Abstract
Renal parenchymal injury in HIV-associated nephropathy (HIVAN) is characterized by epithelial proliferation, dedifferentiation, and apoptosis along the entire length of the nephron. Although apoptotic cell death in HIVAN has been well documented, the mechanism for HIV-induced apoptosis is poorly understood. We investigated here whether the epithelial apoptosis in HIVAN is mediate by NF-κB-activated Fas ligand expression. In human HIVAN and HIV-1 transgenic mouse kidney specimens, the expression of Fas receptor and ligand proteins were markedly up-regulated on epithelium in diseased glomerular and tubulointerstitial compartments when compared to normal. Podocyte cell lines derived from HIV-1 transgenic mice showed a similar up-regulation of Fas receptor expression and de novo expression of Fas ligand by semi-quantitative rt/PCR and Western blotting. In cultured podocytes, crosslinking of the Fas receptor to mimic ligand binding induced caspase 8 activity and apoptosis in both normal and HIVAN podocytes. Since we have demonstrated constitutive NF-κB activity in HIVAN epithelia, we sought evidence for transcriptional control of the Fas ligand expression by NF-κB. Using cultured podocytes, expression of a FasL reporter promoter plasmid was higher in HIVAN podocytes indicating increase transcriptional activity. In addition, chromatin immunoprecipitation assays were performed to demonstrate p65 (RelA) containing complexes bound the Fas ligand promoter, and that suppression of activated NF-κB with a peptide inhibitor could reduce the expression of Fas ligand mRNA in HIVAN podocytes. These results suggest that NF-κB may regulate Fas-mediated apoptosis in HIVAN by controlling the expression of Fas ligand in renal epithelium.
Introduction
In the United States, HIV-associated nephropathy (HIVAN) affects approximately 1% of the overall HIV-1 seropositive population, and has become the third most common cause of renal failure in adult African American men (1,2). A component of the disease process is the direct infection of renal parenchymal cells, including all tubular, parietal and visceral epithelial cell types (3). The pathology of HIVAN is thus largely typified by epithelial cell defects, and includes both a glomerular lesion (collapsing focal segmental glomerulosclerosis) and an equally profound tubulointerstitial lesion that involves all segments of the nephron (4,5). Prior investigations characterizing these epithelial cell defects have shown that the cells undergo a dedifferentiation which includes changes in the expression level and pattern of cell differentiation markers and increased rates of proliferation (6,7). There also is a well described increase in apoptotic cell death, which has been documented in human kidney biopsies; kidneys from animal models of HIVAN; and with in vitro infections of cultured renal epithelial cells (6-12).
In general, a pathologic role for apoptotic cell death has been described in many forms of chronic and acute renal diseases and in transplant rejection. The most common mechanism of apoptosis implicated in the kidney is mediated by Fas (CD95) and Fas ligand (CD178) interactions. Many renal cell types normally express Fas and Fas ligand (FasL), and renal expression of these proteins can be up-regulated in response to various disease processes (13). In HIVAN, a previous report by Conaldi et al. presented evidence for Fas up-regulation during HIV-1 infection of cultured proximal tubular epithelial cells, and suggested a role for Fas-mediated apoptosis in the tubular damage of HIVAN (9).
It has been shown previously that the transcription of the genes for Fas (14-16) and FasL (17-19) are regulated by NF-κB, an inducible transcription factor complex with a principal role in mediating immune and inflammatory processes (20). NF-κB also has a central role in regulating apoptosis. Initially, NF-κB was believed to be exclusively an anti-apoptotic factor based primarily on the analysis of several NF-κB null (knock-out) transgenic mice. This is clearly the case in the development and homeostasis of the immune system; however, this is not a universal observation for all cell types or signaling events. It has become increasingly more evident that NF-κB can have both anti-apoptotic and pro-apoptotic functions depending on the stimulus, cell type, and differentiation state (21). The pro-apoptotic functions of NF-κB are associated with its ability to increase the expression of well known pro-apoptotic genes, such as Fas and FasL, and also possibly through mechanisms that repress anti-apoptotic gene expression (22).
In HIV-1 infected immune cells, NF-κB is a necessary host transcription factor that is required for both elongation of initial, Tat-independent viral transcripts and also for producing the high levels of Tat-dependent viral gene expression needed for productive infection (23). It also has been shown in chronically infected immune cells that NF-κB is altered to a “persistently activated” state, thus assuring the abundant expression of the viral genes (24). Similar to infected immune cells, we have shown previously that HIV-1 gene expression in the kidney is also dependent on NF-κB transactivation (25), and we have new evidence that suggests NF-κB is similarly dysregulated to a persistently activated state in HIV-1 expressing kidney cells (26).
Here we present evidence that links the altered NF-κB transactivation with renal epithelial cell apoptosis, a component of the pathogenic process in HIVAN. Using the HIV-1 transgenic mouse model and human biopsy specimens, we found that the expression of Fas and FasL were elevated in diseased kidneys in both glomerular and tubular cells. Since FasL expression is a crucial step leading to activation of the Fas pathway, we investigated the mechanism of FasL upregulation in HIV-1 expressing renal epithelial cells. Using conditionally-immortalized podocyte cell lines from the HIV-1 transgenic mouse model, we observed elevated levels of Fas and FasL mRNA and protein in podocytes. This enhanced level of FasL mRNA in HIVAN podocytes could be reduced with treatment of an NF-κB inhibitor, and we further demonstrated the role of NF-κB by showing its direct binding to the FasL promoter in vivo, as well as elevated FasL promoter activity in HIVAN podocytes. These data suggest that the HIV-induced activation of NF-κB is associated with de novo FasL expression and subsequent podocyte apoptosis, and thus may contribute to the glomerular lesion in HIVAN.
Materials and Methods
Mouse model, cell lines and plasmids
The HIV-1 transgenic mouse model develops a renal disease very similar to human HIVAN and has been described in detail (27,28). The podocyte cell lines derived from this mouse model also have been described previously, and include both transgenic podocytes isolated from a heterozygous adult mouse (“HIVAN podocytes”), and normal podocytes prepared from a normal littermate (29). The cell lines, conditionally immortalized with a temperature sensitive SV40 T antigen, were propagated under permissive conditions and used for experiments after growth for 8-10 days at nonpermissive conditions as previously described (30). The soluble peptide inhibitor for NF-κB SN50 and control peptide SN50M, were purchased from BIOMOL (Plymouth Meeting, PA) and used at 0.1mg/ml. SN50 inhibits all p50 containing NF-κBs and can have broad effects on NF-κB activation since multiple NF-κBs contain p50.
A FasL promoter reporter plasmid (pFasLTAL-SEAP) was constructed from pTAL-SEAP (Clontech, Palo Alto, CA) by inserting the previously characterized 750bp murine FasL promoter region (17) into the KpnI/BglII cloning sites. An IκBα dominant negative expression plasmid, pCMV-IκBαM, was purchased from Clontech. As previously reported by the Pollak laboratory (30), cultured podocytes were transiently transfected using FuGENE 6 (Roche, Indianapolis, IN) at a 3:1 lipid to DNA ratio in 24 well dishes (2.5×104 cells/well). Due to the known differences in growth rates of the HIVAN and normal podocytes, all transfected cells were counted at the time of assay and data were normalized to cell number. The secreted alkaline phosphatase (SEAP) reporter is detectable in conditioned media and was assayed 48hour after transfection using a chemiluminescence kit (Great EscAPe, Clontech). Luminescence was measured with a luminometer and data are reported in relative light units (RLU) per 105 cells.
Immunohistochemistry and Western blotting
Immunohistochemistry of human and mouse kidney sections were performed as previously described (5). Fas (M-20) and FasL (N-20) antibodies (Santa Cruz Biotech, Santa Cruz, CA), were used at 1:200 dilution and detected with an avidin-biotin based system (Vectastain ABC elite kit, Vector Laboratories, Burlingame, CA) with horseradish peroxidase-driven chromagen production (AEC substrate, Vector Laboratories). HIV-1 transgenic mouse kidneys and human tissue were formalin-fixed and embedded in paraffin. Human biopsies and normal human nephrectomy tissue were obtained in accordance with all requirements of the Mount Sinai Medical Center and Columbia University Medical Center Institutional Review Boards. All animal studies were conducted in accordance with the animal care and use requirements of Case Western Reserve University.
Western blotting was performed on whole cell extracts using the protocol provided by the primary antibody manufacturer using the above Fas and Fas ligand antibodies (1:1000 dilution). Secondary antibodies (1:2000 dilution) were horseradish peroxidase-conjugated goat anti-rabbit (Jackson ImmunoResearch, West Grove, PA), and detection was by luminescence (ECL, Amersham Biosciences, Piscataway, NJ) according to manufacturer's instructions.
Apoptosis assays
Apoptosis detection in cell cultures was determined with a colorimetric assay for caspase-8 activity (ApoAlert, Clontech, Palo Alto, CA) used according to manufacturer's instructions with 106 cells. Apoptotic cells also were quantified using a fluorescent nuclear dye method as previously described (32). Data are presented as percent DAPI-enhanced late apoptotic nuclei per total nuclei in randomly-selected high power fields. Fas apoptosis was induced using a crosslinking anti-Fas antibody (Jo2, Pharmingen, San Diego, CA) at 5μg/ml for 4 hours. An anti-FasL antibody (Pharmingen) was used at 10μg/ml for a 16 hour pretreatment.
Reverse transcription (rt)/PCR and quantitative PCR
For reverse transcription, RNA from podocyte cell lines was extracted using TRIzol (Invitrogen, Carlsbad, CA), and 1μg of total cellular RNA was used for synthesis of cDNA (SuperScript II, Invitrogen, Carlsbad, CA) using random priming. For standard PCR, amplification conditions and primers for murine Fas and FasL coding regions were as previously described (33). For semi-quantitative PCR using standard PCR amplification conditions, a range of dilutions of input cDNA template were amplified for GAPDH to determine the amount of cDNA needed to visualize linear amplification of products resolved on agarose gels. This linear range of input cDNAs (equivalent for GAPDH amplification between samples) subsequently was used for amplification of Fas and FasL. Primer pairs used for semi-quantitative PCR for NF-κB target genes were as follows: mouse interleukin (IL)-6 forward: TAGTCCTTCCTACCCCAATTTCC and reverse: TTGGTCCTTAGCCACTCCTTC, mouse NF-κB p50 forward: GGAGGCATGTTCGGTAGTGG and reverse: CCCTGCGTTGGATTTCGTG. For quantitative (real time) PCR (LightCycler FastStart DNA Master SYBR Green I, Roche Diagnostics, Indianapolis, IN), relative quantitation with external standards (GAPDH) and analysis with the second derivative maximum method were used. Data are presented as the absolute number of mRNA copies. Quantitative PCR primers for murine FasL were forward: CATCACAACCACTCCCACTG and reverse: GTTCTGCCAGTTCCTTCTGC (GenBank accession: S76752). Quantitative PCR amplification conditions were as follows; for FasL: melt at 96°C for 10sec, anneal at 68°C for 5sec, extend at 72°C for 10sec; and for GAPDH (primers purchased from Clontech): melt at 96°C for 10sec, anneal at 60°C for 5sec, extend at 72°C for 10sec; all for 45 cycles.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed using a kit from Upstate Biotechnologies (Lake Placid, NY) with the following modifications. Formaldehyde crosslinking was stopped with 125mM glycine for 5 minutes at room temperature. After reversal of crosslinks, eluates were precipitated overnight at −20°C with 2.5 volumes ethanol. Precipitated material was resuspended in 100μl TE, and digested with 0.2mg/ml proteinase K and 0.1mg/ml DNase-free RNase A for 1hr at 45°C. DNA was purified using a QiaQuick spin column (Qiagen, Valencia, CA) and eluted with 30μl of kit elution buffer, with 3μl of the purified DNA used for PCR. An antibody against p65 (RelA c-19, Santa Cruz Biotech) was used to immunoprecipitate NF-κB-containing chromatin. PCR primers for the murine FasL promoter were derived from available sequence (GenBank accession: AF045739) and were forward: ACAGGCCTCTCAGGACACAC and reverse: TAAGGTTTCCGCAGTCAAGG. These primers flank the only functional NF-κB site in the mouse promoter as previously identified (19). Positive control primers were for the IκBα promoter and have been described (34). Standard PCR (30 cycles) was performed with AmpliTaq Gold (Applied Biosystems, Foster City, CA) using the manufacturer's recommended conditions with an annealing temperature of 50°C.
Statistical analysis
All graphed data represent three independent experiments performed in triplicate. Data are presented as the average ± standard deviation with probability determined by Student's t test (two tailed, two sample equal variance).
Results
A possible role for Fas-mediated apoptosis in HIVAN has been suggested from in vitro infections of cultured tubular epithelial cells (9). To further this observation, we studied the in vivo expression pattern of Fas and FasL in human biopsy specimens and kidneys from the HIV-1 transgenic mouse model. In HIVAN biopsies, both cystic tubules and sclerosed glomeruli expressed abundant levels of Fas and FasL in comparison to normal nephrectomy tissue (Figure 1). In serial sections, it appeared that the same epithelium expressed both Fas and FasL, suggesting concurrent expression of both proteins. In comparison to both collapsing and non-collapsing idiopathic FSGS, the most abundant glomerular expression of Fas and FasL was observed in HIVAN. The most abundant tubular expression, however, was seen in non-collapsing FSGS, which would be consistent with a recent report by Erkan et al. (35). In glomeruli of both collapsing and non-collapsing idiopathic FSGS, focal areas of faint Fas staining were observed. This Fas expression co-localized with faint staining for FasL in collapsing idiopathic FSGS, whereas very little glomerular FasL expression was detected in non-collapsing FSGS.
Figure 1.
Fas and FasL expression in HIVAN, collapsing and non-collapsing idiopathic FSGS biopsies, and normal nephrectomy tissue. Serial sections shown for all except normal tissue. In HIVAN, immunolocalization for Fas and FasL expression appeared in similar areas of tubular epithelium (arrowheads), and expression of both proteins was abundant in glomeruli in a podocyte distribution. In collapsing idiopathic FSGS, staining for Fas and FasL could be detected in focal areas of glomerular epithelial cell hyperplasia (arrows), but at qualitatively lower levels as compared to HIVAN. In non-collapsing idiopathic FSGS, abundant expression of especially Fas was observed in tubules; however, the expression level in glomeruli was low in comparison. Expression in normal tissue was low for Fas and undetectable for FasL.
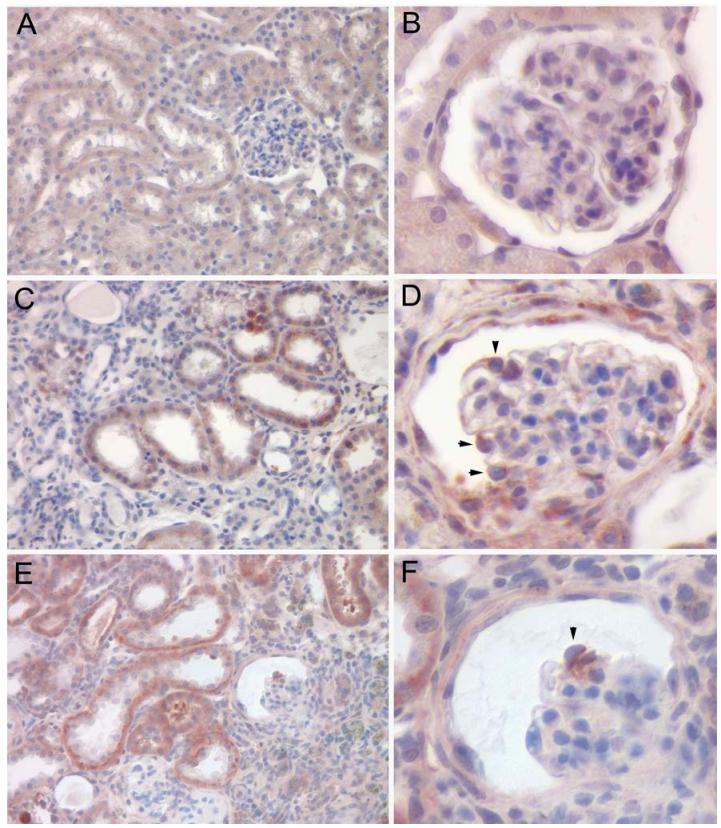
In the HIV-1 transgenic mouse model, the expression and distribution of Fas and Fas ligand were compared between normal and diseased transgenic mouse kidneys (Figure 2). Similarly to the human biopsies, cystic tubules and collapsed glomeruli had clearly elevated levels of Fas and FasL as compared to normal mouse kidney tissue. The glomerular expression appeared to be associated with podocytes; the glomerular cell type previously shown to be infected in the human disease and also the glomerular cell type that expresses the transgene in our mouse model. Thus, the cell types that express the HIV-1 genome are also the cells with evidence of elevated expression of Fas and FasL.
Figure 2.
Fas and FasL expression in the HIV-1 transgenic mouse model. Immunohistochemistry for Fas and FasL expression in normal mouse kidneys (A,B) and in HIV-1 transgenic mouse kidneys with disease (C-F). Fas staining (A-D) was more abundant in the HIV-1 transgenic kidneys with pronounced staining in dilated tubules and in podocytes (arrow heads). FasL staining (E-F) was also very intense in the HIV-1 transgenic kidneys, and was seen in dilated tubules and in a collapsed glomerulus (arrow head). Sections were counterstained with hematoxylin.
To investigate this role of Fas-mediated apoptosis in HIVAN, we used podocyte cell lines established from normal and HIV-1 transgenic mice. Using a standard method to verify the mechanism of apoptosis involved the Fas pathway, cultured podocytes were incubated with a Fas crosslinking antibody that artificially provides a stimulus to activate the Fas pathway. The induction of apoptosis was monitored by both caspase 8 activity, and also with a nuclear dye to detected both live and dead cells (Figure 3). By quantifying the number of pycnotic nuclei, there was a significantly higher basal level of apoptosis in HIVAN cells as compared to normal (approximately fourfold), which is consistent with previously reported apoptotic rates in HIV-1 infected podocytes (32). In addition, a statistically significant increase in the number of apoptotic cells in both cell types was observed with the addition of a crosslinking anti-Fas antibody (Figure 3B). Similarly, the basal level of caspase activity was significantly higher in the HIVAN podocytes as compared to normal (Figure 3C), suggesting the HIVAN podocytes have an existing caspase 8 stimulus that is absent or reduced in normal podocytes. Treatment with a standard dose (5μg/ml) of the anti-Fas antibody similarly increased caspase activity in both cell types (Figure 3C), however the maximal level of assayable caspase 8 activity was lower in the HIVAN cells at this dose and treatment interval. Preliminary survey experiments indicated that the normal and HIVAN cells responded differently depending on the dose and length of treatment with the anti-Fas antibody. The HIVAN cells generated more caspase 8 activity at lower doses of anti-Fas likely due to the higher expression of Fas (see below); and had less total caspase activity with longer treatment periods likely reflecting the complete decomposition of apoptotic cells that died early in the treatment period which were missed by the endpoint assay (data not shown). These studies indicated that Fas is functionally expressed on both normal and HIVAN cells and that activation of the Fas pathway is at least one mechanism of apoptosis in podocytes. The anti-Fas antibody treatments also induced cell death as determined by the nuclear dye assay which monitors the loss of plasma membrane integrity. This would indicate that the increased caspase 8 activity is representative of later apoptotic events and cell death.
Figure 3.
Fas-mediated apoptosis in normal and HIVAN podocytes. A. Confluent normal and HIVAN podocytes cultures were treated with anti-Fas antibody, followed by detection of cell death using a fluorescent staining method. Scale bar is 20μm and all panels are the same magnification. HIVAN podocytes are considerably smaller in size and do not spread as much as normal podocytes. Horizontal arrows indicate pycnotic nuclei, vertical arrows indicate mitotic figures. B. Quantification of apoptosis shown in A for both untreated and anti-Fas antibody treated (“+αFas”) normal and HIVAN podocytes. The DAPI-enhanced and fluorescently bright nuclei with pycnotic changes (non-mitotic) were quantified as a percentage of total nuclei. A higher level of apoptosis was detected in the HIVAN podocytes as compared to normal, and in both cell lines, treatment with the αFas antibody stimulated higher levels of apoptosis. C. Normal and HIVAN podocytes were treated with an anti-Fas antibody (“+αFas”) and assayed for caspase 8 activity, the initiator caspase for Fas-mediated apoptosis. Treatment with a species and isotype-matched control antibody (“+Ab control”) was not statistically different from untreated cells.
Since the immunohistochemistry results indicated the levels of Fas and FasL are increased in vivo, the steady-state mRNA levels of Fas and FasL were compared between normal and HIVAN podocytes. Using a semiquantitative rt/PCR technique (Figure 4A), both normal and HIVAN podocytes expressed Fas, and the level of Fas expression in the HIVAN podocyte was increased (approximately 10 fold) over normal. With this technique, however, there was no detectable basal expression of FasL in normal podocytes, but abundant expression was detected in HIVAN podocytes. This would suggest that HIV-1 may induce de novo expression of FasL in podocytes. The mRNA level for both genes paralleled the protein expression levels as detected by Western blotting (Figure 4B). Thus, the composite of these studies with both human and mouse tissues and cells have consistently shown that in the setting of HIV-1 gene expression, Fas and FasL expression are elevated in renal epithelial cells. The observation that FasL expression was only detected in HIVAN podocytes may provide an explanation for the higher basal level of apoptosis detected by the caspase assays in Figure 3. In addition, since mRNA levels were elevated, it would be logical that a transcriptional event plays a fundamental role in the observed increased expression.
Figure 4.

Fas and FasL gene expression in normal and HIVAN cultured podocytes. A. Semi-quantitative rt/PCR to determine relative differences in mRNA levels by using varying amounts of input template cDNA (0.1 to 100ng) normalized to the amplification of a housekeeping gene (GAPDH). The expression of Fas was increased approximately 10 fold in HIVAN podocytes. Using this method, the expression of FasL was not detectable in normal (WT) podocytes. However, it was clearly detectable in the 10 and 100ng amplifications reactions for HIVAN podocytes, suggesting newly activated expression of FasL. B. Western blots comparing the Fas and FasL protein levels between normal (WT) and HIVAN podocytes. Similar to the rt/PCR results, the expression of Fas was found to be elevated in HIVAN podocytes, and the expression of FasL although undetectable in normal podocytes was observed in HIVAN podocytes (α-tubulin as a loading control).
We have recently shown that HIV-1 expression in renal epithelial cells, similar to infected leukocytes, causes a persistent or constitutive activation of NF-κB (26). Previous studies have shown that the promoter for the FasL gene (human and mouse) contains functional NF-κB binding sites that are critical to its transcriptional regulation. Thus, these observations would suggest a possible connection between the HIV-induced NF-κB activation and the increased FasL gene expression. To functionally demonstrate a role for NF-κB in FasL gene expression, normal and HIVAN podocytes were transfected with the pFasLTAL-SEAP reporter plasmid to measure FasL promoter activity (Figure 5A). In these transfections, the transcriptional activity of the FasL promoter was found to be higher in HIVAN podocytes as compared to normal podocytes. The basal promoter activity of the pTAL-SEAP reporter plasmid alone was 6673±116 RLU/105 cells, and was not statistically different than the level of expression of the pFasLTAL-SEAP plasmid in normal podocytes. Thus, similar to the Northern and Western blots in Figure 4, essentially no expression of the FasL promoter was observed in normal podocytes. In addition, co-transfection of the pFasLTAL-SEAP with a constitutively-expressed dominant negative IκBα, a strong inhibitor of NF-κB, resulted in reduced FasL promoter activity in the HIVAN cells. These studies suggest that NF-κB activation induces FasL expression in HIVAN podocytes, and that suppression of NF-κB can reduce expression to the level seen in normal podocytes. To further demonstrate NF-κB's role in FasL expression, HIVAN podocytes were treated with a soluble peptide inhibitor of NF-κB that blocks the ability of an activated NF-κB complex to bind target DNA sequences (Figure 5B). RNA was harvested and the expression of FasL mRNA was assayed by quantitative (real time) PCR. Blocking NF-κB suppressed the HIV-1-induced increase in FasL expression in HIVAN podocytes by 10 fold, whereas a control treatment had no effect. Cell viability was lower for the SN50 treated cells, but FasL expression in all samples was normalized to GAPDH expression. The effect of NF-κB activity on FasL expression appeared to be a direct transcriptional regulation of the FasL gene as determined by chromatin immunoprecipitation (ChIP) assays, a method that identifies in vivo DNA-protein interactions. ChIP assays (Figure 5C) were performed in HIVAN podocytes using an antibody against p65 (RelA), the transactivating subunit of the NF-κB complex. The ChIP assay showed that RelA bound the FasL promoter within a defined region that contains the functional NF-κB site. These studies demonstrate that an NF-κB RelA-containing complex directly bound the FasL promoter in vivo, and would suggest that the status of NF-κB activation directly correlates with the level of FasL mRNA. The direct association of HIV-1-induced NF-κB activation and FasL gene expression would suggest a mechanism for increased Fas-mediated apoptosis in HIVAN through the renal epithelial cell production of FasL.
Figure 5.
Role of NF-κB in FasL gene expression. A. Expression of a FasL promoter reporter plasmid (pFasLTAL-SEAP) in normal and HIVAN podocytes. The murine FasL promoter was cloned into a SEAP reporter plasmid and transfected into normal and HIVAN podocytes. SEAP activity was measured in conditioned media by a chemiluminescence assay and is reported as relative light units (RLU)/105 cells. Similar to the expression of the native FasL gene, the expression of the reporter construct was significantly higher in HIVAN podocytes as compared to normal (*P<0.01). Co-transfection with a dominant negative mutant of IκBα (“IκBαM”), a repressor of NF-κB, decreased the expression of the FasL reporter plasmid in HIVAN cells. B. Inhibition of NF-κB activity reduced the steady state mRNA level of FasL. HIVAN podocytes cultures were treated with a soluble peptide inhibitor of activated NF-κB, SN50, and also a control mutant peptide, SN50M, which has no effect on NF-κB function. FasL mRNA was quantified by real time PCR, normalized to GAPDH levels, with data presented as absolute number of mRNA copies. The expression of FasL was not significantly different between untreated (640 copies) and SN50M control treated (561 copies) cells, however the SN50 treatment significantly reduced FasL mRNA levels (60 copies) as compared to both the untreated and control treated cells (*P<0.05, **P<0.005). C. ChIP assay using an anti-RelA (p65) antibody. Immunoprecipitated chromatin was purified and used for standard PCR amplification using mouse FasL promoter-specific primers. Amplification of the IκBα promoter was used as a positive control since its promoter is known to be bound by NF-κB. The antibody minus samples indicated there was no non-specific carry over of chromatin through the procedure. D. To rule out a potential effect of Fas/FasL signaling itself activating NF-κB, HIVAN podocytes were treated with a neutralizing FasL antibody (“+αFasL”) and RNA was harvested for rt/PCR. Various known direct NF-κB target genes were analyzed including IL-6, FasL, and NF-κB p50 using semi-quantitative PCR with serial dilutions of input template (GAPDH as a normalization control). There were no changes in the expression level of these direct NF-κB target genes with the inhibition of FasL/Fas signaling.
It has recently been shown that Fas/FasL signaling, in addition to its well known role in regulating apoptosis, may have additional, apoptosis-independent functions, such as participating in fibrosis and inflammation (36). With NF-κB's central role in mediating inflammatory processes, it is possible that FasL signaling may be activating NF-κB in podocytes, as has been shown to occur in other cell types and some cancers (37-39). To exclude the possibility that the observed increases in NF-κB activation was in response to Fas/FasL signaling and not an HIV-1-induced event, HIVAN podocytes were treated with a neutralizing anti-FasL antibody (Figure 5D). Following treatment, RNA was harvested and the expression level of three NF-κB target genes (IL-6, FasL, and NF-κB p50) was quantified to determine if the treatment changed the expression level. The mRNA half life for IL-6 is approximately 1 to 3 hours (40). Since no change was observed in NF-κB target genes expression with this treatment, it suggests Fas signaling is not making a significant contribution to the NF-κB activation state in HIVAN podocytes.
Discussion
During innate and acquired immune responses, many cells undergo apoptosis in response to viral infection. This results in the elimination of infected cells with a consequent reduction in the release of progeny virus. This protective response, however, comes at a high cost to the host, as abnormal apoptosis contributes to tissue destruction and pathogenesis. This is no more evident than during HIV-1 infection, where the devastating effect on the immune system is due to the apoptotic killing of T helper lymphocytes (41,42). These cells are destroyed by both cytotoxic T lymphocyte-mediated killing of infected cells and also to a greater extent the killing of uninfected (bystander) cells via activation induced cell death, a normal homeostatic process of the immune system mediated by Fas. HIV-1 induced apoptosis contributes to pathology in other organ systems as well, including cardiomyocyte cell death in HIV-1 cardiomyopathy, and in CNS cell death which contributes to AIDS dementia complex and HIV-induced encephalopathy (43-45). Similar to studies presented here, Ghorpade et al. also have implicated NF-κB-induced FasL expression as the likely mechanism of astrocyte cell death in AIDS dementia (46). This may suggest HIV-induced Fas-mediated apoptosis is a common mechanism of cell death in non-lymphoid cell types, and underscores the importance of the Fas pathway in host pathogenesis.
Our studies have centered on the HIV-induced NF-κB activation and its contribution to host cell dysfunction such as the apoptotic defect in HIVAN. Although NF-κB has more well known activities in protecting cells from apoptosis, it can also induce apoptosis through the up-regulation of pro-apoptotic genes such as Fas and FasL. The ability of one transcription factor to participate in such mutually exclusive functions as being both pro-apoptotic and anti-apoptotic stems from the fact that NF-κB is not a single transcription factor with one mechanism of activation. NF-κB is a multiprotein complex which is comprised of either homo or heterodimers of DNA binding subunits and transactivating subunits, which are bound by an inhibitor, IκB. There are multiple genes which code for these subunits, and eleven unique NF-κB dimers have been confirmed in vivo (20). The highly important IκB phosphorylation events occur through a multiprotein kinase complex known to participate in numerous signal transduction cascades (47). Cells typically express many NF-κB subunit proteins, and we have found cultured podocytes express at least seven (26). Thus, the combinational diversity and multiple activation mechanisms of the NF-κB pathway even in a single cell type such as the podocyte is extraordinarily complex. It is possible that NF-κB activation in one setting could promote apoptosis and in another setting suppress apoptosis, and would depend on varied aspect of the NF-κB activation cascade including the initiating stimulus; the composition of the NF-κB complex; and its affinity for target gene enhancers.
The fact that NF-κB can induce the expression of both Fas and FasL in one cell establishes a mechanism where FasL does not need to be supplied in trans via an immune cell or through the circulation. This dual expression of both Fas and FasL by epithelial cells appears to have a specific role in the immune response to many viral, bacterial and parasitic infections. A recent study investigating cytotoxic bacterial infections in the lung also found that the airway epithelial cells dually expressed both Fas and FasL to induce apoptosis (48). By using bone marrow reconstitution experiments using lpr (Fas deficient) and gld (FasL deficient) mutant mice, the apoptotic process was shown to be mediated exclusively by the Fas and FasL expressed by the epithelial cells, and did not involve any contribution of FasL from the immune system. In the absence of this apoptotic response, all animals developed a lethal systemic infection. Thus, it was suggested that this mechanism inherent in lung epithelia was a critical innate immune response to limit the infection to a local area and prevent systemic spread. Thus, it may be possible that this increase in Fas-mediated apoptosis observed in HIVAN, may be an intrinsic, first-line defense mechanism of renal epithelia in response to the infection or the presence of viral proteins.
Alternatively, this higher rate of apoptosis may reflect the abnormally high rate of proliferation also characteristic of HIVAN. Mechanisms controlling proliferation and apoptosis share common cell cycle regulatory proteins, and events that activate proliferation can also activate apoptosis (49). In the setting of a high proliferation rate, increased apoptosis is believed to function as a brake to delimit the maximal rate of proliferation and control tissue size (50). However in HIVAN, even with an elevated apoptotic rate, the level of proliferation remains out-of-balance resulting in a net increase in the overall size of the kidney, including podocyte hypertrophy, hyperplasia and pseudocrescent formation. Therefore, a connection between apoptosis and proliferation in HIVAN may exist, but likely represents a complicated relationship of varied aspects of host-virus interactions. One possible connection between these pathological processes could be the HIV-induced activation of NF-κB. Constitutive NF-κB activation has emerged as a hallmark of malignant transformation in lymphomas and solid organ tumors through the up-regulation of cyclin D1, and it has been shown recently that the proliferative defect in HIVAN podocytes is a cyclin D1-dependent cell cycle progression (51,52). Thus, many aspects of HIVAN pathogenesis are likely to center on the dysregulation of NF-κB induced by the virus.
Whether the increased apoptosis in HIVAN is a compensatory mechanism in the setting of increased proliferation, or an innate anti-viral mechanism in the renal epithelial cells is currently under investigation. In either case, identification of the mechanism of NF-κB activation will be important in understanding HIVAN pathogenesis. This mechanism may involve specific effects of individual viral proteins interfering with signal transduction cascades activating NF-κB-specific kinases. Recent studies have made a strong case for Nef as the likely candidate protein in the proliferative and dedifferentiation aspects of HIVAN pathogenesis (53,54). Alternatively NF-κB activation may result from systemic oxidative stress responses known to occur during HIV-1 infection (55). Reactive oxygen species can act as second messengers for activating NF-κB either released systemically from activated immune cells in a general inflammatory process; or through intracellular events involving interactions with host redox-regulating molecules and individual viral proteins (56). With a better understanding of the role of specific viral proteins, as well as key host responses such as NF-κB activation, it may be possible to identify novel and specific targets for the treatment of HIVAN.
Acknowledgements
We thank Drs. Jeffrey Schelling and Peter Nelson for critical review of the manuscript and Dr. Andrew O'Connor for assistance with the statistical analysis. This work was presented in abstract form at the annual meeting of the American Society of Nephrology and was supported by NIH grants DK61395 and DK62672. We dedicate this work to the memory of Ruth G. Abramson, M.D. (1934-2004).
References
- 1.Ross MJ, Klotman PE. HIV-associated nephropathy. Aids. 2004;18:1089–1099. doi: 10.1097/00002030-200405210-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kopp JB, Winkler C. HIV-associated nephropathy in African Americans. Kidney Int.Suppl. 2003:S43–S49. doi: 10.1046/j.1523-1755.63.s83.10.x. [DOI] [PubMed] [Google Scholar]
- 3.Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, Burns GC, D'Agati VD, Winston JA, Klotman ME, Klotman PE. Renal epithelium is a previously unrecognized site of HIV-1 infection. J.Am.Soc.Nephrol. 2000;11:2079–2087. doi: 10.1681/ASN.V11112079. [DOI] [PubMed] [Google Scholar]
- 4.D'Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18:406–421. [PubMed] [Google Scholar]
- 5.Ross MJ, Bruggeman LA, Wilson PD, Klotman PE. Microcyst formation and HIV-1 gene expression occur in multiple nephron segments in HIV-associated nephropathy. J.Am.Soc.Nephrol. 2001;12:2645–2651. doi: 10.1681/ASN.V12122645. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Gubler MC, Beaufils H. Dysregulation of podocyte phenotype in idiopathic collapsing glomerulopathy and HIV-associated nephropathy. Nephron. 2002;91:416–423. doi: 10.1159/000064281. [DOI] [PubMed] [Google Scholar]
- 7.Barisoni L, Kriz W, Mundel P, D'Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 8.Barisoni L, Bruggeman LA, Mundel P, D'Agati VD, Klotman PE. HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int. 2000;58:173–181. doi: 10.1046/j.1523-1755.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- 9.Conaldi PG, Biancone L, Bottelli A, Wade-Evans A, Racusen LC, Boccellino M, Orlandi V, Serra C, Camussi G, Toniolo A. HIV-1 kills renal tubular epithelial cells in vitro by triggering an apoptotic pathway involving caspase activation and Fas upregulation. J.Clin.Invest. 1998;102:2041–2049. doi: 10.1172/JCI3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodi I, Abraham AA, Kimmel PL. Apoptosis in human immunodeficiency virus-associated nephropathy. Am.J.Kidney Dis. 1995;26:286–291. doi: 10.1016/0272-6386(95)90648-7. [DOI] [PubMed] [Google Scholar]
- 11.Alpers CE. Light at the end of the TUNEL: HIV-associated thrombotic microangiopathy. Kidney Int. 2003;63:385–396. doi: 10.1046/j.1523-1755.2003.00743.x. [DOI] [PubMed] [Google Scholar]
- 12.Kapasi AA, Patel G, Franki N, Singhal PC. HIV-1 gp120-induced tubular epithelial cell apoptosis is mediated through p38-MAPK phosphorylation. Mol.Med. 2002;8:676–685. [PMC free article] [PubMed] [Google Scholar]
- 13.Ortiz A, Lorz C, Egido J. New kids in the block: the role of FasL and Fas in kidney damage. J.Nephrol. 1999;12:150–158. [PubMed] [Google Scholar]
- 14.Zheng Y, Ouaaz F, Bruzzo P, Singh V, Gerondakis S, Beg AA. NF-kappa B RelA (p65) is essential for TNF-alpha-induced fas expression but dispensable for both TCR-induced expression and activation-induced cell death. J.Immunol. 2001;166:4949–4957. doi: 10.4049/jimmunol.166.8.4949. [DOI] [PubMed] [Google Scholar]
- 15.Darville MI, Eizirik DL. Cytokine induction of Fas gene expression in insulin-producing cells requires the transcription factors NF-kappaB and C/EBP. Diabetes. 2001;50:1741–1748. doi: 10.2337/diabetes.50.8.1741. [DOI] [PubMed] [Google Scholar]
- 16.Chan H, Bartos DP, Owen-Schaub LB. Activation-dependent transcriptional regulation of the human Fas promoter requires NF-kappaB p50-p65 recruitment. Mol.Cell Biol. 1999;19:2098–2108. doi: 10.1128/mcb.19.3.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crist SA, Griffith TS, Ratliff TL. Structure/function analysis of the murine CD95L promoter reveals the identification of a novel transcriptional repressor and functional CD28 response element. J.Biol.Chem. 2003;278:35950–35958. doi: 10.1074/jbc.M306220200. [DOI] [PubMed] [Google Scholar]
- 18.Holtz-Heppelmann CJ, Algeciras A, Badley AD, Paya CV. Transcriptional regulation of the human FasL promoter-enhancer region. J.Biol.Chem. 1998;273:4416–4423. doi: 10.1074/jbc.273.8.4416. [DOI] [PubMed] [Google Scholar]
- 19.Matsui K, Fine A, Zhu B, Marshak-Rothstein A, Ju ST. Identification of two NF-kappa B sites in mouse CD95 ligand (Fas ligand) promoter: functional analysis in T cell hybridoma. J.Immunol. 1998;161:3469–3473. [PubMed] [Google Scholar]
- 20.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat.Rev.Mol.Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 21.Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-kappaB is the answer--role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 22.Campbell KJ, Rocha S, Perkins ND. Active repression of antiapoptotic gene expression by RelA(p65) NF-kappa B. Mol.Cell. 2004;13:853–865. doi: 10.1016/s1097-2765(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 23.Karn J. Tackling Tat. J.Mol.Biol. 1999;293:235–254. doi: 10.1006/jmbi.1999.3060. [DOI] [PubMed] [Google Scholar]
- 24.Roulston A, Lin R, Beauparlant P, Wainberg MA, Hiscott J. Regulation of human immunodeficiency virus type 1 and cytokine gene expression in myeloid cells by NF-kappa B/Rel transcription factors. Microbiol.Rev. 1995;59:481–505. doi: 10.1128/mr.59.3.481-505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruggeman LA, Adler SH, Klotman PE. Nuclear factor-kappa B binding to the HIV-1 LTR in kidney: implications for HIV-associated nephropathy. Kidney Int. 2001;59:2174–2181. doi: 10.1046/j.1523-1755.2001.00732.x. [DOI] [PubMed] [Google Scholar]
- 26.Martinka S, Bruggeman LA. Persistent NF-kB activation in renal epithelial cells in HIV-associated nephropathy. J Biol Chem in review. 2005 doi: 10.1152/ajprenal.00208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickie P, Felser J, Eckhaus M, Bryant J, Silver J, Marinos N, Notkins AL. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology. 1991;185:109–119. doi: 10.1016/0042-6822(91)90759-5. [DOI] [PubMed] [Google Scholar]
- 28.Kopp JB, Klotman ME, Adler SH, Bruggeman LA, Dickie P, Marinos NJ, Eckhaus M, Bryant JL, Notkins AL, Klotman PE. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc.Natl.Acad.Sci.U.S.A. 1992;89:1577–1581. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz EJ, Cara A, Snoeck H, Ross MD, Sunamoto M, Reiser J, Mundel P, Klotman PE. Human immunodeficiency virus-1 induces loss of contact inhibition in podocytes. J.Am.Soc.Nephrol. 2001;12:1677–1684. doi: 10.1681/ASN.V1281677. [DOI] [PubMed] [Google Scholar]
- 30.Mundel P, Reiser J, Mejia Zuniga, Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 31.Yao J, Le TC, Kos CH, Henderson JM, Allen PG, Denker BM, Pollak MR. Alpha-actinin-4-mediated FSGS: an inherited kidney disease caused by an aggregated and rapidly degraded cytoskeletal protein. PLoS.Biol. 2004;2:e167. doi: 10.1371/journal.pbio.0020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson PJ, Gelman IH, Klotman PE. Suppression of HIV-1 expression by inhibitors of cyclin-dependent kinases promotes differentiation of infected podocytes. J.Am.Soc.Nephrol. 2001;12:2827–2831. doi: 10.1681/ASN.V12122827. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz-Arduan A, Danoff TM, Kalluri R, Gonzalez-Cuadrado S, Karp SL, Elkon K, Egido J, Neilson EG. Regulation of Fas and Fas ligand expression in cultured murine renal cells and in the kidney during endotoxemia. Am.J.Physiol. 1996;271:F1193–F1201. doi: 10.1152/ajprenal.1996.271.6.F1193. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 35.Erkan E, Garcia CD, Patterson LT, Mishra J, Mitsnefes MM, Kaskel FJ, Devarajan P. Induction of Renal Tubular Cell Apoptosis in Focal Segmental Glomerulosclerosis: Roles of Proteinuria and Fas-Dependent Pathways. J.Am.Soc.Nephrol. 2005;16:398–407. doi: 10.1681/ASN.2003100861. [DOI] [PubMed] [Google Scholar]
- 36.Jarad G, Wang B, Khan S, DeVore J, Miao H, Wu K, Nishimura SL, Wible BA, Konieczkowski M, Sedor JR, Schelling JR. Fas activation induces renal tubular epithelial cell beta 8 integrin expression and function in the absence of apoptosis. J.Biol.Chem. 2002;277:47826–47833. doi: 10.1074/jbc.M204901200. [DOI] [PubMed] [Google Scholar]
- 37.Legembre P, Barnhart BC, Peter ME. The relevance of NF-kappaB for CD95 signaling in tumor cells. Cell Cycle. 2004;3:1235–1239. doi: 10.4161/cc.3.10.1194. [DOI] [PubMed] [Google Scholar]
- 38.Kreuz S, Siegmund D, Rumpf JJ, Samel D, Leverkus M, Janssen O, Hacker G, Dittrich-Breiholz O, Kracht M, Scheurich P, Wajant H. NFkappaB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. J.Cell Biol. 2004;166:369–380. doi: 10.1083/jcb.200401036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, Burns K, Hahne M, Kennedy N, Kovacsovics M, Tschopp J. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr.Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- 40.Vannier E, Dinarello CA. Histamine enhances interleukin (IL)-1-induced IL-6 gene expression and protein synthesis via H2 receptors in peripheral blood mononuclear cells. J.Biol.Chem. 1994;269:9952–9956. [PubMed] [Google Scholar]
- 41.Gougeon ML, Montagnier L. Programmed cell death as a mechanism of CD4 and CD8 T cell deletion in AIDS. Molecular control and effect of highly active anti-retroviral therapy. Ann.N.Y.Acad.Sci. 1999;887:199–212. doi: 10.1111/j.1749-6632.1999.tb07934.x. [DOI] [PubMed] [Google Scholar]
- 42.Badley AD, Pilon AA, Landay A, Lynch DH. Mechanisms of HIV-associated lymphocyte apoptosis. Blood. 2000;96:2951–2964. [PubMed] [Google Scholar]
- 43.Twu C, Liu NQ, Popik W, Bukrinsky M, Sayre J, Roberts J, Rania S, Bramhandam V, Roos KP, MacLellan WR, Fiala M. Cardiomyocytes undergo apoptosis in human immunodeficiency virus cardiomyopathy through mitochondrion- and death receptor-controlled pathways. Proc.Natl.Acad.Sci.U.S.A. 2002;99:14386–14391. doi: 10.1073/pnas.212327899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Y, Kulkosky J, Acheampong E, Nunnari G, Sullivan J, Pomerantz RJ. HIV-1-mediated apoptosis of neuronal cells: Proximal molecular mechanisms of HIV-1-induced encephalopathy. Proc.Natl.Acad.Sci.U.S.A. 2004;101:7070–7075. doi: 10.1073/pnas.0304859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel CA, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J.Virol. 2000;74:9717–9726. doi: 10.1128/jvi.74.20.9717-9726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghorpade A, Holter S, Borgmann K, Persidsky R, Wu L. HIV-1 and IL-1 beta regulate Fas ligand expression in human astrocytes through the NF-kappa B pathway. J.Neuroimmunol. 2003;141:141–149. doi: 10.1016/s0165-5728(03)00222-4. [DOI] [PubMed] [Google Scholar]
- 47.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 48.Grassme H, Kirschnek S, Riethmueller J, Riehle A, von Kurthy G, Lang F, Weller M, Gulbins E. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science. 2000;290:527–530. doi: 10.1126/science.290.5491.527. [DOI] [PubMed] [Google Scholar]
- 49.Shankland SJ, Wolf G. Cell cycle regulatory proteins in renal disease: role in hypertrophy, proliferation, and apoptosis. Am.J.Physiol Renal Physiol. 2000;278:F515–F529. doi: 10.1152/ajprenal.2000.278.4.F515. [DOI] [PubMed] [Google Scholar]
- 50.Hipfner DR, Cohen SM. Connecting proliferation and apoptosis in development and disease. Nat.Rev.Mol.Cell Biol. 2004;5:805–815. doi: 10.1038/nrm1491. [DOI] [PubMed] [Google Scholar]
- 51.Foo SY, Nolan GP. NF-kappaB to the rescue: RELs, apoptosis and cellular transformation. Trends Genet. 1999;15:229–235. doi: 10.1016/s0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- 52.Nelson PJ, Sunamoto M, Husain M, Gelman IH. HIV-1 expression induces cyclin D1 expression and pRb phosphorylation in infected podocytes: cell-cycle mechanisms contributing to the proliferative phenotype in HIV-associated nephropathy. BMC.Microbiol. 2002;2:e26. doi: 10.1186/1471-2180-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He JC, Husain M, Sunamoto M, D'Agati VD, Klotman ME, Iyengar R, Klotman PE. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J.Clin.Invest. 2004;114:643–651. doi: 10.1172/JCI21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sunamoto M, Husain M, He JC, Schwartz EJ, Klotman PE. Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int. 2003;64:1695–1701. doi: 10.1046/j.1523-1755.2003.00283.x. [DOI] [PubMed] [Google Scholar]
- 55.Stehbens WE. Oxidative stress in viral hepatitis and AIDS. Exp Mol.Pathol. 2004;77:121–132. doi: 10.1016/j.yexmp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Nakamura H, Masutani H, Yodoi J. Redox imbalance and its control in HIV infection. Antioxid.Redox.Signal. 2002;4:455–464. doi: 10.1089/15230860260196245. [DOI] [PubMed] [Google Scholar]