Abstract
Local cooling of the brain by the respiratory air is found in many animal species. The mechanism is based on cooling of the nasal vein blood and heat transfer in the cavernous sinus/carotid artery complex and is therefore not active in anaesthetised, intubated animals. The present experiment was made to investigate the effects of oxygen flushing of the nasal cavities in such animals. Nine anaesthetised, intubated male pigs were used. The temperatures in the third ventricle and rectum were measured continuously. Oxygen was infused into the nasal cavities during 10 min periods interrupted by 10 min without flow. The nasal oxygen flow constantly induced a rapid, reversible and flow dependant decrease in brain temperature: 0.25°C ± 0.04, (n = 2) (mean ± SD, n) at <4 l/min; 1.35°C ± 0.78, (n = 20) at 4–6 l/min; and 1.44°C ± 0.62, (n = 6) at >6 l/min. The ventricle temperature decreased 0.59°C ± 0.23, (n = 8) when the animals were transferred to spontaneous respiration and the tracheal tube removed. It may be possible to protect the brain in intubated animals and humans from heat-induced damages by establishment of nasal flushing.
Keywords: carotid artery, cavernous sinus, rete mirabile.
Introduction
Many animal species have developed selective brain cooling mechanisms [3,14]. Respiratory air cools the surface of the nasal cavities, and thus nasal venous blood has a lower temperature than general body temperature. The blood may pass through a superficial or profound route (v. supraorbitalis) to the deep venous sinuses at the base of the brain. Cooled venous nasal blood following the profound route passes through the cavernous venous sinus (CS) on its way to the jugular vein. The internal carotid artery (ICA) penetrates the CS, where the cool venous blood decreases ICA temperature in the counter current transfer system formed. The cooler arterial blood will decrease the brain temperature 1–3°C compared to body temperature [1].
In the pig, the ICA forms a rete mirabile (a set of small parallel arteries) within the CS [18]. The rete increases the area of contact between the arterial and venous blood. If cooling is not needed, the blood will be redirected to the superficial veins, thus avoiding passage of the CS-ICA complex. The human does not have a rete mirabile and it is controversial, whether a local brain cooling mechanism exists in man [5,7].
It has been found that brain and nervous tissue are adversely affected by hyperthermia [10]. Mild hypothermia protects brain function from ischemic and traumatic brain injury, whereas mild hyperthermia worsens ischemic outcome. Brain hyperthermia is known to occur in humans with stroke, cardiac arrest, head injuries, fever, heat stroke, and global ischemia and may aggravate brain damage by increasing vascular permeability leading to oedema, ischemia, and entry of blood-borne excitatory substances including amino acids and calcium ions [10].
Intubation of animals obviously decreases the airflow through the nose. A decrease of the brain cooling may be expected. Intubation may therefore cause an unexpected side effect: the brain arterial blood may not be cooled and the brain reach dangerous high temperatures.
The present experiment in intubated pigs was performed to measure the effect on deep brain temperature of nasal flushing with oxygen.
Materials and methods
Nine immature male pigs (Danish Landrace, 40–50 kg) were obtained and kept in individual cages with free access to water and commercial pig pellets. Pigs were starved overnight before anaesthetic induction with: 1 mL/30 kg BW atropine sc; 1 mL/10 kg BW Dormicum® im (Midazol 5 mg/mL); 1 mL/10 kg BW Sedaperon® im (Azaperone 40 mg/mL); followed 15 min later by approx. 20 mL Rapinovet® (Propofol 10 mg/mL) iv. The pigs were intubated, placed in ventral recumbence on a 38°C water madras, and connected to a respirator. Anaesthesia was maintained with halothane (0.8–2%), O2 30% and N2O 70%. The halothane concentration was adjusted throughout the experiment attempting to maintain the minimum level where the respiratory movements of the animal were suppressed to allow respiration to be depending exclusively on the pump. At this level no pain-related reflexes (corneal, heart rate, blood pressure) are observed. Isotonic saline (3–500 mL/h) was given into an ear vein. The heart rate and ECG were monitored continuously. The pigs were kept at a normal pCO2 based on regular venous blood gas samples through adjustments of the respiratory volume provided by the respirator.
A bore hole 14-mm was made in the skull, with a manual drill, through the parietal bone 20 mm from the midline and 20 mm above the lamboid suture. A disposable catheter (Becker EDMS ventricular Catheter, Medtronic) for intraventricular brain pressure measurements was inserted approximately 4 cm with the tip pointing towards the third ventricle. The correct position of the tip within the third ventricle was verified when free back-flow of CNS fluid was observed after which the metal guide was removed. A Cu/CuNi thermoprobe (diameter 0.8 mm, ELLAB, Roedovre, Denmark; http://www.ELLAB.com, model MAA-08500-A, modified with a male luer connector 18 cm from the tip) was inserted into the catheter. Due to the connector, the tip of the thermoprobe was always 5 mm from the tip of the ventricle catheter (at the level of the side holes). Additionally, the connector prevented leakage of CNS fluid.
A rectal thermoprobe (ELLAB MRB-04020-A) was inserted 10 cm. A tympanic thermoprobe (ELLAB MEA-22130-A) was inserted as far as possible. Since the ear canal bend in pigs, it was not possible to verify the exact position of the tip. The probes were fixated with surgical tape.
A soft catheter was inserted approximately 5 cm into each nostril. The catheters were connected to the oxygen outlet via a flowmeter. A needle shaped thermoprobe (ELLAB MKI-15025-A) was inserted close to the nostrils into the plastic tube carrying oxygen. The 4 thermoprobes were connected to a thermometer (ELLAB TM9604) and temperatures were recorded every 2 sec onto an IBM ThinkPad 365X equipped with ELLAB software (PCSOFT96). The changes in the temperatures were calculated from printouts from each experiment.
An airflow (2–10 l/min) was applied for 10-min periods interrupted by 10-min pause. A session of 1-hour duration was included in 2 animals. An air-cooling device based on counter current water cooling could be connected to or bypassed by the oxygen via 2 three-way stop-cocks. The nasal oxygen would therefore be either 23°C or 8°C. An infusion pump equipped with 2 syringes and thin plastic catheters was occasionally used to infuse saline (1 mL/min) into each nasal cavity together with the oxygen to keep the nasal surface humidified.
In 2 pigs, the position of the catheter was documented by a ventriculogram using Omnipaque 300 before taking an X-ray. During the last part of the experiment, the halothane was switched off. When the animal started resisting the respirator, anaesthesia was maintained by phenemal (20 mg/mL) iv, the respirator disconnected, and the tracheal tube removed to obtain spontaneous respiration through the nose. The pig was then euthanised 10 min later by an i. v. overdose of phenemal (200 mg/mL, 20 ml).
Results
Brain temperature was similar to the rectal temperature during the last minute of the control periods (no oxygen flushing). Flushing the nasal cavities with oxygen constantly induced a decrease in brain temperature in all 9 intubated animals. The decrease was observed within minutes, reached a steady state after 5–8 min and reversed within minutes after ceasing the gas flow (Fig. 1). The decrease was flow dependant: 0.25°C ± 0.04, (n = 2) (mean ± sd, (n)) at <4 l/min; 1.35°C ± 0.78, (20) at 4–6 l/min; and 1.44°C ± 0.62, (6) at >6 l/min (Fig. 2 and 3). Cooling or humidifying the gas had no obvious effect (Fig 4). Cooling was observed throughout the 2 one-hour oxygen flushing sessions. Only when using high oxygen flow rates did we observe a small decrease in the tympanic and rectal temperatures.
Figure 1.
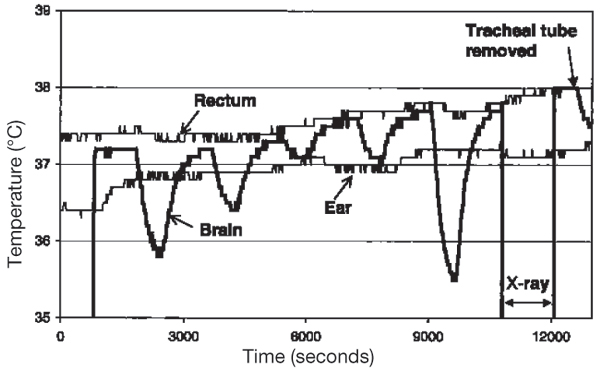
Brain, rectal and ear temperatures in an anaesthetised, intubated pig. The nasal cavities were flushed with oxygen (2–10 l/min) in repeated 10-min periods. The brain temperature was measured in the third ventricle; the position of the probe was controlled by a x-ray. The tracheal tube was removed at the end of the experiment.
Figure 2.
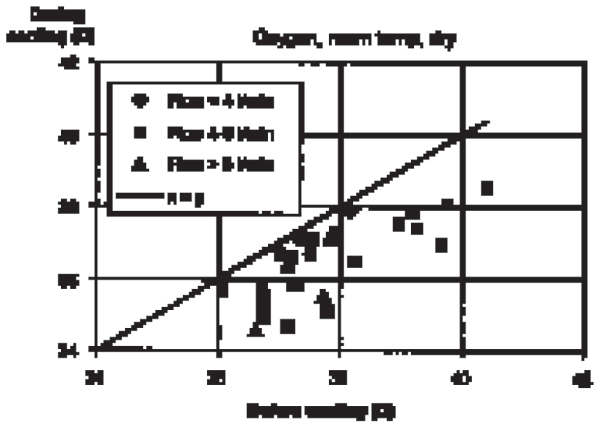
Decrease of brain temperature (third ventricle) in anaesthetised, intubated pigs during nasal flushing with oxygen (2–10 l/min, room temperature, dry).
Figure 3.
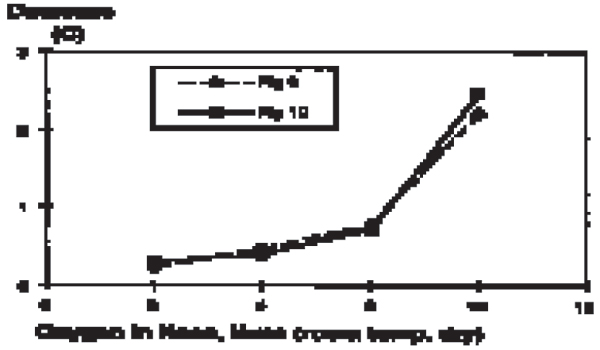
Flow dependent decrease in brain temperature (third ventricle) in anaesthetised, intubated pigs.
Figure 4.
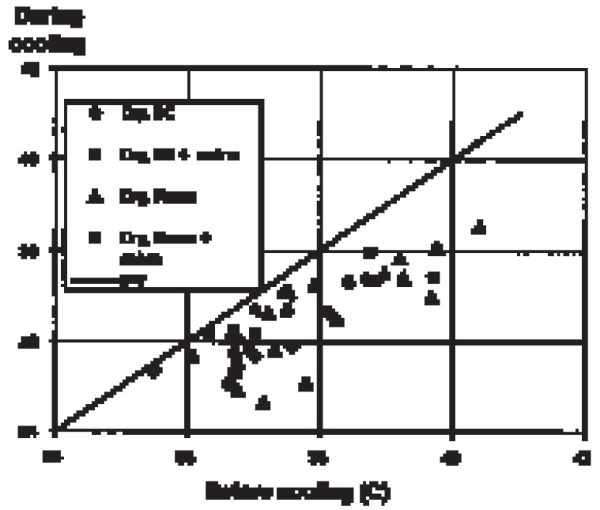
Effect of nasal flushing with oxygen (4–6 l/min, dry or humidified, room temperature or 8°C) on brain temperature (third ventricle) in anaes-thetised, intubated pigs.
Brain temperature decreased 0.59°C ± 0.23, 8 when the pigs were transferred from artificial respiration through the tracheal catheter to spontaneous respiration through the nose (Fig 5).
Figure 5.
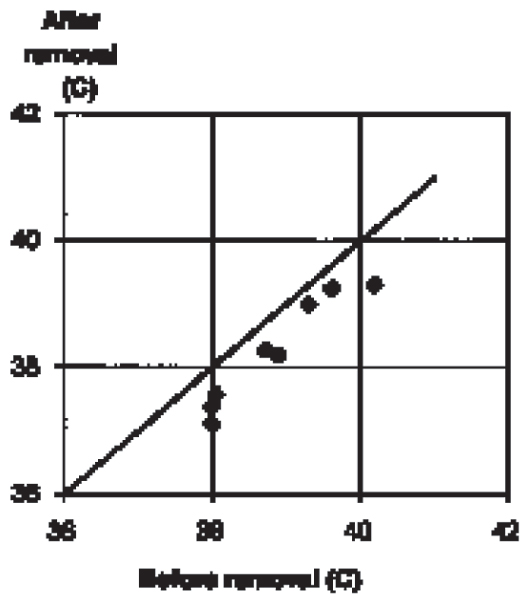
Decrease of brain temperature (third ventricle) following extubation of anaesthetised, intubated pigs. The animals started breathing through the nose. The average decrease in 8 animals was 0.59°C ± 0.23.
Discussion
These experiments show that nasal flushing with oxygen in intubated pigs induces a rapid and reversible cooling of the brain without influencing body temperature. The rapid temperature changes in the third ventricle suggest that the blood circulation carries the cooling effect and that the cooling is not based on heat diffusion from the nose to the centre of the brain through tissues alone. The statements are supported by our previous experiments in rats with a similar technique showing identical cooling irrespectively of whether 2 brain probes were positioned 3 cm apart in either a sagital or a frontal plane [13]. Similarly, a change in the respiratory mode of the pigs from breathing through a tracheal tube to spontaneous ventilation through the nose decreased the brain temperature 0.6°C. Obviously intubation effectively bypasses ventilation through the nasal cavities and therefore the brain-cooling mechanisms, leaving the brain vulnerable to hyperthermia. In contrast to a similar experiment carried out on rats [13], the present study demonstrated that the registered body temperature hardly was affected by nasal flushing with oxygen. It is therefore suggested that the size of the animal exposed to the nasal flushing with oxygen does play a role for the body temperature. Unfortunately, it is not a routine procedure to measure body temperature during large animal anaesthesia; hardly any information is available about the brain temperature. Overheating may unintentionally be induced in large animals during surgery by excessive use of sterile draping as it prevents heat loss. The ears of large pigs should not be covered as they form a large cooling surface, which may induce a lower temperature in the jugular vein blood than in rectum (Einer-Jensen et al. 1992). A rare "allergic" hyperthermic reaction with a genetic background has been found during halothane anaesthesia in pigs (Laizzo 1996). The brain cooling system may delay brain damage slightly but cannot prevent the dramatic increase in temperature.
The rectum is often used to measure body temperature while the tympanic temperature has been taken to express the deep brain temperature [6,9,15]. The present experiment showed that the tympanic temperature was lower than both brain and rectal temperatures, and that the tympanic temperature did not change during the oxygen flushing. The latter may suggest that the tympanic temperature in our hands reflected body temperature (as previously indicated by [19] rather than brain temperature. Due to the bending of the auditory canal in pigs, the position of the probe may have been too inaccurate to reflect the tympanic temperature.
It is not clear whether the cooling mechanism is present in man. It is in general assumed that the rectal temperature is identical to the brain temperature. Some indications of cooling have been found [7], but the results are disputed [5]. It is not even certain that the anatomy of the CS-ICA complex in man is developed to a stage where transfer is possible. However, the potential damage due to overheating of the brain following intubation and the potential benefit of nasal flushing with oxygen in hyperthermic patients warrants further investigation.
Hypothermia has been demonstrated to function as an effective therapeutic tool to reduce infarct volume in experimental animals with cerebral ischemia or/and brain injuries [2,4,11,16,23]. To induce brain cooling in man several techniques including cooling the blood supply of the brain, nasopharyngeal cooling, head cooling, and ice water immersion [8,20-22] have been utilised. The brain temperature was, in general, not measured; the conclusions have instead been based on changes of the body temperature.
In conclusion, the brain temperature in pigs is partly independent of the body temperature due to the local cooling of the arterial brain blood induced by the nasal airflow. The hypotheses should be tested in other animals and man.
Acknowledgments
Acknowledgements
The technical assistance of Ms Lise Larsen is appreciated.
References
- Baker MA. Brain cooling in endotherms in heat and exercise. An R Physiol. 1982;44:85–96. doi: 10.1146/annurev.ph.44.030182.000505. [DOI] [PubMed] [Google Scholar]
- Baker CJ, Onesti ST, Solomon RA. Reduction by delayed hypothermia of cerebral infarction following middle cerebral artery occlusion in the rat: a time-course study. J Neurosurg. 1992;77:438–444. doi: 10.3171/jns.1992.77.3.0438. [DOI] [PubMed] [Google Scholar]
- Bamford OS, Eccles R. The role of sympathetic efferent activity in the regulation of brain temperature. Pflugers Arch. 1983;396:138–143. doi: 10.1007/BF00615518. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ, White RF. Brain cooling during transient focal ischemia provides complete neuroprotection. Neurosci Biobehav Rev. 1997;21:31–44. doi: 10.1016/0149-7634(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Brengelmann GL. Specialised brain cooling in humans? FASEB J. 1993;7:1148–1152. doi: 10.1096/fasebj.7.12.8375613. [DOI] [PubMed] [Google Scholar]
- Burgess KR, Evans JA, Whitelaw WA. No evidence for hypothalamic cooling during nasal cold air breathing in man. Clin Invest Med. 1988;11:134–138. [PubMed] [Google Scholar]
- Cabanac M. Selective brain cooling in humans: "fancy" or fact? FASEB J. 1993;7:1143–1146. doi: 10.1096/fasebj.7.12.8375612. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Jiang JY, Lyeth BG, Jenkins LW, Hamm RJ, Hayes RL. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab. 1991;11:114–121. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- Dickson JA, McKenzie A, McLeod K. Temperature gradients in pigs during whole-body hyperthermia at 42 degrees C. J Appl Physiol. 1979;47:712–717. doi: 10.1152/jappl.1979.47.4.712. [DOI] [PubMed] [Google Scholar]
- Dietrich WD. The importance of brain temperature in cerebral injury. J Neurotrauma Suppl. 1992;2:475–485. [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Globus MY, Ginsberg MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol (Berl) 1994;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- Einer-Jensen N, Hunter RHF, Boegh I, Greve T. Temperature gradients between the jugular vein blood and rectum in anaesthetized, intubated pigs. J Anim Physiol Anim Nutr. 1999;82:305–310. doi: 10.1046/j.1439-0396.1999.00247.x. [DOI] [Google Scholar]
- Einer-Jensen N, Khorooshi MH. Cooling of the brain through oxygen flushing of the nasal cavities in intubated rats: an alternative model for treatment of brain injury. Exp Brain Res. 2000;130:244–247. doi: 10.1007/s002219900230. [DOI] [PubMed] [Google Scholar]
- Elkhawad O. Selective brain cooling in desert animals: the camel (Camelus dromedarius) Comp Biochem Physiol Comp Physiol. 1992;101:195–201. doi: 10.1016/0300-9629(92)90522-R. [DOI] [PubMed] [Google Scholar]
- Haaland K, Steen PA, Thoresen M. Cerebral, tympanic and colonic thermometry in the piglet. Reprod Fertil Dev. 1996;8:125–128. doi: 10.1071/RD9960125. [DOI] [PubMed] [Google Scholar]
- Huh PW, Belayev L, Zhao W, Koch S, Busto S, Ginsberg MD. Comparative neuroprotective efficacy of prolonged moderate intraischemic and postischemic hypothermia in focal cerebral ischemia. J Neurosurg. 2000;92:91–99. doi: 10.3171/jns.2000.92.1.0091. [DOI] [PubMed] [Google Scholar]
- Iaizzo PA, Kehler CH, Zink RS, Belani KG, Sessler DI. Thermal response in acute porcine malignant hyperthermia. Anesth-Analg. 1996;82:782–9. doi: 10.1097/00000539-199604000-00019. [DOI] [PubMed] [Google Scholar]
- Jessen C. Brain cooling: an economy mode of temperature regulation in artiodactyls. News Physiol Sci. 1998;13:281–286. doi: 10.1152/physiologyonline.1998.13.6.281. [DOI] [PubMed] [Google Scholar]
- Jørgensen PF, Willeberg P, m Jensen P, Hansen LL, Northeved Continuous monitering of body temperature in pigs using non-invasive ear canal sensors. Acta Vet Scand. 1986;27:456–60. doi: 10.1186/BF03548159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen G, Bauer R, Walter B. Controlled brain hypothermia by extracorporeal carotid blood cooling at normothermic trunk temperatures in pigs. J Neurosci Methods. 1999;89:167–174. doi: 10.1016/S0165-0270(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Leonov Y, Sterz F, Safar P, Radovsky A, Oku K, Tisherman S, Stezoski SW. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10:57–70. doi: 10.1038/jcbfm.1990.8. [DOI] [PubMed] [Google Scholar]
- Mellergaard P. Changes in human intracerebral temperature in response to different methods of brain cooling. Neurosurgery. 1992;31:671–677. doi: 10.1227/00006123-199210000-00009. [DOI] [PubMed] [Google Scholar]
- Minamisawa H, Smith ML, Siesjo BK. The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of fore-brain ischemia. Ann Neurol. 1990;28:26–33. doi: 10.1002/ana.410280107. [DOI] [PubMed] [Google Scholar]


