. A human adenoviral vector with a chimeric fiber from canine adenovirus type 1 results in novel expanded tropism for cancer gene therapy. Gene Ther. 2005;12:1696–706. doi: 10.1038/sj.gt.3302588. [DOI] [PubMed] [Google Scholar]
- 24.Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, Barker SD, Straughn M, Barnes MN, Alvarez RD, Hemminki A, Curiel DT. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002;8:275–80. [PubMed] [Google Scholar]
- 25.Wu H, Seki T, Dmitriev I, Uil T, Kashentseva E, Han T, Curiel DT. Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus-adenovirus receptor-independent gene transfer efficiency. Hum Gene Ther. 2002;13:1647–53. doi: 10.1089/10430340260201734. [DOI] [PubMed] [Google Scholar]
- 26.Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG, Hakkarainen T, Bauerschmitz GJ, Wang M, Liu B, Cao Z, Alvarez RD, Curiel DT, Hemminki A. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther. 2003;8:449–58. doi: 10.1016/s1525-0016(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 27.Stoff-Khalili MA, Stoff A, Rivera AA, Mathis JM, Everts M, Wang M, Kawakami Y, Waehler R, Mathews QL, Yamamoto M, Rocconi RP, Siegal GP, Richter DF, Dall P, Zhu ZB, Curiel DT. Gene transfer to carcinoma of the breast with fiber-modified adenoviral vectors in a tissue slice model system. Cancer Biol Ther. 2005;4:1203–10. doi: 10.4161/cbt.4.11.2084. [DOI] [PubMed] [Google Scholar]
- 28.Rein DT, Breidenbach M, Wu H, Han T, Haviv YS, Wang M, Kirby TO, Kawakami Y, Dall P, Alvarez RD, Curiel DT. Gene transfer to cervical cancer with fiber-modified adenoviruses. Int J Cancer. 2004;111:698–704. doi: 10.1002/ijc.20295. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson E, Velander P. Gene transfer in wound healing. Br J Surg. 2004;91:1093–4. doi: 10.1002/bjs.4646. [DOI] [PubMed] [Google Scholar]
- 30.Petrie NC, Yao F, Eriksson E. Gene therapy in wound healing. Surg Clin North Am. 2003;83:597–616. vii. doi: 10.1016/S0039-6109(02)00194-9. [DOI] [PubMed] [Google Scholar]
- 31.Andree C, Voigt M, Wenger A, Erichsen T, Bittner K, Schaefer D, Walgenbach KJ, Borges J, Horch RE, Eriksson E, Stark GB. Plasmid gene delivery to human keratinocytes through a fibrin-mediated transfection system. Tissue Eng. 2001;7:757–66. doi: 10.1089/107632701753337708. [DOI] [PubMed] [Google Scholar]
- 32.Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D, Herlyn M, Crombleholme TM. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Rep Reg. 2004;12:497–504. doi: 10.1111/j.1067-1927.2004.12501.x. [DOI] [PubMed] [Google Scholar]
- 33.Crombleholme TM. Adenoviral-mediated gene transfer in wound healing. Wound Rep Reg. 2000;8:460–72. doi: 10.1046/j.1524-475x.2000.00460.x. [DOI] [PubMed] [Google Scholar]
- 34.Sylvester KG, Nesbit M, Radu A, Herlyn M, Adzick NS, Crombleholme TM. Adenoviral-mediated gene transfer in wound healing: acute inflammatory response in human skin in the SCID mouse model. Wound Rep Reg. 2000;8:36–44. doi: 10.1046/j.1524-475x.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 35.Hidaka C, Milano E, Leopold PL, Bergelson JM, Hackett NR, Finberg RW, Wickham TJ, Kovesdi I, Roelvink P, Crystal RG. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J Clin Invest. 1999;103:579–87. doi: 10.1172/JCI5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber M, Limat A, Wagner E, Hohl D. Efficient in vitro transfection of human keratinocytes with an adenovirus-enhanced receptor-mediated system. J Invest Dermatol. 2000;114:661–6. doi: 10.1046/j.1523-1747.2000.00942.x. [DOI] [PubMed] [Google Scholar]
- 37.Short JJ, Pereboev AV, Kawakami Y, Vasu C, Holterman MJ, Curiel DT. Adenovirus serotype 3 utilizes CD80 (B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology. 2004;322:349–5. doi: 10.1016/j.virol.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Sirena D, Lilienfeld B, Eisenhut M, Kalin S, Boucke K, Beerli RR, Vogt L, Ruedl C, Bachmann MF, Greber UF, Hemmi S. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004;78:4454–62. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carson SD, Hobbs JT, Tracy SM, Chapman NM. Expression of the coxsackievirus and adenovirus receptor in cultured human umbilical vein endothelial cells: regulation in response to cell density. J Virol. 1999;73:7077–9. doi: 10.1128/jvi.73.8.7077-7079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spruck CH, III, Gonzalez-Zulueta M, Shibata A, Simoneau AR, Lin MF, Gonzales F, Tsai YC, Jones PA. p16 gene in uncultured tumours. Nature. 1994;370:183–4. doi: 10.1038/370183a0. [DOI] [PubMed] [Google Scholar]
- 41.Dechecchi MC, Melotti P, Bonizzato A, Santacatterina M, Chilosi M, Cabrini G. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J Virol. 2001;75:8772–80. doi: 10.1128/JVI.75.18.8772-8780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 2003;77:13125–3. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 44.Oksala O, Salo T, Tammi R, Hakkinen L, Jalkanen M, Inki P, Larjava H. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem. 1995;43:125–3. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- 45.Marchisio PC, Trusolino L, De Luca M. Topography and biological role of integrins in human skin. Microsc Res Tech. 1997;38:353–60. doi: 10.1002/(SICI)1097-0029(19970815)38:4<353::AID-JEMT3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 46.Contreras JL, Wu H, Smyth CA, Eckstein CP, Young CJ, Seki T, Bilbao G, Curiel DT, Eckhoff DE. Double genetic modification of adenovirus fiber with RGD polylysine motifs significantly enhances gene transfer to isolated human pancreatic islets. Transplantation. 2003;76:252–61. doi: 10.1097/01.TP.0000066361.02042.CA. [DOI] [PubMed] [Google Scholar]
- 47.Elenius K, Jalkanen M. Function of the syndecans – a family of cell surface proteoglycans. J Cell Sci. 1994;107 (Part 11):2975–82. doi: 10.1242/jcs.107.11.2975. [DOI] [PubMed] [Google Scholar]


