Abstract
Tanacetum vulgare L. (Tansy) has been extensively used in folk medicine for treatment of a variety of medical disorders. In the present study, we isolated and purified four acidic polysaccharide fractions (designated T-I to T-IV) from Tansy florets by the sequential use of hot-water extraction, ethanol precipitation, ultra-filtration, anion-exchange, and size-exclusion chromatography. The average Mr of fractions T-I through T-IV was estimated to be 326, 151, 64 and 9 kDa, respectively, as determined by high performance size-exclusion chromatography analysis. Sugar composition analysis revealed that Tansy polysaccharides consisted primarily of galacturonic acid, galactose, arabinose, and rhamnose. Fractions T-II through T-IV contained an arabinogalactan type II structure, as determined by reaction with Yariv reagent. High Mr fractions T-I and T-II exhibited potent macrophage/monocyte-activating activity, enhancing production of reactive oxygen species (ROS), nitric oxide (NO), and tumor necrosis factor α (TNF-α) by J774.A1 murine macrophages, and activating nuclear factor κB (NF-κB) in THP-1 human monocytes. In addition, Tansy polysaccharides stimulated human neutrophil function by greatly enhancing neutrophil myeloperoxidase (MPO) release. Furthermore, the low Mr fraction T-IV had potent complement-fixing activity, which may also contribute to the anti-inflammatory and would-healing properties of Tansy extracts. Taken together, our results provide a molecular basis to explain at least part of the beneficial therapeutic effects of Tansy extracts, and support the concept of using Tansy polysaccharides as an immunotherapeutic adjuvant.
Keywords: Tansy, Immunomodulator, Polysaccharide, Macrophage, Neutrophil, Complement-fixing activity, Reactive oxygen species, Myeloperoxidase
1. Introduction
Humans are continually exposed to a variety of pathogenic microorganisms, and protection from these microbes is achieved by a complex array of immune defense mechanisms [1]. Innate immunity serves as an essential first-line of defense against microbial pathogens and may also influence the nature of the subsequent adaptive immune response [1,2]. Phagocytic cells, such as macrophages and neutrophils, play a key role in innate immunity because of their ability to recognize, ingest, and destroy many pathogens by oxidative and non-oxidative mechanisms [3]. Thus, approaches designed to nonspecifically enhance innate immune mechanisms could increase defense against microbial infections.
During the past three decades, a number of bioactive polysaccharides and polysaccharide-protein complexes have been isolated from mushrooms, fungi, yeast, algae, lichens, and plants, and these compounds have attracted significant attention because of their immunomodulatory and anti-tumor effects. For example, botanical polysaccharides have been shown to increase macrophage cytotoxic activity against tumor cells and microorganisms, activate phagocytosis, increase reactive oxygen species (ROS) and nitric oxide (NO) production, and enhance secretion of a variety of cytokines and chemokines [4]. Moreover, most plant-derived polysaccharides are relatively non-toxic and do not cause severe side effects, which is a major problem associated with immunomodulatory bacterial polysaccharides and other synthetic compounds [5].
A common plant with beneficial medicinal properties is Tanacetum vulgare L. or Tansy, which belongs to the genus Tanacetum and the family Compositae (Asteraceae). Tansy is a perennial native to Europe and Asia; however, it was introduced to the United States for medicinal and horticultural purposes and now grows wild throughout many states. Extracts from this plant have been used extensively in folk medicine for treating rheumatism, ulcers, fever, and digestive disorders, and compounds isolated from this plant have also been reported to exhibit antibacterial and antihelminthic activity [6,7]. Indeed, a number of biologically active low Mr compounds, such as flavonoids and terpenoids, isolated from this species have been shown to be physiologically active [8]. In addition, polysaccharides isolated from Tansy have been reported to be anti-ulcerogenic and anti-atherogenic [7]. Interestingly, the latter activity has been suggested to be due to the ability of Tansy polysaccharides to bind low-density lipoprotein [7]. Aside from these studies, little else is known regarding the medicinal properties of Tansy polysaccharides, and essentially nothing is known regarding their potential immunomodulatory properties.
In the present studies, we isolated and purified four polysaccharide fractions from aqueous extracts of Tansy florets and investigated their effects on innate immune cell function. We found that Tansy polysaccharides had potent immunomodulatory and anti-inflammatory activities, including modulation of macrophage and neutrophil functions, as well as complement-fixation activity. Thus, the immunomodulatory activities of Tansy polysaccharides likely contribute to the known therapeutic effects of Tansy extracts.
2. Materials and methods
2.1. Reagents
β-glucosyl Yariv reagent [1,3,5-tri-(4-β-D-glucosopyranosyloxyphenyl-azo)-2,4,6-trihydroxybenzene] was purchased from Biosupplies Australia (Parkville, Australia). Gum arabic was purchased from Fluka BioChemica (Buchs, Switzerland) and 8-amino-5-chloro-7-phenylpyridol[3,4-d]pyridazine-1,4-(2H,3H)dione (L-012) was purchased from Wako Chemicals (Richmond, VA). Carbozole, sulfamic acid, DEAE-cellulose, Sepharose 6B, Sephadex G-50, galacturonic acid, galactose, arabinose, rhamnose, glucose, diphenylamine, aniline, anthrone, thiourea, trifluoroacetic acid (TFA), N-(1-naphthyl)ethylenediamine, sulfanilamide, horseradish peroxidase (HRP), Histopaque 1077, lipopolysaccharide (LPS) from Escherichia coli K-235, o-phenylene diamine, phorbol-12-myristate-13-acetate (PMA), antibody-sensitized sheep erythrocytes, sodium nitrite (NaNO2), and gelatin veronal buffer (GVB) were purchased from Sigma Chemical Co. (St. Louis, MO).
2.2 Purification and fractionation of polysaccharides
Florets of T. vulgare L. were collected around Bozeman, MT during the period of general flowering, and ∼1.1 kg were dried, ground, and extracted with 10 L boiling distilled H2O for 1 hr. The aqueous extract was centrifuged at 2,000×g for 15 min, and a 4-fold volume of ethanol was added to the supernatant to precipitate polysaccharides overnight at 4°C. The precipitate was pelleted by centrifugation, re-dissolved in distilled H2O, sonicated for 10 min, and centrifuged at 80,000×g for 1 hr. The supernatant was then filtered through a 0.22 μm filter and concentrated in an Amicon concentrator with a 5 kDa Amicon PM5 membrane (Millipore, Billerica, MA) to obtain a crude extract (yield of 1.8% by weight).
The crude polysaccharide extract was further purified using ion-exchange chromatography on a DEAE-cellulose column equilibrated with 0.05 M Tris-HCl buffer (pH 8.0). The column was washed with equilibration buffer, and bound material was eluted with equilibration buffer containing 2 M NaCl. The eluate was concentrated and then fractionated by size-exclusion chromatography (SEC) on a Sepharose 6B column (2.5×92 cm) equilibrated with 0.01 M sodium citrate buffer (pH 7.0) containing 0.15 M NaCl and eluted with the same buffer at a flow rate of 22 ml/hr. The last fraction collected was concentrated and further separated by SEC on a Sephadex G-50 column (2.5×92 cm) equilibrated and eluted with 0.01 M Tris-HCl buffer (pH 7.4) containing 0.15 M NaCl at a flow rate of 22 ml/hr.
The carbohydrate elution profile was determined by the phenol H2SO4 method [9], modified to a microplate format, and absorbance was measured at 488 nm using a SpectraMax Plus microplate reader (Molecular Devices, Palo Alto, CA). Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Pierce Biotechnology, Inc., Rockford, IL), according to the manufacturer's protocol. The relevant fractions were pooled and concentrated. For analysis of biological activity, the fractions were diluted in Hanks balanced salt solution (HBSS) to a concentration of 5 mg/ml and filtered through sterile 0.22 μm filters.
2.3. Characterization of polysaccharide fractions
Pooled fractions were analyzed for protein content using a modified Lowry assay with bovine serum albumin as the standard [10]. Uronic acid content was determined by the sulfamate–carbozole method [11]. Possible LPS contamination in the samples was evaluated using a Limulus Amebocyte Lysate (LAL) assay kit (Cambrex, Walkersville, MD).
For nuclear magnetic resonance (NMR) analysis, samples (5 mg) were dissolved in deuterium oxide (0.5 ml), and 1H NMR spectra were recorded on a Bruker DRX-600 spectrometer (Bruker BioSpin, Billerica, MA) at 20°C using 3-(trimethylsilyl)-propionic 2,2,3,3,-d4 acid sodium salt as an internal reference.
The presence of arabinogalactan in the samples was detected radial gel diffusion in 1% agarose gels containing 100 μg/ml β-glucosyl Yariv reagent, which selectively interacts with and precipitates compounds containing type II arabinogalactan structures [12]. Four μl of the polysaccharide samples (10 mg/ml) were loaded into the wells, and the samples were incubated at room temperature for 24 hr in a humid atmosphere. A positive reaction was identified by a reddish circle around the well, and arabic gum (4 mg/ml) served as a positive control.
The homogeneity and average Mr of the polysaccharide fractions were determined by high performance size-exclusion chromatography (HP-SEC) using a Shimadzu Class VP HPLC and Shodex OHpak SB-804 HQ column (8 mm × 300 mm) or TSK-GEL G3000W×I column (7.8 mm × 300 mm) eluted with 50 mM sodium citrate buffer, pH 7.5, containing 0.15 M NaCl and 0.01% NaN3 at a flow rate of 0.3 ml/min. Peaks were detected using a refractive index detector (RID-10A; Shimadzu, Torrance, CA). Average Mr of the polysaccharide fractions was estimated by comparison with the retention times of pullulan standards (P-800, 400, 200, 100, 50, 20, and 10; Phenomenex, Torrance, CA) or polyethylene glycol standards (PEG-11000, 5000, 3600, 1000, 600; Pressure Chemical Co., Pittsburgh, PA).
For monosaccharide composition analysis, samples were hydrolyzed at 100°C for 6 hr with 3 M TFA, and the resulting samples were separated by thin-layer chromatography (TLC) on Whatman silica gel 60 plates with monosaccharide standards for reference [13]. The TLC plates were developed with butanol/acetic acid/water (3:1:1), and bands were visualized by spraying the plates with aniline-diphenylamine reagent (2% aniline, 2% diphenylamine, and 8.5% H3PO4 acid in acetone) and heating at 100°C for 10 min. Individual monosaccharide bands were scraped from the plate, extracted with H2O, and quantified using a colorimetric method with monosaccharide standards. Briefly, the extracts were mixed with anthrone reagent (0.2% anthrone and 1% thiourea in H2SO4). After heating at 100°C for 10 min, absorbance was measured at 620 nm.
2.4. Cell culture
Murine macrophage J774.A1 cells were cultured in Dulbecco's Modified Eagle's Medium (Invitrogen) supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 μg/ml streptomycin, and 100 U/ml penicillin at 37°C in a humidified atmosphere containing 5% CO2. Cells were grown to confluence in sterile tissue culture flasks and gently detached by scraping. Cell number and viability were assessed microscopically using trypan blue exclusion.
Human monocyte THP1-Blue cells obtained from InvivoGen (San Diego, CA) were cultured in RPMI 1640 medium supplemented with fetal bovine serum (10% v/v), zeocin (100 μg/ml) and blasticidin S (10 μg/ml) at 37° C in a humidified atmosphere containing 5% CO2. These cells are stably transfected with a secreted embryonic alkaline phosphatase (SEAP) gene that is under the control of a promoter inducible by nuclear factor κB (NF-κB).
2.5. Analysis of NF-κB activation
Activation of NF-κB was measured using an alkaline phosphatase reporter gene assay in THP1-Blue human monocytic cells. One week before an experiment, the cells were preactivated overnight with PMA (50 ng/ml) to induce differentiation and plated in 96-well plates at a cell density of 2×105 cells/well. The cells were then incubated for 5 days with daily media changes. On day 7, Tansy polysaccharide fractions or LPS (50 ng/ml) were added, the cells were incubated for 24 hr, and alkaline phosphatase activity was measured in cell supernatants using QUANTI-Blue mix (InvivoGen, San Diego, CA). Activation of NF-κB is reported as the absorbance at 655 nm and compared with positive control samples (LPS).
2.6. Isolation of human neutrophils
Human neutrophils were purified in accordance with a protocol approved by the Institutional Review Board at Montana State University. Briefly, neutrophils were isolated from peripheral blood of healthy donors using dextran sedimentation followed by Histopaque 1077 gradient separation and hypotonic lysis of erythrocytes, as described previously [14]. Isolated neutrophils were washed and resuspended in HBSS without Ca2+ and Mg2+. Cell viability was determined by trypan blue exclusion (>98%), and purity was determined by light microscopy (>95%).
2.7. Analysis of NO production
J774.A1 cells were plated at a density of 1.5×105 cells/well in a final volume of 200 μl in 96-well flat-bottomed tissue culture plates and incubated in medium alone or medium containing various concentrations of polysaccharide fractions or E. coli LPS as a positive control. Cells were incubated at 37°C and 5% CO2 for 24 hr, and 100 μl of the cell culture supernatants were removed and analyzed for NO using a colorimetric method with NaNO2 as the standard [15]. Briefly, supernatants were mixed with an equal volume of Griess reagent, which was prepared by mixing one part of 0.1% (w/v) N-(1-naphthyl)ethylenediamine with one part of 1% (w/v) sulfanilamide in 5% H3PO4 acid. After 20 min, absorbance was measured at 540 nm using a SpectraMax Plus microplate reader.
2.8. Analysis of ROS production
Macrophage ROS production was analyzed using the chemiluminescent probe, L-012, which is highly sensitive for ROS generated in biologically complex systems [16]. Murine J774.A1 macrophages (1.5×105 cells/well) were incubated with various concentrations of polysaccharide fractions or positive control (LPS) for 24 hr. After incubation, 100 μl of culture supernatant (removed for NO analysis) was replaced by an equal volume of HBSS supplemented with 25 μM L-012 and 5 μg/ml HRP, as described previously [17]. The reaction was monitored on a Fluoroscan Ascent FL microtiter plate reader (ThermoElectron, Milford, MA) at 37°C. Chemiluminescence was measured every 2 min for 2 hr and is expressed as the integrated response over this time (arbitrary units).
2.9. Determination of TNF-α
J774.A1 cells were incubated at a density of 1.5×105 cells/well in final volume of 200 μl for 24 h with or without Tansy polysaccharide fractions or LPS. A mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences Pharmigen) was used to detect TNF-α in the cell supernatants. TNF-α concentration was determined by extrapolation from the TNF-α standard curve, according to the manufacturer's protocol.
2.10. Cytotoxicity assay
Cytotoxicity was analyzed with a CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Inc., Madison, WI), according to the manufacturer's protocol. Briefly, J774.A1 cells were cultured at a density of 3×104 cells/well with the polysaccharide fractions for 24 hr at 37°C and 5% CO2. After centrifugation of the samples at 80×g for 5 min, the media was aspirated, and the cells were washed three times with HBSS. Substrate was added, and the samples were analyzed with a Fluoroscan Ascent FL.
2.11. Complement fixation assay
The complement fixation assay was performed as previously described by Diallo et al. [18]. Antibody-sensitized sheep erythrocytes were washed three times with GVB containing 0.5 mM Mg2+ and 0.15 mM Ca2+ (GVB2+) before use. The erythrocytes were resuspended in GVB2+ at a concentration of 2×108 cells/ml, and human serum was diluted with GVB2+ to a concentration giving about 50% hemolysis. Triplicate samples containing 50 μl of each serially-diluted polysaccharide fraction were mixed with 50 μl diluted serum and added to microplate wells and incubated at 37°C. After 30 min, sensitized sheep erythrocytes (50 μl) were added to each well, and the samples were incubated for an additional 30 min at 37°C. After centrifugation (900×g for 5 min), 50 μl of the supernatants were mixed with 200 μl distilled H2O in flat-bottom microplates, and absorbance was measured at 405 nm. 100% lysis was obtained by adding distilled H2O to sensitized sheep erythrocytes. Samples containing GVB2+, serum, and sensitized sheep erythrocytes were used as background controls (Acontrol), while heparin served as a positive control. Inhibition of hemolysis induced by the test samples was calculated by the formula: [(Acontrol - Asample)/ Acontrol]×100%.
2.12. Determination of myeloperoxidase (MPO) activity
Human neutrophils (4×105 cells/well) were incubated in a final volume of 160 μl at 37°C for 1 h with or without Tansy polysaccharide fractions or PMA. After centrifugation of the samples at 80×g for 5 min, 100 μl of the cell culture supernatants were removed and analyzed for MPO activity. Briefly, supernatants were mixed with o-phenylene diamine (0.4 mg/ml) and 1.5 mM H2O2 in phosphate-citrate buffer (pH 5.0). The reaction was stopped after 10 min by addition of 0.1 M H2SO4, and absorbance was measured at 492 nm [19].
3. Results
3.1. Preparation and characterization of Tansy polysaccharides
Acidic polysaccharides obtained from Tansy extracts were fractionated by preparative Sepharose 6B size-exclusion chromatography to obtain four main fractions, which were selected based on total carbohydrate and protein elution profile (designated as T-I, T-II, T-III, and T-IV) (Fig. 1A). Since fraction T-IV contained >40% protein (Table 1), we further separated this fraction by Sephadex G-50 chromatography into three sub-fractions (designated as T-IV-1, T-IV-2, and T-IV-3) (Fig. 1B). Sub-fraction T-IV-3 was almost all protein and contained very little carbohydrate; whereas, fraction T-IV-1 was high in carbohydrate and contained little protein (Table 1).
Fig. 1.
Fractionation of Tansy polysaccharides by size-exclusion chromatography. Panel A. Crude Tansy polysaccharide extract was isolated by DEAE-cellulose chromatography, and the extract was further fractionated using Sepharose 6B column chromatography. Four fractions (designated T-I through T-IV) were collected. Panel B. Fraction T-IV was further purified using Sephadex G-50, and three fractions (designated T-IV-1 through T-IV-3) were selected for further analysis. Total carbohydrate content of the fractions was determined by a phenol-H2SO4 method (detected at 488 nm), while protein content was analyzed using the BCA protein assay (detected at 562 nm).
Table 1.
Biochemical properties of polysaccharide fractions isolated from Tansy
| Polysaccharide Fraction | Average Mr (kDa) | Yariv Test | Protein Content (%) | LPS Content (ng/mg) |
|---|---|---|---|---|
| T-I | 326 | Negative | 1.2 | < 1.0 |
| T-II | 151 | Positive | 3.1 | < 2.5 |
| T-III | 64 | Positive | 7.0 | < 2.5 |
| T-IV | 9 | Positive | 41.2 | < 1.0 |
| T-IV-1 | 42 | Positive | 13.2 | < 1.0 |
| T-IV-2 | 5 | Negative | 39.4 | < 1.0 |
| T-IV-3 | 2 | Negative | 54.0 | < 1.0 |
Analytical HP-SEC of the individual fractions showed that each fraction was represented by a broad, generally symmetrical peak on the chromatograms, suggesting the polysaccharide fractions were relatively homogeneous (data not shown). Analysis of pullulan standards (Mr from 5.9 to 788 kDa) and polyethylene glycol standards (Mr from 0.6 to 11 kDa) resulted in linear calibration curves that allowed us to determine average Mr of the fractions. As shown on Table 1, average Mr of polysaccharide fractions T-I through T-IV was 326, 151, 64 and 9 kDa, respectively, and average Mr of sub-fractions T-IV-1, 2, 3 was 42, 5, 2 kDa, respectively. Note that the average Mr of fractions T-IV-2 and T-IV-3 was ≤5 kDa, which is the pore size in the membrane used for ultrafiltration of the crude sample. Based on the high protein content of these fractions (see Table 1 and Fig. 1B), it is likely that fraction T-IV consisted of non-covalent protein:polysaccharide complexes that dissociated under the buffer/salt/mechanical conditions of the final Sephadex G-50 chromatography step. Indeed, previous studies have shown that charge effects, solution conditions, and membrane surface characteristics can impact ultrafiltration and SEC fractionation, with various organic components being affected differently [20,21].
Analysis of the main fractions using the Yariv test showed that fractions T-II, T-III, and T-IV contained type II arabinogalactan; whereas, fraction T-I tested negative for the presence of arabinogalactan (Table 1). Likewise, analysis of T-IV sub-fractions using the Yariv test showed that only the high molecular weight component (sub-fraction T-IV-1) contained arabinogalactan (Table 1).
Uronic acid was detected in all four main fractions using a sulfamate–carbazole assay, suggesting galacturonic acid (GalA) residues were present in Tansy polysaccharides. The samples were also tested for possible LPS contamination using the LAL assay. As shown in Table 1, all fractions contained <2.5 ng/mg endotoxin, which is considered to be insignificant for various bioactive products [22,23].
Analysis of sugar composition revealed that Tansy polysaccharides consisted primarily of galacturonic acid (GalA), galactose (Gal), arabinose (Ara), and rhamnose (Rha), with GalA and Gal being the dominant monosaccharides (Table 2). These data are in general agreement with the monosaccharide composition of tanacetan, a previously reported polysaccharide from Tansy [24]. Rha and GalA may constitute a rhamnogalacturonan backbone that is either associated with or might be covalently linked to arabinogalactan [25].
Table 2.
Sugar composition of polysaccharide fractions isolated from Tansy
| Polysaccharide Fraction | Galactose (mol %) | Galacturonic Acid (mol %) | Arabinose (mol %) | Rhamnose (mol %) |
|---|---|---|---|---|
| T-I | 44.2 | 39.2 | 13.1 | 3.4 |
| T-II | 48.9 | 27.8 | 19.8 | 3.5 |
| T-III | 38.0 | 41.9 | 18.0 | 2.1 |
| T-IV | 35.9 | 22.8 | 38.4 | 2.9 |
Monosaccharides were identified and quantified based on TLC analysis of known standards.
Very-high-field (600 MHz) 1H NMR was used to characterize the structure of the native Tansy polysaccharides (Fig. 2). The spectra of all of the fractions were similar to each other, suggesting a common backbone structure, and resembled the spectra of tanacetan reported previously [24]. The weak signals present at 3.38-3.45 ppm can be assigned to α-rhamnopyranose (α-Rha p), while the strong signals at 3.68-3.90 ppm were consistent with the presence of β-galactopyranose (β-Gal p) [24]. The signals at 4.05-5.05 ppm were consistent with the presence of α-arabinofuranose (α-Ara f) and α-galacturonopyranose (α-GalA p) residues [24,26]. Additionally, the signals at 1.14-1.20 and 1.97–2.08 ppm can be assigned to methyl groups and N/O-acetyl groups, respectively. Signals corresponding aromatic protons (5.48-8.35 ppm) were observed only in fractions T-IV and T-IV-3. The presence of aromatic resonances is consistent with the high protein content of these two fractions (see Table 1). The paired doublet at 2.48-2.55 ppm in the spectra of fractions T-I through T-IV is due to the presence of sodium citrate bound to the polysaccharides (Fig. 2A, arrow).
Fig. 2.
1H NMR spectra of Tansy polysaccharide fractions. Fractions T-I through T-IV (Panel A) and T-IV-1 through T-IV-3 (Panel B) were dissolved in D2O, and spectra were recorded at 20°C.
3.2. Effect of Tansy polysaccharides on macrophage NO and ROS production
A minimum amount of NO was produced when murine J774.A1 macrophages were incubated with medium alone; whereas, treatment of these cells with polysaccharide fractions T-I, T-II, and T-III resulted in a dose-dependent increase in NO production, with fraction T-II being the most active (Fig. 3A). Indeed, NO production induced by fraction T-II was comparable to that induced by 50 ng/ml LPS. In contrast, fraction T-IV polysaccharides failed to induce NO production, even at the maximal dose.
Fig. 3.
Modulatory effects of Tansy polysaccharide fractions on murine macrophage ROS and NO production. J774.A1 macrophages were incubated for 24 hr with the indicated concentrations of polysaccharide fractions or 50 ng/ml LPS (positive control). NO production was quantified by measuring nitrite in the cell-free supernatants (Panel A), and ROS were monitored using a chemiluminescence detection system, as described (Panel B). Values are the mean ± S.D. of three replicates.
In the absence of any treatment, murine macrophages generated very low levels of ROS (Fig. 4, control); whereas, a dose-dependent enhancement of ROS production was observed in macrophages treated with 200-1600 μg/ml of polysaccharide fractions T-I, T-II, and T-III (Fig. 3B and 4), with fraction T-II being the most active fraction. No stimulation of ROS production was observed with fraction T-IV. Thus, the individual polysaccharide fractions showed a similar pattern with respect to their ability to induce NO and ROS production by murine J774.A1 macrophages.
Fig. 4.
Kinetics of ROS production in murine macrophages stimulated by Tansy polysaccharides. J774.A1 cells were incubated for 24 hr with medium alone (control) or polysaccharide fraction T-I (1600 μg/ml), and chemiluminescence was monitored, as described. Values are the mean ± S.D. of three replicates.
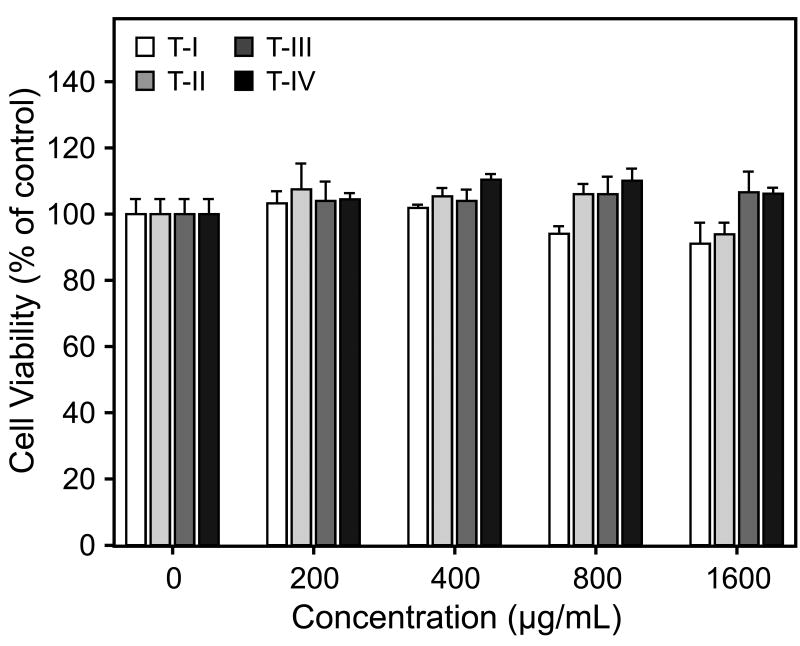
We confirmed that the differential effects of Tansy polysaccharide fractions on macrophage responses were not due to cytotoxic effects on the cells using a cytotoxicity assay. None of the Tansy polysaccharide fractions inhibited proliferation/viability of J774.A1 cells over the entire concentration range of Tansy polysaccharides tested (200-1600 μg/ml) (Fig. 5).
Fig. 5.
Effects of Tansy polysaccharide fractions on cell proliferation. J774.A1 cells were cultured for 24 hr with the indicated concentrations of polysaccharide fractions, and cell viability was determined using the luminescent cell viability assay kit, as described. Values are the mean ± S.D. of three replicates.
3.3. Effect of Tansy polysaccharides on macrophage TNF-α production
A number of immunomodulatory compounds modulate macrophage cytokine and/or chemokine production. Thus, we analyzed the effects of Tansy polysaccharide fractions on murine macrophage TNF-α production. Untreated murine J774.A1 macrophages produced negligible amounts of TNF-α; whereas, incubation of these cells with polysaccharide fractions T-I and T-II modestly enhanced the TNF-α production (Fig. 6). In contrast, fractions T-III and T-IV failed to stimulate this response. As expected, LPS, a strong cytokine inducer, greatly enhanced TNF-α production by murine macrophages (Fig. 6).
Fig. 6.
Modulatory effects of Tansy polysaccharide fractions on murine macrophage TNF-α production. Cultured J774.A1 macrophages were incubated for 24 hr with the indicated concentrations of polysaccharide fractions or 50 ng/ml LPS (positive control). Cell-free supernatants were collected, and extracellular TNF-α secretion was quantified by ELISA, as described. Values are the mean ± S.D. of three replicates.
Effects of Tansy polysaccharide fractions on cell proliferation. J774.A1 cells were cultured for 24 hr with the indicated concentrations of polysaccharide fractions, and cell viability was determined using the luminescent cell viability assay kit, as described. Values are the mean ± S.D. of three replicates.
3.4. Effect of Tansy polysaccharides on the complement system
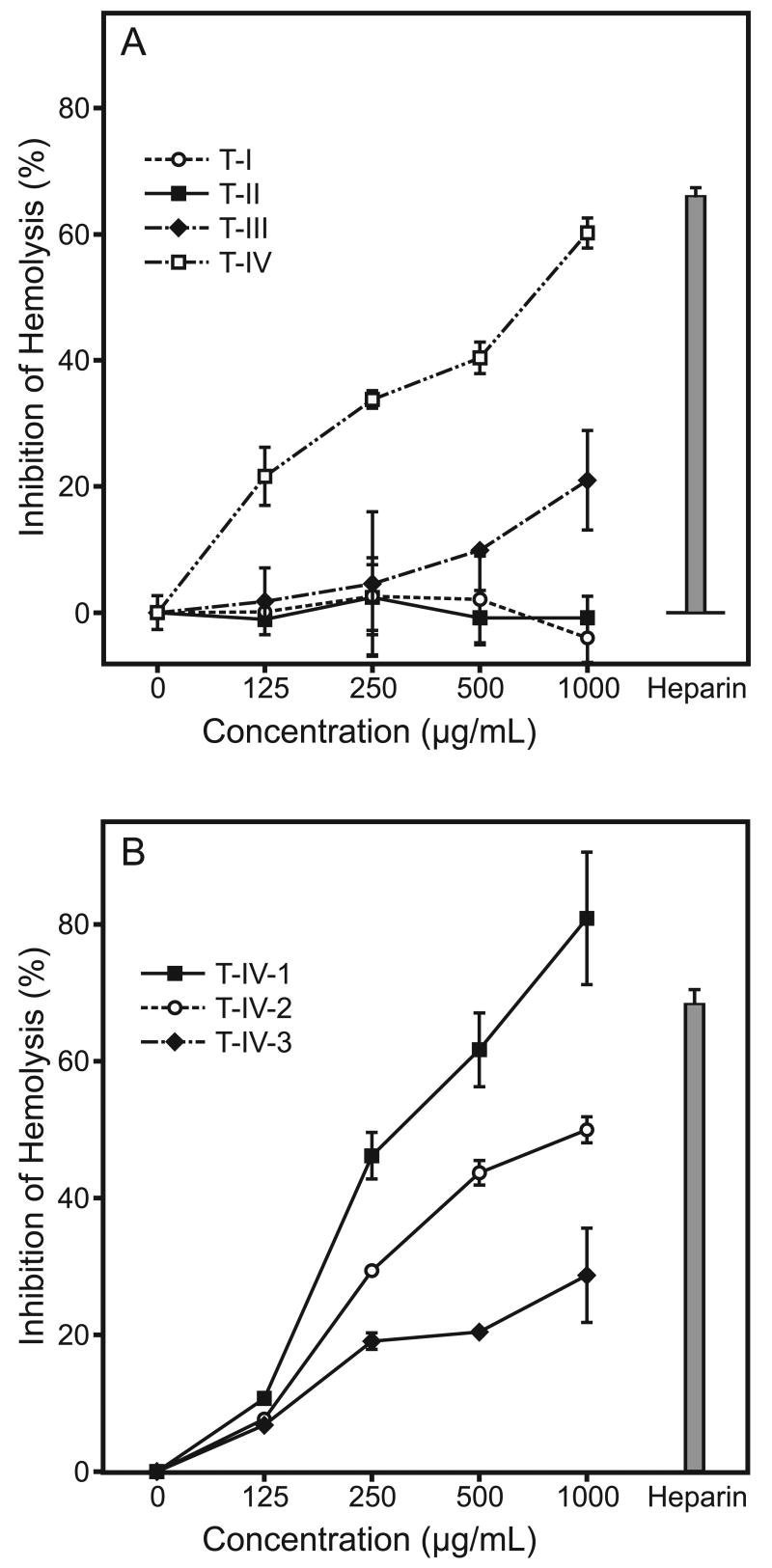
Tansy polysaccharide fractions were tested for their ability to fix complement, as compared to heparin, a known complement fixing agent. As shown in Fig. 7A, addition of fraction T-IV and, to a lesser degree, T-III resulted in dose-dependent inhibition of erythrocyte hemolysis, indicating that these fractions exhibited potent complement fixing activity. Indeed, the level of hemolysis inhibition at 1000 μg/ml of T-IV was comparable to that induced by the positive control heparin (Fig. 7A). In contrast, fractions T-I and T-II failed to fix complement, even at the maximal concentrations.
Fig. 7.
Complement fixing activity of Tansy polysaccharide fractions. The indicated concentrations of each polysaccharide fraction were analyzed for their ability to fix complement, as described. Heparin (150 μg/ml) served as positive control. Values are the mean ± S.D. of three replicates.
To determine whether the carbohydrate or protein component of fraction T-IV contributed to the anti-complement activity, we further evaluated complement fixing activity of the three sub-fractions purified from fraction T-IV. Sub-fraction T-IV-1, which had the least protein, exhibited the highest activity; whereas, sub-fraction T-IV-3, which had the highest protein content, was the least active (Fig. 7B). Thus, these results suggest that the carbohydrate moiety of fraction T-IV was responsible for this biological activity.
3.5. Effect of Tansy polysaccharides on neutrophil MPO release
To determine whether human phagocytes also respond to Tansy polysaccharides, we analyzed polysaccharide effects on MPO release by human neutrophils, which is indicative of granule mobilization. Treatment of neutrophils with all the fractions resulted in a dose-dependent enhancement of MPO release (Fig. 8A). Indeed, the level of MPO release induced by 800 μg/ml of fractions T-I, T-II, and T-IV was comparable to that induced by 100 nM PMA (Fig. 8A). Thus, these results demonstrate an important role for enhancing neutrophil function in the immunomodulatory properties of Tansy polysaccharides. The three sub-fractions purified from fraction T-IV were also tested for their ability to modulate neutrophil function. Sub-fractions T-IV-1 and T-IV-2 dose-dependently stimulated MPO release; whereas, fraction T-IV-3 was much less active (Fig. 8B).
Fig. 8.
Effects of Tansy polysaccharide fractions on neutrophil MPO release. The indicated concentrations of each polysaccharide fraction were incubated with human neutrophils (4×105 cells/well) for 60 min at 37°C. MPO activity was analyzed spectrophotometrically in the cell supernatant as described. PMA (100 nM) served as positive control. Values are the mean ± S.D. of three replicates.
3.6. Effect of Tansy polysaccharides on activation of NF-κB in THP-1 monocytic cells
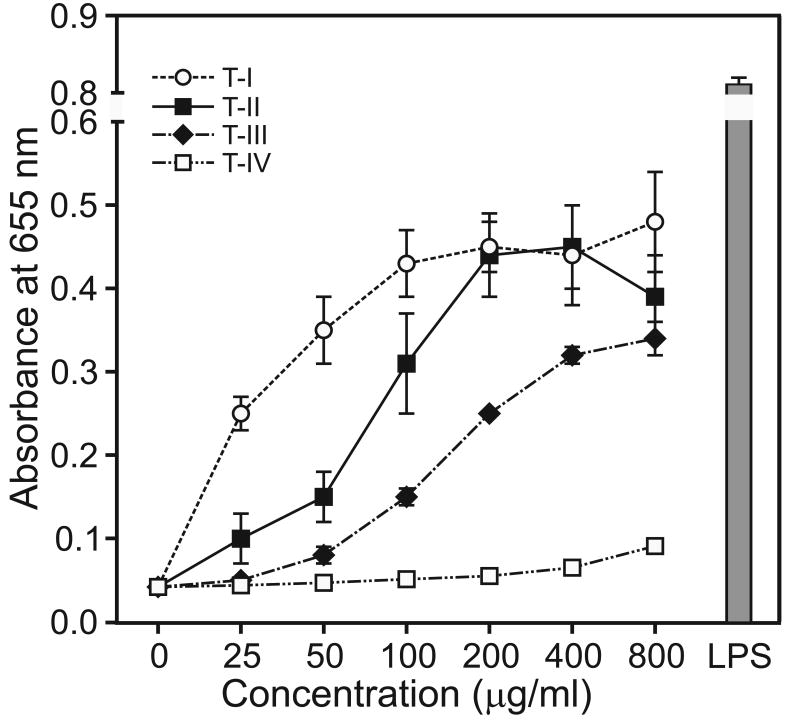
To evaluate signaling pathways involved in the immunomodulatory activity of Tansy polysaccharides, we utilized a transcription factor-based bioassay for NF-κB activation in human THP-1 monocytes. Tansy polysaccharide fractions T-I, T-II, and T-III dose-dependently stimulated NF-κB, as determined by monitoring alkaline phosphatase release (Figure 9), indicating that these polysaccharides potently stimulated the NF-κB activation pathway. Alkaline phosphatase release induced by fractions T-I and T-II was ∼50% of that induced by 50 ng/ml LPS. In contrast, treatment with fraction T-IV resulted in negligible NF-κB activation, even at high concentrations of this polysaccharide fraction.
Fig. 9.
Effects of Tansy polysaccharide fractions on NF-κB activity. Human THP1-Blue monocytes (5×105 cells/well) were incubated for 24 hr with the indicated concentrations of each polysaccharide fraction or 50 ng/ml LPS (positive control). Alkaline phosphatase release was analyzed spectrophotometrically in the cell supernatant, as described. Values are the mean ± S.D. of three replicates.
4. Discussion
Tansy is a commonly grown domestic remedy that is reported to be useful in treating a variety of medical problems, such as kidney weakness, stomach problems, fever, hysteria [6]. Despite the widespread use of Tansy, little is known regarding the active component(s) responsible for its therapeutic effects. In this study, we demonstrate that acidic polysaccharides extracted from Tansy florets can enhance macrophage and neutrophil function and, for one fraction, fix complement, suggesting these properties may contribute to the therapeutic efficacy of Tansy extracts.
We provide initial structural and pharmacological characterization of polysaccharides purified from Tansy florets. All Tansy polysaccharide fractions, except for the high Mr fraction A-I, were type II arabino-3,6-galactans. Type II arabinogalactans have a β-(1,3)-linked galactan backbone with side chains containing arabinose and galactose residues and have been reported to possess a variety of biological activities. Indeed, Nergard et al. [27] proposed that the composition of arabinogalactan side chains is related to the biological activity of plant polysaccharides, and we recently found that biologically-active polysaccharides isolated from Juniperus scopolorum contained type II arabinogalactan [28].
Tansy polysaccharide fraction T-IV and its sub-fraction T-IV-3 exhibited potent complement fixing activity, which may also contribute to the therapeutic potential of Tansy extracts. In this assay, interaction of the antibody with complement protein initiates the sequential complement-activation pathway, resulting in the formation of membrane attack complex (MAC) that leads to erythrocyte lysis. Previous studies showed that Tansy polysaccharides have lipoprotein-binding activity, which was proposed to contribute to their antiatherogenic activity [7]. Likewise, a similar protein-binding property could enable Tansy polysaccharides to bind or fix complement protein, thereby preventing formation of MAC and inhibiting hemolysis. Indeed, Xu et al. [29] recently found that interaction of polysaccharide from Bupleurum smithii with complement proteins resulted in anti-complement activity. The complement system plays an essential role in innate immunity, contributing to inflammatory responses and the destruction and removal of pathogens (reviewed in [30]). However, excessive or uncontrolled complement activation can also contribute to host tissue damage, and therapeutic strategies have been developed to inhibit this process [31]. Likewise, the removal of complement by polysaccharide fixation has also been proposed to be a potential therapeutic strategy for treating inflammatory diseases [27]. A number of reports have shown polysaccharides from different plants can enhance wound healing, and some of these polysaccharides also have anti-complement activity (reviewed in [4]). For example, Samuelsen et al. [32] reported that the wound healing properties of Plantago major L. polysaccharides were at least partly due to their ability to fix complement. Similarly, wound healing properties of polysaccharides from Biophytum petersianum Klotzsch were reported to be related to their effects on the complement system [33]. Indeed, Wagner [34] suggested that the anti-complement properties of plant-derived polysaccharides significantly contribute to their anti-inflammatory properties. Thus, the anti-complement activity of Tansy polysaccharides may represent an important biological activity that could also be considered for further medicinal applications.
Macrophages play key roles in host defense, including phagocytosis of pathogens and apoptotic cells, production of cytokines, and the proteolytic processing and presentation of foreign antigens (reviewed in [35]). Consequently, the identification of substances that can modulate macrophages is of great interest, and it is well established that a wide range of plant polysaccharides exhibit beneficial pharmacological effects via their ability to modulate macrophage function (reviewed in [4]). Thus, it is not surprising that Tansy polysaccharide fractions exhibited macrophage immunomodulatory properties. Fraction T-I and T-II exhibited the most potent immunoenhancing activity and stimulated murine J774.A1 macrophages to produce significant levels of NO and ROS. Both NO and ROS play important roles in a variety of physiological processes and are key participants in host defense (reviewed by [36,37]). In addition, these reactive oxidants have also been shown to be involved in the regulation of apoptosis and immune homeostasis [38].
Tansy polysaccharide fractions T-I and T-II greatly enhanced macrophage production of TNF-α, which is an important cytokine involved in innate immune responses against pathogens [39]. TNF-α plays a key role in inflammation and can act on monocytes and macrophages in an autocrine manner to enhance various functional responses and induce the expression of additional immunoregulatory and inflammatory mediators [40]. Thus, the modulation of macrophage TNF-α production likely contributes to part of the therapeutic effects of Tansy extracts and confirms that Tansy polysaccharides have potent immunomodulatory properties.
Neutrophils are principal effectors of the initial host response to injury or infection and constitute a significant threat to invading bacterial pathogens (reviewed in [41]). Thus, it is significant that Tansy polysaccharides also stimulated neutrophil degranulation and MPO release. Neutrophil granules contain a multitude of antimicrobial and potentially cytotoxic substances that are delivered to the phagosome or to the exterior of the cell following degranulation [42]. MPO is a key enzyme released into the phagosome or the extracellular space upon neutrophil activation, and plays an important role in microbicidal activity by catalyzing the formation of various ROS that can attack microorganisms [43]. Thus, the ability to stimulate neutrophil MPO release could represent an important immunomodulatory property of Tansy polysaccharides. Likewise, other botanical polysaccharides have previously been shown to stimulate neutrophil degranulation. For example, Ross et al. [44] showed that β-glucans from yeast bind to iC3b-receptors (CR3, CD11b/CD18) of neutrophils, stimulating degranulation. Similarly, polysaccharides from Silene vulgaris were found to increase neutrophil MPO activity [19].
Tansy polysaccharides potently activated transcription factor NF-κB in THP-1 human monocytes. Activation of NF-κB controls multiple genes in phagocytes, and target genes regulated by NF-κB include proinflammatory cytokines, chemokines, inflammatory enzymes, adhesion molecules, receptors, and inhibitors of apoptosis (reviewed in [1,45]). Therefore, the ability to activate phagocyte NF-κB signaling provides further evidence that Tansy polysaccharides possess immunomodulatory properties, and confirmed that human phagocytes respond to these compounds. In support of this finding, a number of polysaccharides have been reported previously to activate NF-κB. For example, high Mr polysaccharides from Aloe vera increased NF-κB directed luciferase expression in THP-1 cells [46]. Likewise, high Mr polysaccharides from Spirulina platensis, Aphanizomenon flos-aquae, and Chlorella pyrenoidosa were found to activate NF-κB, leading to increased immune cytokine mRNA levels [47]. It is well known the activation of NF-κB plays a critical role in the transcriptional regulation of TNF-α and inducible NO synthase (iNOS) genes [45]. In addition, TNF-α [48] and NO [49] generated upon cell activation can further activate the NF-κB pathway. Thus, it is reasonable that Tansy polysaccharide fractions T-I and T-II dose-dependently stimulated both macrophage TNF-α and NO production and also activated NF-κB in a similar pattern. In future studies, it will be interesting to determine whether these polysaccharides enhanced production of proinflammatory mediators (e.g., TNF-α and NO) through activation of NF-κB and/or vice versa.
In summary, we isolated four polysaccharide fractions from Tansy florets and demonstrated that these polysaccharides exhibited potent macrophage-activating activities, resulting in modulation of NO, ROS, and TNF-α production. Tansy polysaccharides also stimulated neutrophil MPO release and activated NF-κB signaling in monocytic cells. Lastly, some Tansy polysaccharides had potent complement-fixation activity. Taken together, our data provide a molecular basis to explain at least part of the beneficial therapeutic effects reported for Tansy extracts, suggesting that modulation of phagocytes by Tansy polysaccharides could lead to a decreased susceptibility to microbial infection.
Acknowledgments
We would like to thank Dr. Scott Busse, Montana State University, Bozeman, MT for help in running NMR samples and Liliya Kirpotina, Laura Nelson-Overton, and Daniel Siemsen for technical support. This work was supported in part by Department of Defense grant W9113M-04-1-0001, National Institutes of Health grant RR020185, an equipment grant from the M.J. Murdock Charitable Trust, and the Montana State University Agricultural Experimental Station. The U.S. Army Space and Missile Defense Command, 64 Thomas Drive, Frederick, MD 21702 is the awarding and administering acquisition office. The content of this report does not necessarily reflect the position or policy of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–59. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–74. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 3.Tosi MF. Innate immune responses to infection. J Allerg Clin Immunol. 2005;116:241–49. doi: 10.1016/j.jaci.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 4.Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6:317–33. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Ooi VE, Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr Med Chem. 2000;7:715–29. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- 6.Abad MJ, Bermejo P, Villar A. An approach to the genus Tanacetum L. (Compositae): Phytochemical and pharmacological review. Phytother Res. 1995;9:79–92. [Google Scholar]
- 7.Polle AY, Ovodova RG, Shashkov AS, Ovodov YS. Isolation and general characterization of polysaccharides from tansy Tanacetum vulgare L. Russ J Bioorg Chem. 2001;27:45–49. [Google Scholar]
- 8.Abad MJ, Bermejo P, Villar A. The activity of flavonoids extracted from Tanacetum microphyllum DC. (Compositae) on soybean lipoxygenase and prostaglandin synthetase. Gen Pharmacol. 1995;26:815–19. doi: 10.1016/0306-3623(94)00242-f. [DOI] [PubMed] [Google Scholar]
- 9.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 10.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 11.Filisetti-Cozzi TM, Carpita NC. Measurement of uronic acids without interference from neutral sugars. Anal Biochem. 1991;197:157–62. doi: 10.1016/0003-2697(91)90372-z. [DOI] [PubMed] [Google Scholar]
- 12.van Holst GJ, Clarke AE. Quantification of arabinogalactan-protein in plant extracts by single radial gel diffusion. Anal Biochem. 1985;148:446–50. doi: 10.1016/0003-2697(85)90251-9. [DOI] [PubMed] [Google Scholar]
- 13.Dogsa I, Kriechbaum M, Stopar D, Laggner P. Structure of bacterial extracellular polymeric substances at different pH values as determined by SAXS. Biophys J. 2005;89:2711–20. doi: 10.1529/biophysj.105.061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauss KA, Bunger PL, Crawford MA, McDermott BE, Swearingen R, et al. Variants of the 5′-untranslated region of human NCF2: Expression and translational efficiency. Gene. 2006;366:169–79. doi: 10.1016/j.gene.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Keller R, Geiges M, Keist R. L-arginine-dependent reactive nitrogen intermediates as mediators of tumor cell killing by activated macrophages. Cancer Res. 1990;50:1421–25. [PubMed] [Google Scholar]
- 16.Imada I, Sato EF, Miyamoto M, Ichimori Y, Minamiyama Y, et al. Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal Biochem. 1999;271:53–58. doi: 10.1006/abio.1999.4107. [DOI] [PubMed] [Google Scholar]
- 17.Schepetkin IA, Kirpotina LN, Khlebnikov AI, Quinn MT. High-throughput screening for small-molecule activators of neutrophils: Identification of novel N-formyl peptide receptor agonists. Mol Pharm. 2007;71:1061–74. doi: 10.1124/mol.106.033100. [DOI] [PubMed] [Google Scholar]
- 18.Diallo D, Paulsen BS, Liljeback TH, Michaelsen TE. Polysaccharides from the roots of Entada africana Guill. et Perr., Mimosaceae, with complement fixing activity. J Ethnopharmacol. 2001;74:159–71. doi: 10.1016/s0378-8741(00)00361-5. [DOI] [PubMed] [Google Scholar]
- 19.Popov SV, Popova GY, Ovodova RG, Bushneva OA, Ovodov YS. Effects of polysaccharides from Silene vulgaris on phagocytes. Int J Immunopharmacol. 1999;21:617–24. doi: 10.1016/s0192-0561(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 20.Buffle J, Leppard GG. Characterization of aquatic colloids and macromolecules. 2. Key role of physical structures on analytical results. Environ Sci Technol. 1995;29:2176–84. doi: 10.1021/es00009a005. [DOI] [PubMed] [Google Scholar]
- 21.Schafer AI, Mauch R, Waite TD, Fane AG. Charge effects in the fractionation of natural organics using ultrafiltration. Environmental Science & Technology. 2002;36:2572–80. doi: 10.1021/es0016708. [DOI] [PubMed] [Google Scholar]
- 22.Jahr TG, Ryan L, Sundan A, Lichenstein HS, Skjak-Braek G, et al. Induction of tumor necrosis factor production from monocytes stimulated with mannuronic acid polymers and involvement of lipopolysaccharide-binding protein, CD14, and bactericidal/permeability-increasing factor. Infect Immun. 1997;65:89–94. doi: 10.1128/iai.65.1.89-94.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catchpole B, Hamblin AS, Staines NA. Autologous mixed lymphocyte responses in experimentally-induced arthritis of the Lewis rat. Autoimmunity. 2002;35:111–17. doi: 10.1080/08916930290016619. [DOI] [PubMed] [Google Scholar]
- 24.Polle AY, Ovodova RG, Shashkov AS, Ovodov YS. Some structural features of pectic polysaccharide from tansy, Tanacetum vulgare L. Carbohydrate Polymers. 2002;49:337–44. doi: 10.1023/a:1021810010222. [DOI] [PubMed] [Google Scholar]
- 25.Samuelsen AB, Paulsen BS, Wold JK, Knutsen SH, Yamada H. Characterization of a biologically active arabinogalactan from the leaves of Plantago major L. Carbohydrate Polymers. 1998;35:145–53. [Google Scholar]
- 26.Gane AM, Craik D, Munro SL, Howlett GJ, Clarke AE, et al. Structural analysis of the carbohydrate moiety of arabinogalactan-proteins from stigmas and styles of Nicotiana alata. Carbohydr Res. 1995;277:67–85. doi: 10.1016/0008-6215(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 27.Nergard CS, Diallo D, Michaelsen TE, Malterud KE, Kiyohara H, et al. Isolation, partial characterisation and immunomodulating activities of polysaccharides from Vernonia kotschyana Sch. Bip. ex Walp. J Ethnopharmacol. 2004;91:141–52. doi: 10.1016/j.jep.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Schepetkin IA, Faulkner CL, Nelson-Overton LK, Wiley JA, Quinn MT. Macrophage immunomodulatory activity of polysaccharides isolated from Juniperus scopolorum. Int Immunopharmacol. 2005;5:1783–99. doi: 10.1016/j.intimp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Zhang Y, Zhang J, Chen D. Isolation and characterization of an anti-complementary polysaccharide D3-S1 from the roots of Bupleurum smithii. Int Immunopharmacol. 2007;7:175–82. doi: 10.1016/j.intimp.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–98. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Mollnes TE, Kirschfink M. Strategies of therapeutic complement inhibition. Mol Immunol. 2006;43:107–21. doi: 10.1016/j.molimm.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Samuelsen AB, Paulsen BS, Wold JK, Otsuka H, Yamada H, et al. Isolation and partial characterization of biologically-active polysaccharides from Plantago major L. Phytother Res. 1995;9:211–18. [Google Scholar]
- 33.Inngjerdingen KT, Coulibaly A, Diallo D, Michaelsen TE, Paulsen BS. A complement fixing polysaccharide from Biophytum petersianum Klotzsch, a medicinal plant from Mali, West Africa. Biomacromolecules. 2006;7:48–53. doi: 10.1021/bm050330h. [DOI] [PubMed] [Google Scholar]
- 34.Wagner H. Search for plant derived natural-products with immunostimulatory activity (Recent advances) Pure Appl Chem. 1990;62:1217–22. [Google Scholar]
- 35.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. Am J Physiol Cell Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 37.Quinn MT, Ammons MC, DeLeo FR. The expanding role of NADPH oxidases in health and disease: no longer just agents of death and destruction. Clin Sci. 2006;111:1–20. doi: 10.1042/CS20060059. [DOI] [PubMed] [Google Scholar]
- 38.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–97. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 39.Ghezzi P, Cerami A. Tumor necrosis factor as a pharmacological target. Mol Biotechnol. 2005;31:239–44. doi: 10.1385/MB:31:3:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baugh JA, Bucala R. Mechanisms for modulating TNFα in immune and inflammatory disease. Curr Opin Drug Discov Devel. 2001;4:635–50. [PubMed] [Google Scholar]
- 41.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 42.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–27. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Faurschou M, Kamp S, Cowland JB, Udby L, Johnsen AH, et al. Prodefensins are matrix proteins of specific granules in human neutrophils. J Leukoc Biol. 2005;78:785–93. doi: 10.1189/jlb.1104688. [DOI] [PubMed] [Google Scholar]
- 44.Ross GD, Vetvicka V, Yan J, Xia Y, Vetvicková J. Therapeutic intervention with complement and β-glucan in cancer. Immunopharmacology. 1999;42:61–74. doi: 10.1016/s0162-3109(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 45.Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Pugh N, Ross SA, ElSohly MA, Pasco DS. Characterization of Aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J Agric Food Chem. 2001;49:1030–1034. doi: 10.1021/jf001036d. [DOI] [PubMed] [Google Scholar]
- 47.Pugh N, Ross SA, ElSohly HN, ElSohly MA, Pasco DS. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001;67:737–42. doi: 10.1055/s-2001-18358. [DOI] [PubMed] [Google Scholar]
- 48.Grivennikov SI, Kuprash DV, Liu ZG, Nedospasov SA. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: From simple paradigms to complex mechanisms. Int Rev Cytol. 2006;252:129–61. doi: 10.1016/S0074-7696(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 49.Persichini T, Cantoni O, Suzuki H, Colasanti M. Cross-talk between constitutive and inducible NO synthase: an update. Antioxid Redox Signal. 2006;8:949–54. doi: 10.1089/ars.2006.8.949. [DOI] [PubMed] [Google Scholar]