Abstract
In tottering mice, a point mutation in the gene encoding P-type (Cav2.1) voltage-gated calcium channels results in ataxia, absence epilepsy, and motor dystonia that appear 3–4 weeks postnatal. The aberrant motor behaviors have been linked to cerebellar dysfunction, and adult Purkinje cells (PCs) of tottering mice exhibit calcium-dependent changes in gene transcription suggestive of altered calcium homeostasis. In an attempt to identify early postnatal events important for the development of the behavioral phenotype, we examined calcium channel expression in cerebellar PCs from postnatal days 6 to 15 (P6–15). Whole cell recording was combined with selective calcium channel antagonists to allow discrimination of the various voltage-activated calcium channels types; early age-dependent differences between tottering and wild-type PCs were found. Wild-type PCs experienced a steady increase in P current density over this period, resulting in a two-fold change by P15. In tottering, by contrast, P current density remained unchanged from postnatal days 6 to 8 and was only 25% of the wild-type level by P8. A developmental delay in functional expression was implicated in this early deficit, since ensuing gains over the subsequent week brought tottering P current density close to the wild-type level by P15. At this age, tottering PCs also exhibited a 2.2-fold higher L-type calcium current density than that expressed by wild-type PCs. Increases in N current were apparent at some ages, most strikingly within a subset of tottering PCs at P15. Functional Rand T-type calcium current densities were equivalent to wild-type levels at all ages. We conclude that the tottering mutation brings about selective changes in functional calcium channel expression one to two weeks prior to the appearance of the behavioral deficits, raising the possibility that they represent an early, primary event along the path to motor dysfunction in tottering.
Keywords: voltage, gated Ca2+ channel, channelopathy, neural development
Voltage-gated calcium channels participate in cellular differentiation, neurotransmitter release, synaptic plasticity, and membrane excitability. In humans, mutations in these channels produce abnormalities that include ataxia, familial hemiplegic migraine, absence epilepsy, and/or cerebellar degeneration (Lorenzon and Beam, 2000). In tottering mice, a spontaneous point mutation in the gene encoding the P-type calcium channel (Cav2.1) changes proline to leucine near the putative outer vestibule of the channel’s pore (Fletcher et al., 1996). The impact of this mutation on the biophysical properties of the channel—i.e., gating and permeation—are few and subtle (Wakamori et al., 1998), whereas the behavioral consequences are not—ataxia, absence epilepsy, and paroxysmal dystonia begin ~3–4 weeks postnatal (Green and Sidman, 1962; Noebels and Sidman, 1979).
Evidence suggests the involvement of diverse regions of the central nervous system in these varied neurological abnormalities (Noebels, 1984; Helekar and Noebels, 1991; Campbell and Hess, 1998; Caddick et al., 1999). In the cortex, abnormal spike wave discharges are thought to be an essential trigger for absence seizures (Noebels and Sidman, 1979), as both are eliminated following chemically induced ablation of the locus coerelus (Noebels, 1984). In contrast, the prominent ataxia and dystonia exhibited by tottering animals are both considered of cerebellar origin (Campbell and Hess, 1998; Campbell et al., 1999), albeit through distinct underlying mechanisms, since surgical lesions of the cerebellar vermis eliminate stress-induced dystonia without altering ataxia (Abbott et al., 2000).
The involvement of the cerebellum in motor dysfunction in tottering is not unexpected, since P-type channels contribute ~85% of the total voltage-activated calcium current in Purkinje cells (Mintz et al., 1992), the sole output neuron of the cerebellum. Indeed aberrant PC function in tottering has been reported during optokinetic behavior as evidenced by irregular floccular PC spiking relative to wild-type cells (Hoebeek et al., 2005). PCs of adult tottering mice have also been shown to exhibit a selective 40% reduction in P current density (Wakamori, et al. 1998; but see Dove et al. 1998). In addition, the L-type (Cav1.2) channel mRNA is elevated in adult tottering PCs (Campbell and Hess, 1999), suggesting that changes in calcium channel expression play a role in the tottering phenotype.
Alterations in a number of other proteins have also been reported, some of which may contribute to the motor dysfunction in the mutant (Hess and Wilson, 1991; Austin et al., 1992; Fureman et al., 1999; Sawada and Fukui, 2001; Cicale et al., 2002; Frank-Cannon et al., 2007). In the case of tyrosine hydroxylase (TH), aberrations in calcium channel influx in tottering appear to precipitate abnormal developmental control of expression. In wild-type PCs, TH turns on by P21 and off by P35. By contrast, TH expression persists through adulthood in tottering PCs (Hess and Wilson, 1991; Austin et al., 1992); such aberrant TH expression can be blocked in vivo by administration of L channel antagonists (Fureman et al., 1999). Consistent with this finding, Sawada and Fukui (2001) demonstrated that enhanced Ca2+ influx into non-catecholaminergic neurons in vitro was able to trigger aberrant TH expression. Taken together, such expression studies link Ca2+ influx to control of expression in PCs and raise the possibility that, if alterations in calcium channel complement occur sufficiently early in tottering, they could interfere with normal PC development. We report here results of studies to explore the functional expression of all voltage-activated calcium channel types in PCs from tottering and wild-type mice during the first two weeks of postnatal development, prior to the onset of the behavioral phenotype. Our results provide strong support for the idea that changes in calcium influx may be an early factor precipitating the motor dysfunctions in tottering.
Experimental Procedures
Mice
All procedures with mice were approved by the Tufts Animal Case and Use Committee. Breeding pairs heterozygous for the tottering mutation on the C57BL/6J background (strain name B6.D2-Cacna1atg/J) were purchased from the Jackson Laboratories (Bar Harbor, ME) to establish our colony. Pregnant mice were checked at roughly the same time daily to establish the date of birth to within 24 hours. For simplicity all mice born within this 24-hour period of time were designated as postnatal day 1 (P1). For genotyping, the distal tips of the tails were removed from pups as early as P4, and the animals were tagged using the AIMSTM pup tattoo identification system. The mice used for controls consisted of wild-type offspring of heterozygous parents or offspring of wild type C57BL/6J.
Genotyping
Genotyping was performed following the procedure developed by Wakamori et al. (1998). Briefly, DNA was extracted from tail snips using the DNeasy kit from Qiagen (Valencia, CA) and polymerase chain reaction was used to amplify a region encompassing the T-to-G tottering mutation (Hot-Start kit, Qiagen). Because this point mutation removes an AciI restriction enzyme recognition site present in the wild-type gene, unambiguous assignment of genotypes was possible by AciI digestion and electrophoretic separation of the PCR reaction products on agarose gels.
Cell dissociation and acute culture
A single animal was used to generate PCs for each day’s recording; averages of data reported for each time point were generated from multiple animals and dissociations. Pups were decapitated and the brains rapidly removed and placed into oxygenated ice-cold HEPES buffer (containing 82 mM Na2SO4, 30 mM K2SO4, 5 mM MgCl2, 10 mM HEPES, and 10 mM glucose, pH 7.4). The procedure for dissociation was modified from that of Mintz and Bean (1993). The cerebellar vermis was isolated and gently minced into pieces about 2 mm3 using featherweight razor blades. The tissue was transferred into protease solution (3 mg/ml protease XXIII in HEPES buffer) at 37 °C and digested for 6-7 minutes. The tissue was then rinsed twice with the HEPES buffer and transferred into 2 ml of Minimum Essential Medium with Earle’s salts containing10 mM glucose, 44 mM NaHCO3, 1 mg/ml trypsin inhibitor, 1 mg/ml bovine serum albumin, and 0.1 mg/ml DNase. The tissue was minimally triturated—5 times through a fire polished glass Pasteur pipette with a ~2.0 mm diameter opening, then 10 times through a ~1.25 mm opening. Cells were plated onto tissue culture dishes (Becton-Dickinson) and placed at 37 °C in an O2/CO2 incubator. After an hour, dishes were supplemented with the medium described above after the cells were adequately attached for recording. Immediately prior to recording, cells were removed from the incubator and extracellular recording solution added (5 mM BaCl2, 160 mM tetraethylammonium chloride, 10 HEPES, 1 μM tetrodotoxin, pH 7.4). Cells treated in this way typically remained viable for recording for at least 6 hours after dissociation.
Electrophysiology
Dissociated PCs were visually identified at 400 × using a Zeiss inverted microscope with phase contrast, and were clearly distinguishable from granule or other neurons based on shape and size, ranging from ~15–25 μm in diameter. From P6–P9, PCs typically were oval or round with large eccentric visible nuclei (cf. images in Liljelund et al. 2000); by P15, PCs were typically larger and opaque, with a uniformly smooth appearance. Starting around P9, cells exhibited a combination of these features indicating a transition from the immature to the more mature morphology. P15 cells from tottering mice exhibiting low P-type current were from 3 out of 4 preparations of dissociated cells, and identified independently by 3 electrophysiologists.
Electrodes were pulled from Kimble capillary soda lime glass (Fisher Scientific) and had a typical resistance of 2 MΩ (range 1.5–4) when filled with an internal solution containing 150 mM CsCl, 10 mM HEPES, 5 mM tetracesium BAPTA, and 5 mM MgATP at pH 7.4. A List EPC-7 patch clamp amplifier was used for recording somatic currents in the whole cell mode. Capacitive transients were compensated and total cell capacitance noted from the Cslow dial. Series resistance was compensated, typically to ~30%. Currents were filtered at 3 kHz via a low pass, 8 pole Bessel filter (LPF8, Warner Instruments).
Data acquisition and analysis
Cell stimulation and data acquisition were controlled using Pulse software (HEKA Electronik, Lambrecht/Pfalz, Germany). Data were acquired at 10 or 40 kHz and a p/4 or p/6 protocol, respectively, was employed for subtracting leak currents. Data analysis was carried out off-line using IgorPro (Wavemetrics, Lake Oswego, OR); spreadsheets and statistical calculations were performed with Excel (Microsoft, Redmond, WA). Data are expressed as means ± standard errors of the mean (with statistical significance assessed by a Student’s two-tailed t test).
Ba2+ replaced Ca2+ as the charge carrier in order to minimize calcium-dependent inactivation of the channels, thereby avoiding underestimations of current density (particularly important for L-type channels, which exhibit significant calcium-dependent inactivation, Liang et al., 2003). Since the peak current of PC from P15 mice occasionally exceeded 5 nA when using 5 mM Ba2+, 2.5 mM Ba2+ (and 164 mM tetraethylammonium chloride) was used in some cases to minimize access resistance errors. In this concentration range, Ba2+ current is a linear function of external Ba2+ (Cota and Stefani, 1984); thus, currents measured in 2.5 mM Ba2+ solution were scaled up by a factor of 2 during analysis to allow comparison with other data.
Somatic currents in Purkinje cells slowly increased during the first few minutes following whole cell access (Regan, 1991), and an additional increase accompanied the onset of perfusion of the control solution (i.e., no antagonists). Application of solutions containing Ca2+ channel blockers was initiated only after the voltage-activated current of a cell had reached a stable level in the control solution. Solutions were applied by gravity flow from tubes positioned directly in front of the cell under investigation. As nimodipine-induced inhibition of L current is voltage-dependent, we monitored the progress of inhibition during a test pulse to 0 mV preceded by a strong depolarizing conditioning prepulse (to 80 mV). After reaching a steady-state, full inhibition was measured at the end of a long (500 ms) depolarizing pulse without the conditioning pulse.
Chemical Reagents
All reagents, unless otherwise noted, were obtained from Sigma-Aldrich (St. Louis, MO). Stock solutions were prepared in deionized water, stored as aliquot samples frozen at ca. −60º C unless otherwise indicated, and diluted into the final solution immediately prior to use. Stock concentrations were made for bovine serum albumin, trypsin inhibitor and protease XXIII at 100 mg/ml, DNase I (Roche Applied Science, Indianapolis, IN) at 10 mg/ml, tetrodotoxin (EMD Biosciences, San Diego, CA) at 3 mM (stored at −20º C), ω-agatoxin IVA and ω-conotoxin GVIA (Peptides International, Louisville, KY) at 0.1 mM, nimodipine at 10 mM in dimethysulfoxide. BAPTA was obtained from Invitrogen (Carlsbad, CA). In all experiments employing ω-agatoxin IVA and ω-conotoxin GVIA, bovine serum albumin was added at 0.2 mg/ml to the final working solutions to minimize non-specific binding and, therefore, was also used in the controls.
Results
Functional calcium channel expression in early postnatal PCs
Somatic currents through voltage-activated calcium channels in PCs were recorded using tight seal, whole cell recordings from cells acutely dissociated from wild-type or tottering mouse cerebellar vermis. On average, peak Ba2+ current was significantly lower in tottering than in wild-type PCs at P6 (−1.83±0.26 nA vs. −2.78±0.33 nA, n=12 and 11, respectively, p<0.03). The smaller current in tottering PCs cannot be attributed to a reduction in cell size, because the average membrane capacitance (an indicator of cell membrane surface area) was actually larger in tottering than in wild-type PCs at this age (34.0±4.0 pF vs. 24.7±5.9 pF, respectively, n=11, Fig. 1). This disparity in capacitance persisted until P15. Thus, the average current density in tottering PCs at P6 was ~50% lower than in their wild-type counterparts at P6 (−54±4.6 pA/pF vs. −113.0±9.7 pA/pF, respectively). This indicates that significant functional differences are apparent between PCs of the two genotypes as much as two weeks before the behavioral dysfunctions appear (Green and Sidman, 1962).
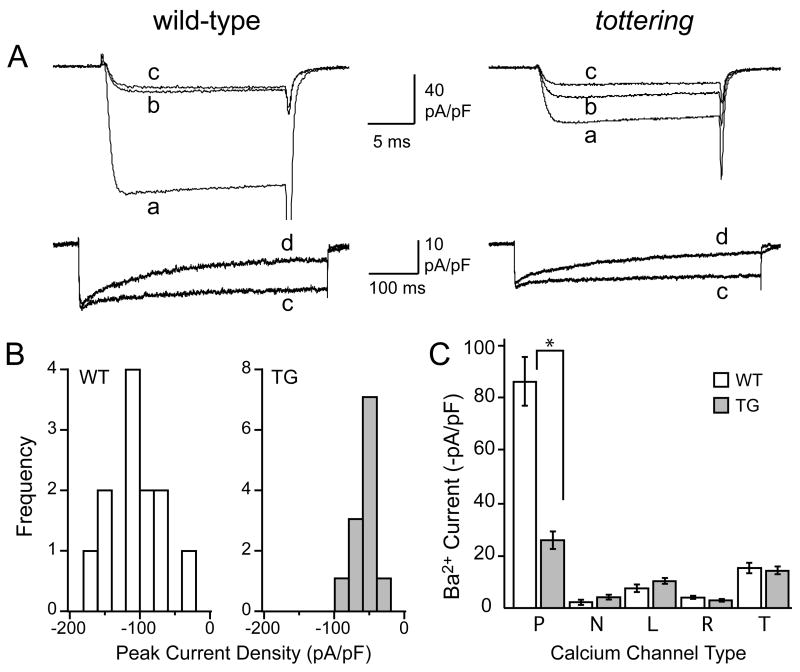
Figure 1. Ca2+ channel complement in PCs at postnatal day 6.
A) Representative current recordings from 6 day-old wild-type and tottering PCs (as marked) in response to depolarizing voltage steps from −80 to 0 mV for 20 ms (upper panels) or from −80 to −10 mV for 500 ms (lower panels). Superimposed currents were taken under control conditions (a), then during exposure to ω-agatoxin IVA (b), then ω-agatoxin IVA + ω-conotoxin GVIA (c), then ω-agatoxin IVA + ω-conotoxin GVIA + nimodipine (d), each at saturating concentration, as noted in the text. B) Amplitude histograms illustrating the distribution of peak Ba2+ current densities measured in the sample of P6 wild-type (WT) and tottering (TG) PCs. C) Histogram illustrating the peak Ba2+ current densities through the pharmacologically-isolated calcium channel types recorded from wild-type (white bars) and tottering (grey bars) PCs (*, p<0.001).
To identify which types of voltage-activated calcium channels were altered in these cells, we measured Ba2+ currents before and after exposure to saturating concentrations of selective Ca2+ channel antagonists. As shown in Fig. 1A and C, the P-type channel antagonist, ω-agatoxin IVA (300 nM), inhibited an average 77±9% (n=11) of voltage-activated current in wild-type PCs at P6. In contrast, the tottering P-type current averaged only 46±3% of the total at P6 (n=11), significantly different from wild-type (p<0.001). The remaining “non-P type” current was not significantly different in the two groups of cells at this age (−28±3 pA/pF for wild-type and −29±2 pA/pF for tottering, n=11 for both). This result indicated that the reduced current density of immature tottering PCs can be attributed to a selective reduction in the functional expression of P-type channels.
In the continued presence of ω-agatoxin IVA to block P channels, N-type currents were isolated by applying the selective N channel antagonist, ω-conotoxin GVIA (at 1 μM). Overall, N-type current densities were equivalent for wild-type and tottering PCs at P6, −5±2 pA/pF (n=4, wild-type) and −7±1 pA/pF (n=5, tottering) (Fig. 1C). Thus, unlike P channels, functional expression of N channels in PCs was unaltered at this early postnatal age.
L-type current was then isolated by the addition of the selective L channel antagonist, nimodipine (1 μM), applied in the continuous presence of the P- and N-type channel antagonists. Because the efficacy of blockade by dihydropyridine antagonists, such as nimodipine, is enhanced at depolarized potentials (Sanguinetti and Kass, 1984), we measured the inhibitory effect at the end of a prolonged (500 ms) depolarizing test pulse (Fig. 1A, lower panels). The average density of nimodipine-sensitive current at P6 was also not significantly different in the tottering and wild-type PCs, being −7.7±1.6 pA/pF in wild-type and −10.8±0.9 pA/pF in tottering (n=8 for both).
Although the T- and R-type calcium channels are less amenable to pharmacological isolation than the other Ca2+ channel types, they can be distinguished by taking advantage of their different inactivation kinetics. T-type channels undergo rapid and complete inactivation during prolonged depolarization (Perez-Reyes, 2006) whereas R-type channels inactivate more slowly (Stotz and Zamponi, 2001). Thus, by applying a sufficiently long depolarizing voltage step (500 ms), the T-type current fully inactivates, appearing as a transient (Fig. 1A lower panels). After inactivation of the T-type current, with P-, N- and L-type channels blocked, the sustained current remaining was R-type current. At P6, wild-type and tottering PCs exhibited similar levels of R- and T-type current densities. R-type current densities were −4.2±0.6 pA/pF (n=4) in wild-type and −3.2±0.5 pA/pF (n=5) in tottering, and T-type current densities were -15.5±1.8 pA/pF (n=4) in wild-type and −14.6±2 pA/pF (n=5) in tottering. Thus, P-type channels were the only calcium channels whose functional expression was significantly affected in tottering PCs at P6.
Alterations in functional calcium channel expression in PCs with postnatal age
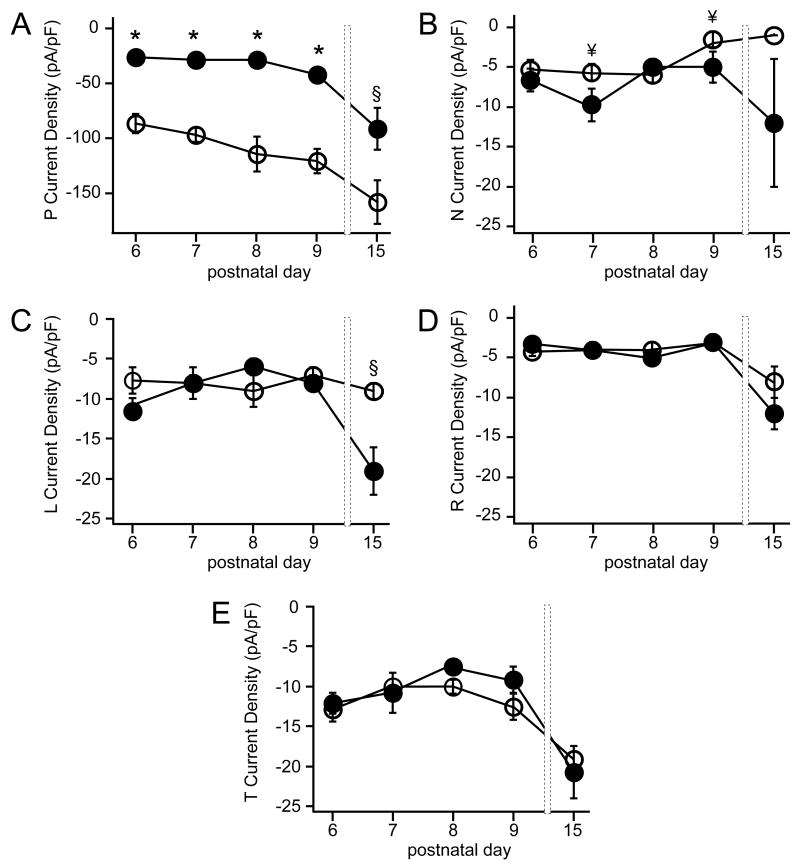
To compare the developmental changes in PC calcium channels from wild-type and tottering mice, voltage-activated current densities were measured over time between P6 to P15. Wild-type PCs exhibited a steady increase in total current density from P6-P9 and by P15 had increased overall by 1.7 fold (Fig. 2). Current densities of PCs from tottering exhibited a very different developmental progression; between P6 and P9, the total current density remained constant (at ca. −55 pA/pF). By P8–9, the total current density in tottering represented only ~40% of that in wild-type PCs. Between P8 and P15, however, a 2.7-fold increase in average current density occurred in tottering PCs. This delayed increase was so pronounced that, by the end of the second postnatal week, the total current density in tottering PCs reached levels not significantly different from those in wild-type cells (Fig. 2).
Figure 2. Functional Ca2+ channel density changes during postnatal development.
Plot illustrating the mean current density over time for PCs dissociated from wild-type (white circles) or tottering (black circles) animals from the ages shown on the abscissa. Error bars represent standard errors of the mean (n = 8–24 cells/point; *, p<0.001).
To determine which Ca2+ channel types contributed to the changes in current density, we used the pharmacological dissection of currents described above and in Figure 1. The steady rise in wild-type current density observed throughout the time period examined was due largely to an increase in P channels (Figs. 3A, 4A,C). Similarly, in tottering, the P current underlay the majority of the changes in total current density (Figs. 3A, 4A,C), increasing 3.2-fold between P8–P15.
Figure 3. Developmental changes in Ca2+ channel complement.
Plots illustrating the mean current densities through the different Ca2+ channel types (noted on the ordinates) measured in PCs dissociated from wild-type (white circles) or tottering (black circles) animals from the ages shown on the abcissae. Error bars represent standard errors of the mean (n = 4–21 cells/point; *, p<0.001, §, p<0.01, ¥, p<0.05).
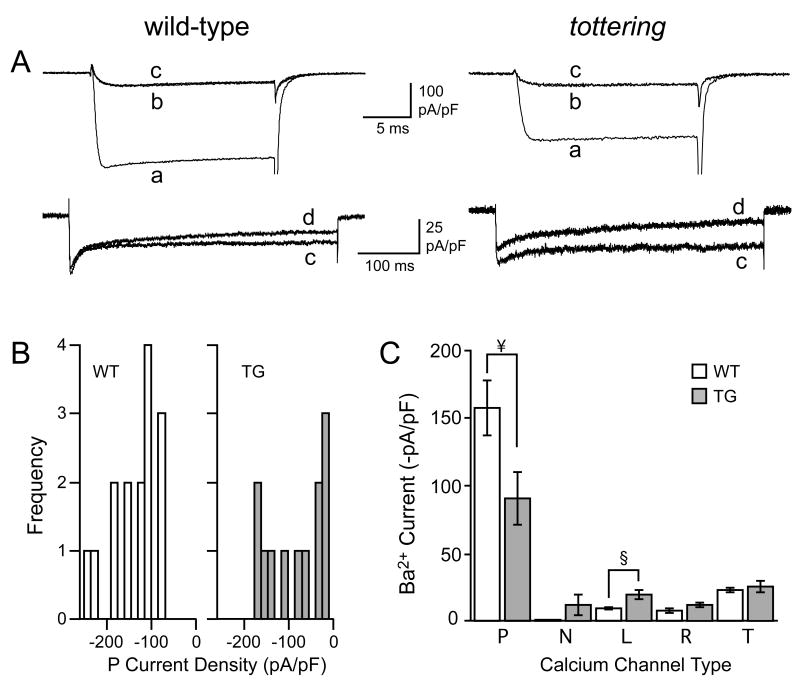
Figure 4. Ca2+ channel complement in PCs at postnatal day 15.
A) Representative current recordings from 15 day-old wild-type and tottering PCs (as marked) in response to depolarizing voltage steps from −80 to 0 mV for 20 ms (upper panels) or from −80 to −10 mV for 500 ms (lower panels). Superimposed currents were taken under control conditions (a), then during exposure to ω-agatoxin IVA (b), then ω-agatoxin IVA + ω-conotoxin GVIA (c), then ω-agatoxin IVA + ω-conotoxin GVIA + nimodipine (d), each at saturating concentration, as noted in the text. B) Amplitude histograms illustrating the distribution of peak Ba2+ current densities measured in the samples of P15 wild-type (WT) and tottering (TG) PCs. C) Histogram illustrating the peak Ba2+ current densities through the pharmacologically-isolated calcium channel types recorded from P15 wild-type (white bars) and tottering (grey bars) PCs (§, p<0.01; ¥, p<0.05).
We next tested whether concomitant changes in density occurred in other channel types as well, producing a change in channel complement as previously reported for adult tottering cerebella (Zhou et al., 2003). For both wild-type and tottering PCs between P6 and P9, the current through L-type channels was small and unchanging (at ca. −8 pA/pF). Over the ensuing week, however, L current density increased almost two-fold in tottering PCs, while it was unchanged in wild-type PCs (−16.3±2.4 pA/pF in tottering and −8.7±1.8 in wild-type, n=9, p=0.002) (Figs. 3C 4A,C). Thus, tottering PCs exhibit aberrant functional expression of L-type Ca2+ channels nearly a week before the appearance of motor deficits.
Functional expression of N-type channels in wild-type PCs dropped more than 3.5-fold from P6 to P15, from −5.3±1.2 pA/pF (n=4) to −1.5±0.4 pA/pF (n=14), p=0.001 (Figs. 3B, 4A,C). In tottering PCs, a deviation from wild-type was seen for the N-type current at P7 and P9 (also see below). Levels of R- and T-type channels were equivalent for wild-type and tottering PCs over this entire developmental window (Fig. 3D and 3E). Taken together, these results indicate that the mutation in the P-type channel in tottering is selectively accompanied by functional increases in L-type channels (and N-type at some ages), while R-, and T-type channels remained relatively unchanged.
Qualitative differences in the variance of the wild-type and tottering PCs became apparent by P15. Wild-type PCs were relatively homogeneous with regard to their Ca2+ channel complement, with high levels of P current, prominent T current, low levels of L current, and virtually no N current. Tottering PCs as a group showed more variation, particularly in regard to the levels of P current density as indicated by the non-normal distribution of values (Fig. 4B, right). Two groups of tottering PCs were apparent, one of which exhibited values for mean P current density remarkably close to the wild-type value of −157±20 pA/pF (n=16). This group of cells was in the majority (7/12) with a mean P current density of −132±19 pA/pF, whereas the remaining 5 cells exhibited a significantly lower density, −31±3 pA/pF (p<0.001). These two groups of tottering PCs were otherwise indistinguishable based on morphological criteria, cell capacitance, and other physiological characteristics of PCs (see Experimental Procedures). Furthermore, two of these 5 cells with a low P-type current also exhibited a mean N-type current density that was significantly higher than that calculated from the entire sample of P15 tottering PCs (−73±3 pA/pF vs. −5.4±4.8 pA/pF, n=2 and 10 respectively, p<0.001). The current densities of all other Ca2+ channel types were equivalent in the two groups. These data indicate that the tottering mutation results in anomalous patterns of expression in a subset of PCs.
Discussion
Results reported here demonstrate that the P-type calcium channel mutation in tottering gives rise to significant changes in PC calcium channel complement over the first two weeks of postnatal development. Early in this window (by P8), the only manifestation of the mutation was a ~75% reduction in P current density, while later in development (by P15) a two-fold enhancement of L current density was apparent in tottering PCs as compared to their wild-type counterparts. Given that the ataxia and seizures characteristic of the mutant do not manifest before 3 weeks of age, these results suggest the possibility that changes in calcium channel complement may be an early precipitating factor for the behavioral phenotype.
The reductions in macroscopic P current in postnatal tottering PCs reported here are most simply explained by a reduction in the surface expression of P channels, since the mutation has little effect on the gating and permeation properties of the channel itself (Wakamori et al., 1998; Pietrobon, 2002). However, a differential expression of alternative splice variants in wild-type and tottering PCs, for example, (Chaudhuri et al., 2005) or alterations in channel modulation (Mintz and Bean, 1993; Zhou et al., 2003) might also contribute to the changes in current density reported here. Although such a possibility cannot be ruled out, our results suggest that P-type calcium channels in PCs from tottering appear on the surface membrane later in development, reach a lower density, and exhibit more heterogeneity than their wild-type counterparts.
Given the variety of calcium-dependent processes known to contribute to normal PC function—gene expression, intracellular Ca2+ homeostasis, spontaneous firing properties and synaptic plasticity—the aberrations in calcium channel complement reported here are likely to have multiple functional consequences.
Consequences of postnatal deficits in P-type current
Voltage-gated calcium channels are key regulators of calcium-dependent gene transcription (Finkbeiner and Greenberg, 1998). Although L channels may be the dominant transcriptional regulators, P channels also contribute both to calcium-dependent transcription (Fields et al., 2001, Sutton et al., 1999) and to nuclear Ca2+ levels in early postnatal PCs (Gruol et al., 2005). Thus, the results reported here showing early 75% reductions in P-type current density and later two-fold increases in L-type current density in PCs during these first two weeks after birth are likely to bring about significant changes in transcription in the mutant and produce multiple effects. Further, such effects would likely not be limited to PCs, as other cell types with dominant expression of P channels may be similarly affected.
The Ca2+ influx through calcium channels is also selectively coupled to a number of other effector responses in PCs that might contribute to motor dysfunction in tottering. Of particular note are both large and small conductance calcium-activated potassium channels (BK and SK), which are key negative regulators of the spontaneous firing in PCs (Womack and Khodakhah, 2002). BK and SK channels are themselves selectively activated by Ca2+ influx through P-type Ca2+ channels in PCs (Womack et al., 2004), making it likely that changes in P channel expression directly affect PC excitability. In fact, blockade of P-type Ca2+ channels in wild-type PCs dramatically affects PC firing rates, as does direct blockade of the potassium channels (Womack and Khodakhah, 2002). Furthermore, the selective blockade of SK channels in vivo, through application of the channel antagonist apamin, promotes ataxia (Lallement et al., 1995). In support of this proposed mechanism for motor dysfunction in tottering, potassium channel blockers have been shown to inhibit stress-induced dystonia (Weisz et al., 2005). Another calcium channel mutant, ducky—which also displays reduced P-type current—exhibits altered frequency and regularity of spontaneous firing as well (Donato et al., 2006). Thus, the reductions in P-type Ca2+ channels that we report here for tottering would be expected to significantly alter PC firing rates and, thereby, promote motor abnormalities.
Consequences of late postnatal variability in Ca2+ channel complement
It is unclear whether the P15 tottering PCs characterized by unusually low P current density represent a functionally distinct population of PCs that persist into adulthood or, alternatively, disappear still later in postnatal development. The finding of cell-to-cell variability in PCs is not unprecedented. Considerable variation has been reported for calcium-dependent inactivation of calcium current in P10 wild-type PCs (Chaudhuri et al., 2005); furthermore, only subpopulations of PCs from tottering and leaner (another P channel mutant) exhibit elevated tyrosine hydroxylase expression (Austin et al., 1992). The two PC populations reported here would likely have significantly different firing properties based on their different calcium channel complements. Furthermore, such inhomogeneity (if found in the flocculus as well as the vermis) may underlie, in part, the reported decrease in the regularity of spiking recorded from PCs in the flocculus of tottering animals—the activity responsible for controlling eye movement; such circuit “noise” may help to explain tottering’s motor deficits (Hoebeek et al., 2005).
The differences in Ca2+ channel complement between the majority and minority samples, may serve to resolve a long-standing discrepancy between the results of Wakamori et al. (1998, in which low adult tottering current density was reported) and Dove et al. (1998, in which no significant differences were found). Our results indicate that the ratio of the two populations in a sample would be one key determinant of whether significant reductions are found on average.
Consequences of late postnatal increase in L-type current
As with P channels, changes in L channel expression are also likely to have acute physiological consequences. The increased expression of L channels that we report in two-week-old postnatal PCs is likely to be maintained in adult tottering animals, as increased L-type mRNA has been reported in adult PCs (Doyle et al., 1997; Campbell and Hess, 1999). Abundant pharmacological evidence also supports this conclusion. L channel antagonists (including nimodipine and nifedipine) administered to adult tottering animals, for example, prevent stress-induced dystonia, but are without effect in wild-type animals (Campbell and Hess, 1999). This finding, together with evidence that PCs in the cerebellar vermis are a focal point for such stress-induced seizures (Campbell and Hess, 1998; Abbott et al., 2000), raises the possibility that early elevations in Ca2+ influx through L channels in PCs bring about at least some of the motor dysfunction in tottering. Further support for this idea comes from experiments on wild-type animals in which the L channel agonist, BayK 8644, was applied in vivo and produced dystonia at concentrations well below those that induce seizures (Campbell and Hess, 1999). Finally, dantrolene-induced inhibition of intracellular Ca2+ release via ryanodine receptors in PCs (which are, themselves, activated by Ca2+ influx through L channels) also eliminates dystonia (Raike et al., 2006). Interestingly, the ataxia (likely also of cerebellar origin) was unaltered by dihydropyridine administration (Campbell and Hess, 1999), indicating that reversible changes in L channel current do not directly underlie the ataxia in tottering.
The changes in L channel expression in PCs are interesting because Ca2+ influx through L channels is known to be a particularly important physiological regulator of these neurons. For example, L channel current underlies spontaneous, rhythmic oscillations in intracellular Ca2+ concentration in young (P4–P7) PCs (Liljelund, et al., 2000). Similar spontaneous Ca2+ oscillations, reported in other neurons, are known to alter several developmental processes, including neurite outgrowth and neurotransmitter phenotype (Spitzer et al., 1994; Gu and Spitzer, 1995; Gomez and Spitzer, 1999). In wild-type PCs, Ca2+ oscillations normally subside after P7 (Liljelund et al., 2000), at a time when P-type current is rising rapidly. Thus, we might anticipate that, in P15 tottering PCs, the unusually high L channel density and reduced P channel density might help to sustain Ca2+ oscillations (and the burst firing that drives them) well beyond their appropriate developmental window, thereby maintaining the cells in a relatively immature state.
A two-fold elevation of the L current in postnatal PCs was also reported for cells from the P channel knockout mouse (Jun et al., 1999); as with tottering, the knockout exhibits dystonia and absence seizures and delayed cerebellar development. These animals do not survive to adulthood. Tottering is a mild Ca2+ channelopathy, by contrast (Lorenzon and Beam, 2000), with significant levels of P-type current. The fact that the L current elevation is triggered in both mouse models suggests that L channels are tightly regulated and readily upregulated following changes in P channel expression. It is tempting to propose that other P-type calcium channelopathies will also exhibit such compensatory L channel upregulation, even following modest reductions in P current. In addition, it would be intriguing if early treatment with L-type channel antagonists could abrogate or delay the development of motor dysfunction (as discussed above for dystonia in adult tottering) and/or prevent progression to cell death and degeneration that is characteristic of the most severe calcium channelopathies.
Acknowledgments
This work was supported by grants to K.D. from the National Institutes of Health, NS41322 and NS16483.
Abbreviations
- PCs
Purkinje Cells
- TH
Tyrosine Hydroxylase
- HEPES
4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid
- BAPTA
1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- pA
picoampere
- pF
picofarad
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LC, Bump M, et al. Investigation of the role of the cerebellum in the myoclonic-like movement disorder exhibited by tottering mice. Mov Disord. 2000;15(Suppl 1):53–59. doi: 10.1002/mds.870150710. [DOI] [PubMed] [Google Scholar]
- Austin MC, Schultzberg M, et al. Expression of tyrosine hydroxylase in cerebellar Purkinje neurons of the mutant tottering and leaner mouse. Brain Res Mol Brain Res. 1992;15:227–240. doi: 10.1016/0169-328x(92)90113-p. [DOI] [PubMed] [Google Scholar]
- Caddick SJ, Wang C, et al. Excitatory but not inhibitory synaptic transmission is reduced in lethargic (Cacnb4(lh)) and tottering (Cacna1atg) mouse thalami. J Neurophysiol. 1999;81:2066–2074. doi: 10.1152/jn.1999.81.5.2066. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Hess EJ. Cerebellar circuitry is activated during convulsive episodes in the tottering (tg/tg) mutant mouse. Neuroscience. 1998;85:773–783. doi: 10.1016/s0306-4522(97)00672-6. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Hess EJ. L-type calcium channels contribute to the tottering mouse dystonic episodes. Mol Pharmacol. 1999;55:23–31. doi: 10.1124/mol.55.1.23. [DOI] [PubMed] [Google Scholar]
- Campbell DB, North JB, et al. Tottering mouse motor dysfunction is abolished on the Purkinje cell degeneration (pcd) mutant background. Exp Neurol. 1999;160:268–278. doi: 10.1006/exnr.1999.7171. [DOI] [PubMed] [Google Scholar]
- Chaudhuri D, Alseikhan BA, et al. Developmental activation of calmodulin-dependent facilitation of cerebellar P-type Ca2+ current. J Neurosci. 2005;25:8282–8294. doi: 10.1523/JNEUROSCI.2253-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicale M, Ambesi-Impiombato A, et al. Decreased gene expression of calretinin and ryanodine receptor type 1 in tottering mice. Brain Res Bull. 2002;59:53–58. doi: 10.1016/s0361-9230(02)00841-9. [DOI] [PubMed] [Google Scholar]
- Cota G, Stefani E. Saturation of calcium channels and surface charge effects in skeletal muscle fibres of the frog. J Physiol. 1984;351:135–154. doi: 10.1113/jphysiol.1984.sp015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Page KM, et al. The ducky(2J) mutation in Cacna2δ2 results in reduced spontaneous Purkinje cell activity and altered gene expression. J Neurosci. 2006;26:12576–12586. doi: 10.1523/JNEUROSCI.3080-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove LS, Abbott LC, et al. Whole-cell and single-channel analysis of P-type calcium currents in cerebellar Purkinje cells of leaner mutant mice. J Neurosci. 1998;18:7687–7699. doi: 10.1523/JNEUROSCI.18-19-07687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J, Ren X, et al. Mutations in the Cacnl1a4 calcium channel gene are associated with seizures, cerebellar degeneration, and ataxia in tottering and leaner mutant mice. Mamm Genome. 1997;8:113–120. doi: 10.1007/s003359900369. [DOI] [PubMed] [Google Scholar]
- Fields RD, Eshete F, et al. Regulation of gene expression by action potentials: dependence on complexity in cellular information processing. Novartis Found Symp. 2001;239:160–172. doi: 10.1002/0470846674.ch13. discussion 172-166, 234-140. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Greenberg ME. Ca2+ channel-regulated neuronal gene expression. J Neurobiol. 1998;37:171–189. [PubMed] [Google Scholar]
- Fletcher CF, Lutz CM, et al. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Zeve DR, et al. Developmental expression of neuronal nitric oxide synthase in P/Q-type voltage-gated calcium ion channel mutant mice, leaner and tottering. Brain Res. 2007;1140:96–104. doi: 10.1016/j.brainres.2005.10.082. [DOI] [PubMed] [Google Scholar]
- Fureman BE, Campbell DB, et al. L-type calcium channel regulation of abnormal tyrosine hydroxylase expression in cerebella of tottering mice. Ann N Y Acad Sci. 1999;868:217–219. doi: 10.1111/j.1749-6632.1999.tb11289.x. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- Green MC, Sidman RL. Tottering--a neuromuscular mutation in the mouse and its linkage with oligosyndacylism. J Hered. 1962;53:233–237. doi: 10.1093/oxfordjournals.jhered.a107180. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Netzeband JG, et al. Contribution of L-type channels to Ca2+ regulation of neuronal properties in early developing Purkinje neurons. Cerebellum. 2005;4:128–139. doi: 10.1080/14734220510007969. [DOI] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Helekar SA, Noebels JL. Synchronous hippocampal bursting reveals network excitability defects in an epilepsy gene mutation. Proc Natl Acad Sci U S A. 1991;88:4736–4740. doi: 10.1073/pnas.88.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess EJ, Wilson MC. Tottering and leaner mutations perturb transient developmental expression of tyrosine hydroxylase in embryologically distinct Purkinje cells. Neuron. 1991;6:123–132. doi: 10.1016/0896-6273(91)90127-l. [DOI] [PubMed] [Google Scholar]
- Hoebeek FE, Stahl JS, et al. Increased noise level of Purkinje cell activities minimizes impact of their modulation during sensorimotor control. Neuron. 2005;45:953–965. doi: 10.1016/j.neuron.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Jun K, Piedras-Renteria ES, et al. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci U S A. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallement G, Fosbraey P, et al. Compared toxicity of the potassium channel blockers, apamin and dendrotoxin. Toxicology. 1995;104:47–52. doi: 10.1016/0300-483x(95)03120-5. [DOI] [PubMed] [Google Scholar]
- Liang H, DeMaria CD, et al. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–960. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- Liljelund P, Netzeband JG, et al. L-Type calcium channels mediate calcium oscillations in early postnatal Purkinje neurons. J Neurosci. 2000;20:7394–7403. doi: 10.1523/JNEUROSCI.20-19-07394.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzon NM, Beam KG. Calcium channelopathies. Kidney Int. 2000;57:794–802. doi: 10.1046/j.1523-1755.2000.00917.x. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Bean BP. Block of calcium channels in rat neurons by synthetic omega-Aga-IVA. Neuropharmacology. 1993;32:1161–1169. doi: 10.1016/0028-3908(93)90010-z. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Venema VJ, et al. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992;355:827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Noebels JL. A single gene error of noradrenergic axon growth synchronizes central neurones. Nature. 1984;310:409–411. doi: 10.1038/310409a0. [DOI] [PubMed] [Google Scholar]
- Noebels JL, Sidman RL. Inherited epilepsy: spike-wave and focal motor seizures in the mutant mouse tottering. Science. 1979;204:1334–1336. doi: 10.1126/science.572084. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular characterization of T-type calcium channels. Cell Calcium. 2006;40:89–96. doi: 10.1016/j.ceca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. Calcium channels and channelopathies of the central nervous system. Mol Neurobiol. 2002;25:31–50. doi: 10.1385/MN:25:1:031. [DOI] [PubMed] [Google Scholar]
- Raike RS, Yin AH, et al. Release of store-operated calcium within cerebellum is necessary for the transient neurological dysfunction in the Cacna1a mouse mutant tottering. Soc Neurosci Abstr. 2006;32:78.9. [Google Scholar]
- Regan LJ. Voltage-dependent calcium currents in Purkinje cells from rat cerebellar vermis. J Neurosci. 1991;11:2259–2269. doi: 10.1523/JNEUROSCI.11-07-02259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Kass RS. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984;55:336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Sawada K, Fukui Y. Expression of tyrosine hydroxylase in cerebellar Purkinje cells of ataxic mutant mice: its relation to the onset and/or development of ataxia. J Med Invest. 2001;48:5–10. [PubMed] [Google Scholar]
- Spitzer NC, Gu X, et al. Action potentials, calcium transients and the control of differentiation of excitable cells. Curr Opin Neurobiol. 1994;4:70–77. doi: 10.1016/0959-4388(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Stotz SC, Zamponi GW. Structural determinants of fast inactivation of high voltage-activated Ca2+ channels. Trends Neurosci. 2001;24:176–181. doi: 10.1016/s0166-2236(00)01738-0. [DOI] [PubMed] [Google Scholar]
- Sutton KG, McRory JE, et al. P/Q-type calcium channels mediate the activity-dependent feedback of syntaxin-1A. Nature. 1999;401:800–804. doi: 10.1038/44586. [DOI] [PubMed] [Google Scholar]
- Wakamori M, Yamazaki K, et al. Single tottering mutations responsible for the neuropathic phenotype of the P-type calcium channel. J Biol Chem. 1998;273:34857–34867. doi: 10.1074/jbc.273.52.34857. [DOI] [PubMed] [Google Scholar]
- Weisz CJ, Raike RS, et al. Potassium channel blockers inhibit the triggers of attacks in the calcium channel mouse mutant tottering. J Neurosci. 2005;25:4141–4145. doi: 10.1523/JNEUROSCI.0098-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Chevez C, et al. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24:8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Characterization of large conductance Ca2+-activated K+ channels in cerebellar Purkinje neurons. Eur J Neurosci. 2002;16:1214–1222. doi: 10.1046/j.1460-9568.2002.02171.x. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Turner TJ, et al. Enhanced G protein-dependent modulation of excitatory synaptic transmission in the cerebellum of the Ca2+ channel-mutant mouse, tottering. J Physiol. 2003;547:497–507. doi: 10.1113/jphysiol.2002.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]