Abstract
Cryptosporidium is a common cause of gastroenteritis and is associated with severe life-threatening illness among immunocompromised individuals. This review aimed to assess the efficacy of interventions for the treatment and prevention of cryptosporidiosis among immunocompromised patients. A search of Medline, Embase and other electronic databases was carried out up to August 2005. Two reviewers independently extracted data and assessed study quality. The relative risk for each intervention was calculated. Seven trials involving 169 participants were included. Nitazoxanide and paramomycin were associated with a relative risk (RR) of reduction in the duration and frequency of diarrhoea of 0.83 [95% confidence interval (CI) 0.36, 1.94] and 0.74 (95% CI 0.42, 1.31), respectively, showing no evidence of effectiveness. Nitazoxanide led to significant evidence of oocyst clearance compared with placebo with a RR of 0.52 (95% CI 0.30, 0.91). The effect was not significant for HIV-seropositive participants (RR 0.71, 95% CI 0.36, 1.37). HIV-seronegative participants on nitazoxanide had a significantly higher relative risk of achieving parasitological clearance of 0.26 (95% CI 0.09, 0.80) based on a single study. No other intervention was associated with either a reduction in diarrhoea, mortality or a significant parasitological response. This review confirms the absence of evidence for effective agents in the management of cryptosporidiosis. The results indicate that nitaxozanide reduces load of parasites and may be useful in immunocompetent individuals. The absence of effective therapy highlights the importance of preventive interventions in this group of patients.
Keywords: cryptosporidiosis, meta-analysis, systematic review, therapeutics
Introduction
Cryptosporidiosis in humans is usually caused by the coccidial parasites Cryptosporidium hominis and Cryptosporidium parvum. Cryptosporidiosis affects immunocompetent, particularly children under the age of 5 years, and immunocompromised individuals worldwide, especially HIV-infected individuals. It causes diarrhoea lasting about 1–2 weeks, extending up to 2.5 months among the immunocompetent and a more severe life-threatening illness among immunocompromised individuals [1]. The World Health Organization's guideline for drinking water classifies Cryptosporidium as a pathogen of significant public health importance, contributed in part by the organisms' low infective dose and resistance to conventional water treatment such as chlorination [2].
Cryptosporidium has been responsible for major outbreaks [3, 4] as well as sporadic cases of gastroenteritis in the developed world [5]. Cryptosporidium is also widespread in the developing world, with 10–30% of individuals being asymptomatic cyst excretors [6]. The frequency of cryptosporidiosis worldwide is often dependent on HIV status.
Treatment of underlying immunosuppression with antiretrovirals has been found to reduce the severity of cryptosporidiosis in HIV+ patients [7, 8]; however, thisis not an option in immunocompromised patients without HIV. Effective treatment for cryptosporidiosis will be a useful adjunct to antiretroviral therapy, particularly in developing world settings where antiretrovirals are either too expensive or not available. Cancer and post-transplant patients would also benefit if effective treatment were available.
Several antimicrobials have been proposed for the treatment of cryptosporidiosis among the immunocompromised, with clinical trials suggesting potential benefits of treatment with nitazoxanide, paromomycin, rifabutin and macrolides. The aim of this review was to assess the efficacy of interventions for the treatment of cryptosporidiosis among immunocompromised patients.
Methods
Search strategy
We searched Medline, Embase and other electronic databases up to August 2005. A search strategy that combines a highly sensitive filter for randomized controlled trials and subject-specific terms (Cryptosporidiosis or Cryptosporidium or Cryptosporidium parvum) was used. We did not have any language or publication status restrictions. For details of the search strategy see reference 9 [9].
Study eligibility and application of inclusion and exclusion criteria
Two reviewers independently reviewed all identified titles and abstracts. The full text of all articles considered relevant by either reviewer was then assessed. Studies were independently selected for inclusion. All randomized controlled trials of treatment interventions were included.
Data extraction
Two reviewers independently extracted data in a standard form. Disagreements were resolved by consensus. The following information was recorded: study setting; year of study; patient characteristics (age and cause of immunosuppression), intervention and control given, cointervention (such as use of antiretrovirals), duration of diarrhoea, location, outcome measures and the source of funding.
Assessment of methodological quality
Allocation concealment and the Jadad Scale [10] were used to assess the methodological quality of the included studies. This scale scores three dimensions of study quality: randomization, blinding and study withdrawals. Conflicts in coding were resolved by discussion.
Statistical analysis
A meta-analysis was performed to generate summary point estimates and corresponding confidence intervals for the relevant outcomes. Evidence for statistical heterogeneity of results was assessed using Cochrane Q χ2 test and I2 statistic. A significance level of <0.10 and I2 >50% was interpreted as evidence of heterogeneity. If significant heterogeneity was found, the results from the random effects model were emphasized and the relevant factors explored in subgroup analysis where data were available. Analyses were performed with Cochrane's Review Manager (Version 4.2.7). The potential for publication bias was assessed by visually examining funnel plots for evidence of asymmetry.
Results
The initial search identified 1503 potentially relevant titles. Twenty-one papers in full text were considered for inclusion into the review. Fourteen papers were excluded due to the nonrandomized nature of the study [11–18], the use of healthy, immunocompetent adults or children [19–22], single patient trial [23] or the lack of adequate placebo or comparator [24]. Seven randomized, double-blind trials involving 169 participants were included. There were 130 adults with AIDS enrolled in five studies.
Methodological quality
Three studies had a Jadad score of five [25–27], whilst the remaining scored four [28–31]. The method of randomization (computer generated or table of random number list) was appropriate in three studies [25, 27, 28]. The remaining four studies [26, 29–31] did not describe the method of randomization. Within three of these studies [25, 29, 31] patient and investigators blinding was described. Concealment of allocation was clear in three of seven studies. Four studies did not describe the method of allocation concealment [28, 29, 30, 31].
Duration of diarrhoea, mortality and parasitological clearance
The random effects summary estimate of the relative risk (RR) for resolution of diarrhoea for the two studies [25, 32] evaluating nitazoxanide was 0.83 [95% confidence interval (CI) 0.36, 1.94], showing no evidence of effectiveness compared with placebo (Figure 1). Amadi et al. had data on deaths which showed a RR of 0.61 (95% CI 0.22, 1.63) among all 96 children based on five and eight deaths in the intervention and control arms, respectively. Nitazoxanide led to a significant parasitological response compared with placebo among all children with a RR of 0.52 (95% CI 0.30, 0.91). The effect was not significant for HIV-seropositive participants (RR 0.71, 95% CI 0.36, 1.37). HIV-seronegative participants on nitazoxanide had a significantly higher RR of achieving parasitological clearance of 0.26 (95% CI 0.09, 0.80) based on a single study.
Figure 1.
Studies evaluating the effectiveness of nitazoxanide for the treatment of cryptosporidiosis
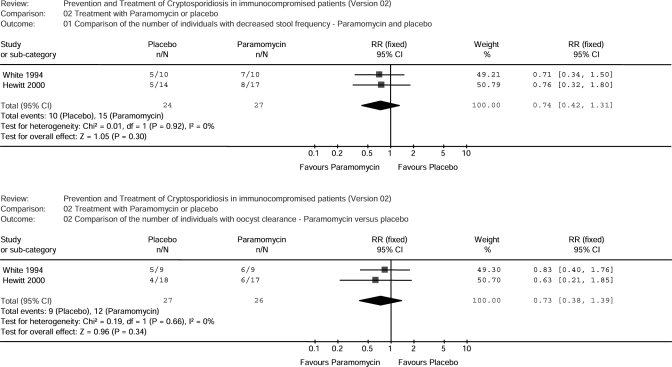
Two studies [28, 31] showed no evidence that paramomycin is more effective in reducing the frequency of diarrhoea than placebo with a summary RR of 0.74 (95% CI 0.42, 1.31) (Figure 2). The use of paramomycin does not significantly lead to a parasitological response (0.73, 95% CI 0.38, 1.39).
Figure 2.
Studies evaluating the effectiveness of paramomycin for the treatment of cryptosporidiosis
Only one study [27] compared the effect of spiramycin with placebo. No outcome data were presented on diarrhoea duration or frequency. There was no difference in mortality between the two arms of the trial (RR 0.43, 95% CI 0.04, 4.35). Spiramycin did not significantly lower oocyst concentration compared with placebo (RR 0.88, 95% CI 0.37, 2.05).
The RR for decreased stool frequency for those on bovine dialysable leucocyte extract with placebo was 0.19 (95% CI 0.03, 1.19) from one study [26]. There was no evidence of a significant decrease in stool volume [mean difference of 4.74 (95% CI 0.75, 8.73)] or of reduced oocyst concentration (RR 0.24, 95% CI 0.04, 1.44).
A pilot study [30] of five patients randomized to receive bovine hyperimmune colostrums showed no evidence in improvement as assessed by volume of stool following 10 days' infusion of bovine colostrums (RR 3.00, 95% CI 0.61, 14.86). There was no evidence of a reduction in oocyst concentration per ml of stool (RR 0.27, 95% CI 0.02, 3.74).
Secondary outcome measures (adverse effects) occurred infrequently in all studies. A variety of adverse effects were reported. None of the papers reported sufficiently similar results to allow a meta-analysis of adverse effects. Similarly, none of the individual trials reported data on tolerability to allow a comparison. In one study [29], nitazoxanide use was ‘probably related’ to a case of viral myocarditis (none in placebo group) and two cases of vomiting (one in placebo group). In another study [25], 58 adverse events were observed but none was considered related or possibly related to the blinded intervention. Twelve adverse events were reported by 11 patients in the nitazoxanide treatment group, compared with 14 adverse events reported by 13 patients in the placebo group. The adverse events included abdominal pain, dyspepsia, constipation, yellow discoloration of urine, dysuria and dry mouth. Two adult patients had episodes of dizziness which resulted in discontinuation of therapy. No side-effects of paramomycin were noted in one study [28], while the second study [31] did not report adverse events. The spiramycin study did not report adverse events. Patients on bovine dialysable leucocyte extract experienced no adverse signs, while bovine hyperimmune colostrum caused nausea and vomiting in two patients and mild abdominal cramps in one patient. One control patient on bovine colostrum also had nausea and vomiting.
Discussion
The studies presented in this review are disparate in design and several are small in size. Based upon the paucity of evidence, we were not able to demonstrate the effectiveness of any therapeutic agent in the treatment of immunocompromised patients with cryptosporidiosis. A significant effect on parasitological clearance was observed with nitazoxanide when all patient groups were included. A randomized, double-blind, placebo-controlled trial was excluded because the target population is not immunocompromised [19]. This study found nitazoxanide treatment reduced the duration of both diarrhoea (P < 0.0001) and oocyst shedding (P < 0.0001). The effect of nitazoxanide in immunocompromised patients needs investigating in a larger trial. Given the severity of this infection in immunocompromised individuals, nitazoxanide may be worth considering in this group of patients pending the availability of further evidence for efficacy. At present the use of fluid and electrolyte replacement and antimotility agents may be the only option for most immunocompromised patients.
The absence of effective therapy highlights the need to ensure that infection is avoided. Unfortunately, evidence for the effectiveness and cost effectiveness of preventive interventions is also lacking. Studies of interventions for the prevention of diarrhoea (not specifically due to cryptosporidiosis) have recently been reviewed [33]. The authors reported that multiple interventions, hygiene and water quality improvement measures significantly reduce the levels of diarrhoeal illness.
Since the emergence of AIDS, medical interest in the diagnosis and management of cryptosporidiosis has increased dramatically. There are currently several published practice guidelines for the prevention and treatment of cryptosporidiosis [34–36]. Unfortunately, most of these guidelines rely on poor-quality studies. For HIV-infected patients, highly active antiretroviral therapy, which can lead to complete resolution of clinical symptoms and oocysts [37, 38], is the mainstay of preventing and managing cryptosporidiosis.
Nitazoxanide is rapidly hydrolysed to an active metabolite, tizoxanide (desacetyl-nitazoxanide) following oral administration. The exact mechanism of action on Cryptosporidium remains unclear, but data from anaerobic protozoa and bacteria suggest that tizoxanide interferes with the pyruvate, ferredoxin oxidoreductase, enzyme-dependent electron transfer reaction, which is essential to anaerobic energy metabolism [39]. Paramomycin, on the other hand, is a non-absorbable aminoglycoside, which binds to prokaryotic ribosomes resulting in a decrease in protein synthesis [40].
The development of new therapies must rely on knowledge of Cryptosporidium biology. Progress in developing tissue culture systems capable of sustaining C. parvum infection for in vitro test of therapies has been achieved only to a limited degree [41]. A large number of antimicrobial drugs have been tested in animals and humans infected with Cryptosporidium with no clear evidence of consistent effectiveness against this parasite [42]. The completion of the genome sequence of C. parvum [43] provides an important opportunity to understand the mechanisms of resistance, identify targets and produce candidate agents. Recent advances in our understanding of the mechanism of resistance have shed light on the reasons for treatment failure. Unlike other parasites, Cryptosporidium salvages pyrimidine and purine bases from its host [44], thus remaining resistant to antifolate drugs which usually inhibit the de novo synthesis of these nucleotides. C. parvum relies on inosine 5′-monophosphate dehydrogenase (IMPDH) to produce guanine nucleotides and is highly susceptible to IMPDH inhibition [45]. Both ribavirin and mycophenolic acid, which inhibit IMPDH, have been shown to have dose-dependent effects on C. parvum development. It appears very likely, based on these observations, that more effective drugs for cryptosporidiosis will be designed. Large-scale randomized controlled trials and cost-effectiveness studies of agents to treat cryptosporidiosis especially among immunocompromised patients are needed.
The studies were limited by variation in important indicators of quality such as allocation concealment, description of randomization, as well as power and drop-out rates. The paramomycin study by White et al. had a high drop-out rate and was underpowered. The study by Nord et al. was very small (five participants). For three interventions, only a single study was identified, therefore a meta-analysis was not possible. Due to the small number of studies, identified formal assessment of publication bias using funnel plots and Egger's test was not possible.
In conclusion, there is no evidence to support the role of chemotherapeutic agents in the management of cryptosporidiosis among immunocompromised individuals. Some evidence of effectiveness for nitazoxanide in a combined population of immuno-competent and -compromised individuals was identified and is worth further study. Supportive management including rehydration therapy, electrolyte replacement and antimotility agents will remain the main treatment strategies until better drugs emerge.
Acknowledgments
Competing interests: None declared.
We are grateful to Lee Hooper for comments on the review.
References
- 1.Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15:145–54. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havelaar A, Boonyakarnkul T, Cunliffe D, Grabow W, Sobsey M, Giddings M, Magara Y, Ohanian E, Toft P, Chorus I, Cotruvo J, Howard G, Jackson P. Guidelines for Drinking Water Quality Water Borne Pathogens. 3. Geneva: WHO; 2003. [Google Scholar]
- 3.Cicirello HG, Kehl KS, Addiss DG, Chusid MJ, Glass RI, Davis JP, Havens PL. Cryptosporidiosis in children during a massive waterborne outbreak in Milwaukee, Wisconsin: clinical, laboratory and epidemiologic findings. Epidemiol Infect. 1997;119:53–60. doi: 10.1017/s0950268897007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacKenzie WR, Hoxie NJ, Proctor ME, Gradus MS, Blair KA, Peterson DE, Kazmierczak JJ, Addiss DG, Fox KR, Rose JB. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161–7. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 5.McLauchlin J, Amar C, Pedraza-Diaz S, Nichols GL. Molecular epidemiological analysis of Cryptosporidium spp. in the United Kingdom: results of genotyping Cryptosporidium spp. in 1,705 fecal samples from humans and 105 fecal samples from livestock animals. J Clin Microbiol. 2000;38:3984–90. doi: 10.1128/jcm.38.11.3984-3990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Current WL, Garcia LS. Cryptosporidiosis. Clin Microbiol Rev. 1991;4:325–58. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr A, Marriott D, Field A, Vasak E, Cooper DA. Treatment of HIV-1-associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet. 1998;351:256–61. doi: 10.1016/S0140-6736(97)07529-6. [DOI] [PubMed] [Google Scholar]
- 8.Maggi P, Larocca AM, Quarto M, Serio G, Brandonisio O, Angarano G, Pastore G. Effect of antiretroviral therapy on cryptosporidiosis and microsporidiosis in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis. 2000;19:213–7. doi: 10.1007/s100960050461. [DOI] [PubMed] [Google Scholar]
- 9.Abubakar I, Aliyu SH, Arumugam C, Hunter P, Usman N. Prevention and treatment of cryptospordiosis in immunocompromised patients. Cochrane Database Syst Rev. 2007. CD004932. [DOI] [PubMed]
- 10.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 11.Amenta M, Dalle Nogare ER, Colomba C, Prestileo TS, Di Lorenzo F, Fundaro S, Colomba A, Ferrieri A. Intestinal protozoa in HIV-infected patients: effect of rifaximin in Cryptosporidium parvum and Blastocystis hominis infections. J Chemother. 1999;11:391–5. doi: 10.1179/joc.1999.11.5.391. [DOI] [PubMed] [Google Scholar]
- 12.Dionisio D, Orsi A, Sterrantino G, Meli M, Di Lollo S, Ibba ML, Trotta M, Pozzi M, Sani L, Leoncini F. Chronic cryptosporidiosis in patients with AIDS: stable remission and possible eradication after long-term, low dose azithromycin. J Clin Pathol. 1998;51:138–42. doi: 10.1136/jcp.51.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fichtenbaum CJ, Zackin R, Feinberg J, Benson C, Griffiths JK. Rifabutin but not clarithromycin prevents cryptosporidiosis in persons with advanced HIV infection. AIDS. 2000;14:2889–93. doi: 10.1097/00002030-200012220-00010. [DOI] [PubMed] [Google Scholar]
- 14.Sprinz E, Mallman R, Barcellos S, Silbert S, Schestatsky G, Bem DD. AIDS-related cryptosporidial diarrhoea: an open study with roxithromycin. J Antimicrob Chemother. 1998;41(Suppl. B):85–91. doi: 10.1093/jac/41.suppl_2.85. [DOI] [PubMed] [Google Scholar]
- 15.Flanigan TP, Ramratnam B, Graeber C, Hellinger J, Smith D, Wheeler D, Hawley P, Heath-Chiozzi M, Ward DJ, Brummitt C, Turner J. Prospective trial of paromomycin for cryptosporidiosis in AIDS. Am J Med. 1996;100:370–2. doi: 10.1016/S0002-9343(97)89499-5. [DOI] [PubMed] [Google Scholar]
- 16.Smith NH, Cron S, Valdez LM, Chappell CL, White AC., Jr Combination drug therapy for cryptosporidiosis in AIDS. J Infect Dis. 1998;178:900–3. doi: 10.1086/515352. [DOI] [PubMed] [Google Scholar]
- 17.Uip DE, Lima AL, Amato VS, Boulos M, Neto VA, Bem DD. Roxithromycin treatment for diarrhoea caused by Cryptosporidium spp. in patients with AIDS. J Antimicrob Chemother. 1998;41(Suppl. B):93–7. doi: 10.1093/jac/41.suppl_2.93. [DOI] [PubMed] [Google Scholar]
- 18.Saez-Llorens X, Odio CM, Umana MA, Morales MV. Spiramycin vs. placebo for treatment of acute diarrhea caused by Cryptosporidium. Pediatr Infect Dis J. 1989;8:136–40. [PubMed] [Google Scholar]
- 19.Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of Nitazoxanide. J Infect Dis. 2001;184:103–6. doi: 10.1086/321008. [DOI] [PubMed] [Google Scholar]
- 20.Okhuysen PC, Chappell CL, Crabb J, Valdez LM, Douglass ET, DuPont HL. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin Infect Dis. 1998;26:1324–9. doi: 10.1086/516374. [DOI] [PubMed] [Google Scholar]
- 21.Hellard ME, Sinclair MI, Forbes AB, Fairley CK. A randomized, blinded, controlled trial investigating the gastrointestinal health effects of drinking water quality. Environ Health Perspect. 2001;109:773–8. doi: 10.1289/ehp.01109773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woolf GM, Townsend M, Guyatt G. Treatment of cryptosporidiosis with spiramycin in AIDS. An ‘N of 1’ trial. J Clin Gastroenterol. 1987;9:632–4. doi: 10.1097/00004836-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg PD, Cello JP. Treatment of severe diarrhea caused by Cryptosporidium parvum with oral bovine immunoglobulin concentrate in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:348–54. doi: 10.1097/00042560-199612010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Kadappu KK, Nagaraja MV, Rao PV, Shastry BA. Azithromycin as treatment for cryptosporidiosis in human immunodeficiency virus disease. J Postgrad Med. 2002;48:179–81. [PubMed] [Google Scholar]
- 25.Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360:1375–80. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- 26.McMeeking A, Borkowsky W, Klesius PH, Bonk S, Holzman RS, Lawrence HS. A controlled trial of bovine dialyzable leukocyte extract for cryptosporidiosis in patients with AIDS. J Infect Dis. 1990;161:108–12. doi: 10.1093/infdis/161.1.108. [DOI] [PubMed] [Google Scholar]
- 27.Wittenberg DF, Miller NM, van den Ende J. Spiramycin is not effective in treating cryptosporidium diarrhea in infants: results of a double-blind randomized trial. J Infect Dis. 1989;159:131–2. doi: 10.1093/infdis/159.1.131. [DOI] [PubMed] [Google Scholar]
- 28.Hewitt RG, Yiannoutsos CT, Higgs ES, Carey JT, Geiseler PJ, Soave R, Rosenberg R, Vazquez GJ, Wheat LJ, Fass RJ, Antoninievic Z, Walawander AL, Flanigan TP, Bender JF. Paromomycin: no more effective than placebo for treatment of cryptosporidiosis in patients with advanced human immunodeficiency virus infection. AIDS Clinical Trial Group. Clin Infect Dis. 2000;31:1084–92. doi: 10.1086/318155. [DOI] [PubMed] [Google Scholar]
- 29.Rossignol JF, Hidalgo H, Feregrino M, Higuera F, Gomez WH, Romero JL, Padierna J, Geyne A, Ayers MS. A double-blind placebo-controlled study of nitazoxanide in the treatment of cryptosporidial diarrhoea in AIDS patients in Mexico. Trans R Soc Trop Med Hyg. 1998;92:663–6. doi: 10.1016/s0035-9203(98)90804-5. [DOI] [PubMed] [Google Scholar]
- 30.Nord J, Ma P, DiJohn D, Tzipori S, Tacket CO. Treatment with bovine hyperimmune colostrum of cryptosporidial diarrhea in AIDS patients. AIDS. 1990;4:581–4. doi: 10.1097/00002030-199006000-00015. [DOI] [PubMed] [Google Scholar]
- 31.White AC, Jr, Chappell CL, Hayat CS, Kimball KT, Flanigan TP, Goodgame RW. Paromomycin for cryptosporidiosis in AIDS: a prospective, double-blind trial. J Infect Dis. 1994;170:419–24. doi: 10.1093/infdis/170.2.419. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Maboud AI, Rossignol JF, el Kady MS, Mostafa MS, Kabil SM. Cryptosporidiosis in Benha, study of some recent modalities in diagnosis and treatment. J Egypt Soc Parasitol. 2000;30:717–25. [PubMed] [Google Scholar]
- 33.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM., Jr Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 34.Hunter PR. Advice on the response from public and environmental health to the detection of cryptosporidial oocysts in treated drinking water. PHLS Advisory Committee on Water and the Environment. Commun Dis Public Health. 2000;3:24–7. [PubMed] [Google Scholar]
- 35.CDC. Cryptosporidium in water. CDC Guidelines on How to Protect Yourself. Centers for Disease Control and Prevention. AIDS TreatNews. 1995:7–8. [PubMed] [Google Scholar]
- 36.Kaplan JE, Masur H, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons – 2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR Recomm Report. 2002;51:1–52. [PubMed] [Google Scholar]
- 37.Grube H, Ramratnam B, Ley C, Flanigan TP. Resolution of AIDS associated cryptosporidiosis after treatment with indinavir. Am J Gastroenterol. 1997;92:726. [PubMed] [Google Scholar]
- 38.Miao YM, Awad-El-Kariem FM, Franzen C, Ellis DS, Muller A, Counihan HM, Hayes PJ, Gazzard BG. Eradication of cryptosporidia and microsporidia following successful antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25:124–9. doi: 10.1097/00042560-200010010-00006. [DOI] [PubMed] [Google Scholar]
- 39.Gilles HM, Hoffman PS. Treatment of intestinal parasitic infections: a review of nitazoxanide. Trends Parasitol. 2002;18:95–7. doi: 10.1016/s1471-4922(01)02205-x. [DOI] [PubMed] [Google Scholar]
- 40.Principles and Practice of Infectious Diseases. Philadelphia, PA: Elsevier Churchill Livingstone; 2005. [Google Scholar]
- 41.Theodos CM, Griffiths JK, D'Onfro J, Fairfield A, Tzipori S. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob Agents Chemother. 1998;42:1959–65. doi: 10.1128/aac.42.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mead JR. Cryptosporidiosis and the challenges of chemotherapy. Drug Resist Updat. 2002;5:47–57. doi: 10.1016/s1368-7646(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 43.Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, Deng M, Liu C, Widmer G, Tzipori S, Buck GA, Xu P, Bankier AT, Dear PH, Konfortov BA, Spriggs HF, Iyer L, Anantharaman V, Aravind L, Kapur V. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–5. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- 44.Striepen B, Pruijssers AJ, Huang J, Li C, Gubbels MJ, Umejiego NN, Hedstrom L, Kissinger JC. Gene transfer in the evolution of parasite nucleotide biosynthesis. Proc Natl Acad Sci USA. 2004;101:3154–9. doi: 10.1073/pnas.0304686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umejiego NN, Li C, Riera T, Hedstrom L, Striepen B. Cryptosporidium parvum IMP dehydrogenase: identification of functional, structural, and dynamic properties that can be exploited for drug design. J Biol Chem. 2004;279:40320–7. doi: 10.1074/jbc.M407121200. [DOI] [PubMed] [Google Scholar]