Abstract
Aims
We sought to optimize a quantitative noninvasive method to determine the concentration in their glands of origin of biochemical markers of compartments of the male genital tract as the first step towards validation of a novel method for estimation of drug concentrations in these male genital tract compartments.
Methods
Sixty-eight men participated. We compared four collection devices to split ejaculate into fractions. Fractions were assayed for fructose and prostate specific antigen (PSA) as unique markers of the seminal vesicle and prostate, respectively. Seminal vesicle fructose and prostatic PSA were estimated using a linear regression method, based on fructose-PSA axis intercepts, and compared with an older method which solves a simultaneous series of equations.
Results
A five-compartment collection device performed best with mean (95% confidence interval) PSA vs. fructose r2 of 0.84 (0.71, 0.98, P < 0.001). Using resampling simulations, glandular PSA and fructose estimates were highly variable and often implausible when using only two fractions. Using our method, the prostate contributed 37–44% to the whole ejaculate and the seminal vesicle contributed 55–61%. The novel regression method was highly correlated (r2 ≥ 0.98) with older methods.
Conclusions
We developed a noninvasive quantitative method of male genital tract biochemical marker estimation using a five-compartment tray to collect three to five ejaculate fractions. Our novel regression method is quantitative and more fully developed than older methods. This noninvasive method for determining glandular marker concentrations should be useful to provide quantitative estimates of drug concentrations in these glands.
Keywords: fructose, male genital tract pharmacology, prostate, prostate specific antigen, semen, semen vesicle
Introduction
Rational therapeutics is best informed, when possible, by knowledge of the pharmacokinetics and pharmacodynamics of a drug at its site of action, especially where this is poorly reflected by pharmacokinetics in the blood. The accessory glands of the male genital tract are anatomically and biochemically diverse sites of action for drugs in several therapeutic categories, including prostate cancer, prostatitis, and HIV infection, and there is ample evidence of discordance between blood and accessory gland pharmacokinetics [4]. Particularly in HIV infection, where semen to blood concentration ratios of the most potent antiretroviral (ARV) drugs are very low, there is interest in understanding the mechanisms by which the male genital tract acts as a pharmacologic sanctuary for HIV with implications both for HIV transmission and resistance [4, 5]. Further, quantitative assessment of pathogen and immune cell concentration from individual glands, also especially relevant in the case of HIV infection and possibly prostatitis, could provide pharmacodynamic data to inform optimization of drug regimens to reduce the local pathogen burden.
Currently, there are no quantitative, noninvasive methods for estimation of drug penetration into individual compartments of the male genital tract. Direct measures of drug concentration in male accessory glands, like tissue biopsy, are invasive and not amenable to repeated sampling within a dosing interval which would be useful to investigate gland specific pharmacokinetic parameters. Prostatic massage as a means to assess prostate gland drug concentration may be contaminated by seminal vesicle secretions expressed by the massage, repeated assessment is uncomfortable, and volumes are often very small [6–8]. Successful seminal vesicle cannulation has been reported, but it also suffers from invasiveness and greatly limited repeatability during a dosing interval [9].
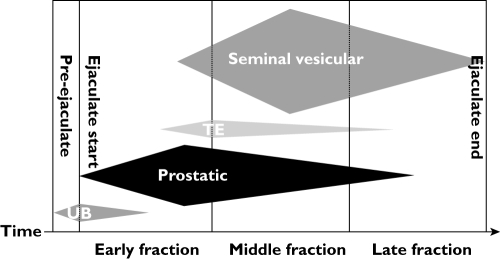
Split ejaculate sampling, the collection of the semen from a single ejaculation as multiple fractions, is a noninvasive method that has been used by numerous investigators to assess qualitatively drug and endogenous chemical concentrations in fluid from the largest of the male accessory glands [6, 10–17]. This method takes advantage of the sequential release into the urethra of fluid from different male accessory glands and incomplete mixing of these fluids prior to emergence from the urethra (Figure 1). When collected in discrete fractions, this heterogeneity, and therefore the relative contribution from each gland, is quantifiable by measurement of biochemical markers unique to each of the major glands: for example, fructose or phosphoryl choline for the seminal vesicle, prostate specific antigen (PSA) or acid phosphatase for the prostate, spermatozoa or alpha-glucosidase for the testicular-epididymal component. None of these markers is present in fluid from the urethral (Littre's) and bulbourethral (Cowper's) glands. Once the glandular contribution to each fraction is determined, measurement of the concentration of a drug in each fraction allows calculation of the drug concentration in each of the glands. The split ejaculate method is also amenable to repeated sampling.
Figure 1.
Heterogeneity of ejaculate fractions. This schematic representation depicts the proportional contribution and temporal (left to right) sequence of four sources of seminal fluid: first, urethral and bulbourethral glands (‘UB’), <10% of total ejaculate volume; second, prostatic, 20–40%; third, testicular-epididymal (‘TE’), <10%; finally, seminal vesicular, 50–80%. Collecting semen in sequential fractions results in heterogeneous composition of each fraction due to varied contributions from biochemically different glands
While there are several reports using ejaculate fractions to assess qualitatively the association of drugs with the early ‘prostatic’ or late ‘seminal vesicle’ fraction (primarily of antibiotics for prostatitis), neither quantitative estimation of gland specific drug concentrations, nor comparison of collection methods, nor development of statistical methods have been reported. In order to fill this gap and move toward validation of a quantitative, statistically assessable method, we performed a series of studies to compare collection methods for their ability to maximize the biochemical heterogeneity among fractions. Next, we modified Lundquist's original computational method to reduce measurement error and avoid the subjective selection of only some ejaculate fractions while arbitrarily excluding others. Finally, we developed a computationally simpler linear regression method to estimate glandular marker concentrations in the seminal vesicle and prostate.
Methods
Subjects
We enrolled four cohorts of volunteers, each cohort using a different ejaculate collection device. Subjects were excluded for active medical problems, but HIV infection was not an exclusion criterion. The Johns Hopkins Medicine Institutional Review Board approved the study protocol and all subjects provided informed consent prior to enrolment. These subjects formed four cohorts defined by the four semen collection devices which differed primarily in the number of compartments each contained (see section below). Each cohort was recruited by advertisement in local print media, from a research subject registry, and by word-of-mouth among volunteers. Cohorts were enrolled sequentially over time as we explored the performance of each of the four devices, in turn. Because these quantitative methods had not previously been used, variability data to inform formal sample size estimates were not available. Sample size in each cohort was 36 (cohort A), 10 (cohort B), 12 (cohort C), and 10 (cohort D). Some subjects participated in more than one cohort given recruiting limitations and the extension of the studies over several years.
Collection devices
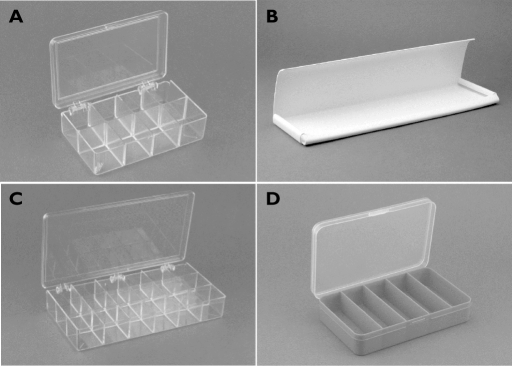
Cohort A used an acrylic hinged lid multicompartment organizer box (similar to UPC# 740512111114, CreateForLess, Inc. Portland, OR) divided into eight square compartments (two rows and four columns) to fractionate the ejaculate (Figure 2A). From these compartments, all visible semen was removed to form three fractions. Sometimes, very small volumes from adjacent compartments were combined into one fraction to provide adequate sample volume for the later assays. Cohort B used a simple one compartment v-shaped trough cut from commercially available vinyl material (Figure 2B). Up to five fractions of ejaculate were aspirated from the vinyl tray in sequence attempting to capture 200 µl per fraction. Cohort C used an 18 compartment (three rows and six columns) acrylic collection tray (similar to UPC# 740512111107, CreateForLess, Inc. Portland, OR), in which three–five fractions were formed, sometimes combining multiple fractions into one from multiple chambers (Figure 2C). Cohort D used a five compartment blue plastic collection tray (UPC# 652695062728, Darice, Inc. Strongsville, OH). This hinged lid organizer box had a single row of compartments divided across the short dimension into five rectangular compartments from which fractions were taken as they fell into each section; unlike the collection device for Cohort A and C, no fractions were combined to form larger samples (Figure 2D).
Figure 2.
Collection devices. A) cohort A, collection tray with eight square compartments (four columns × two rows). B) cohort B, collection trough for one continuous sample to be fractionated manually. C) cohort C, collection tray with 18 square compartments (six columns × three rows). D) cohort D, collection device with five rectangular compartments (single row of five compartments)
Sample collection procedures
Subjects were asked to refrain from ejaculation for 48 h prior to study participation in order to maximize ejaculate volumes. (In unpublished studies we have demonstrated that PSA and fructose are shown to be affected by sampling intervals as short as 8 h). A semen sample was collected by masturbation into the collection device. Subjects were instructed to wipe away and discard visible pre-ejaculate fluid which formed at the urethral orifice during masturbation, but prior to ejaculation. For the multichambered collection device cohorts (A, C, and D), when ejaculation of seminal fluid occurred, subjects directed the emerging seminal fluid stream across the open face of the collection device, making one pass in a single direction (right-to-left or left-to-right) across the longer axis of the device. In this way, the seminal stream fell into the separate compartments of the collection device in sequence with discrete fractions of ejaculate defined by the existing compartments of the device. The final drops of ejaculate were milked from the urethra into the last compartment (either the extreme right or left) of the collection device. Subjects were instructed to deposit semen in at least three compartments in the collection device. For subjects using the trough (cohort B), the emerging ejaculate stream was directed into and along the crease of the trough in a single direction (right-to-left or left-to-right). Discrete fractions from the trough collections were not defined by the device, which had no compartments, but by the sequential removal of discrete fractions by the technician after liquefaction.
Semen sample processing
The collection device with freshly ejaculated semen was placed in the negative flow hood for 20–30 min at room temperature to allow the semen to liquefy. Liquefied semen fractions were transferred, using positive displacement pipettes, into sterile tared 1.5 ml Eppendorf tubes, weighed, and centrifuged (800 × g, 10 min, 4°C). After centrifugation, supernatant-seminal plasma was aliquoted into tared prelabelled cryo-vials and weighed. For the PSA assays, 10 µl of each supernatant was transferred into a sterile 1.8 ml NUNC cryo-vial (#377267) containing 90 µl (1 : 10) of Hybritech PSA Sample Diluent (Beckman Coulter, #37206) and further diluted to 1 : 20 000 using PSA sample diluent. These and all other aliquots were stored at −75°C until analysis.
Fructose assay
For cohorts A and B, seminal plasma fructose concentration was determined by the enzymatic reduction of fructose by sorbitol dehydrogenase [18], which required 170 µl seminal plasma and had a lower limit of quantification of ∼6 nm. In order to reduce the sample volume requirements for use in future studies when fructose, PSA, and drug or pathogen concentrations must simultaneously be assessed, we changed assay methods for cohorts C and D. For these latter cohorts, we used the Fructose Assay Kit (FA-20, Sigma-Aldrich, Inc., St Louis, MO) based on the coupled enzymatic conversion of fructose to 6-phosphogluconate with spectrophotometric readout at 340 nm [19], which we validated for use in human seminal plasma. An initial ZnSO4 and NaOH de-proteination step was used. This fructose assay uses 25 µl of seminal plasma, has a linear range between 6 and 66 mm, sensitivity of 10%, specificity near 100%, and interday and intraday assay variations <10%. Because there are no cross subject comparisons of fructose concentrations involved in the split ejaculate method in which only within subject fructose concentrations are included in calculations, we did not cross-validate the absolute fructose concentrations between the assays.
Prostate specific antigen (PSA) assay
We validated an existing commercial PSA assay, Hybritech PSA (Beckman coulter, Fullerton, CA), for use with seminal plasma [20]. The assay is a two-site immunoenzymatic (‘sandwich’) method using mouse monoclonal antibody in alkaline phosphatase conjugate and paramagnetic particles coated with a second mouse monoclonal antibody. A chemiluminescent substrate is added to produce light directly proportional to the amount of PSA in the sample. The only modification required for use with seminal plasma was an initial 20,000-fold dilution. The dynamic range of the assay was from 0.008 ng ml−1 to 150 ng ml−1. The day to day variability of the QC samples was less than 3% and standard curves all have a CV of less than 15%.
Sperm counts
We used a Makler chamber to determine the sperm counts. Four counts (two on each side of the Makler chamber) were averaged. Sperm counts were performed on all fractions, though in only four subjects in cohort D and not in other cohorts, since we developed this capability only late in the study in preparation for using sperm count as a marker for the testicular-epididymal compartment in future studies.
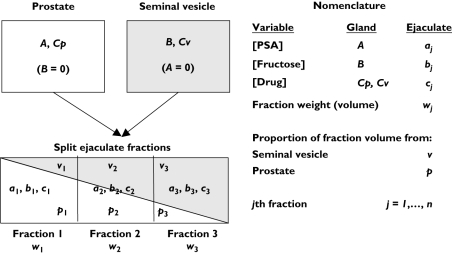
Split ejaculate method and nomenclature
The objective of the split ejaculate method is to provide parameter estimates of drug concentration (Cp) in the prostate (upper white box, Figure 3) and in the seminal vesicle (Cv, grey box). One first estimates the concentration of unique glandular markers in their glands of origin, namely PSA in the prostate, A, and the fructose in the seminal vesicle, B. The method assumes that the prostate and seminal vesicle empty their contents into the forming seminal stream in sequence such that the prostatic contribution falls over time (white wedge within the rectangle [whole ejaculate]) while the seminal vesicle contribution rises (grey wedge). In practice, the method involves collection of ejaculate in j fractions (three shown in Figures 1 and 2) from individuals with measurement of [PSA], aj and [fructose], bj in each fraction. The drug concentration, cj, used for the calculation of prostatic (Cp) and seminal vesicle (Cv) drug concentration was not measured in these developmental studies.
Figure 3.
Schematic representation and nomenclature for split ejaculate calculations based on prostate and seminal vesicle contributions to semen. Capital letters represent concentrations in the glands and lowercase letters represent variables in the ejaculate (concentrations, weights, contributing gland proportions). Subscripts represent fraction number, j
Methodologic assumptions
The split ejaculate method makes the following assumptions: 1) each gland releases its contents once and in sequence with the other gland, 2) contents released from each gland are homogeneous, 3) heterogeneity exists among ejaculate fractions due to incomplete mixing of contents from each gland and 4) there are inconsequential contributions from glands other than the seminal vesicle and prostate.
Analytical methods
In these developmental studies, we employed two analytical methods to quantify the split ejaculate results: 1) our modification of the method previously described by Lundquist which requires solution of a series of linear equations (‘Modified Lundquist’ method) and 2) a linear regression approach (‘Regression method’).
Simultaneous series of equations (‘Modified Lundquist’ method)
We modified the classical split ejaculate method most thoroughly developed by Lundquist [10] in several ways by 1) restricting attention to only two glands rather than three, thereby simplifying calculations, 2) allowing the elimination of fraction weights, 3) using all possible combinations of fraction pairs rather than only selecting some and 4) summarizing all possible solutions with use of medians rather than describing selected pairs individually.
The Modified Lundquist method involves three steps. First, one estimates the unique gland-specific marker concentrations in their glands of origin, here prostatic PSA (A) and seminal vesicular fructose (B), based on marker concentrations, PSA (aj) and fructose (bj), in each pairwise combination of semen fractions by solving a series of two linear equations (see Appendix 1 for full derivation). Second, one estimates the contribution of each gland to each fraction of ejaculate (pj and vj) using A, B, and the marker concentration in each fraction (aj and bj). In this step, the sum of pj and vj is assumed to be 1, since the contribution from the other glands is ignored. Lundquist originally used weights of each fraction in this step and these are necessary for three compartment calculations, but our modification eliminates the use of volume or weight, thereby avoiding the introduction of experimental error in this step. Third, the concentration of the drug of interest in the prostate (Cp) and the seminal vesicle (Cv) is calculated using the fraction contributed by each gland to each fraction (pj and vj) and the drug concentration in each fraction (cj) by solving another series of two equations. This third step is not done in the developmental studies presented here, but this step is included to demonstrate the final steps when drug concentrations are being calculated. Minimally, two samples are sufficient to determine the values, Cp and Cv. Since more than three fractions were obtained to improve the robustness of parameter estimates the Cp and Cv values reported for each individual were based on the median of all possible pairwise values.
Linear regression (‘Regression’ method)
In principle, since PSA and fructose arise only in the prostate and seminal vesicle, respectively, if one could sample the very first, infinitesimally small, unmixed semen fraction, one would find only PSA at the concentration in its gland of origin and fructose would be absent: aj = A and bj = 0. Similarly, in the last fraction, aj = 0 and bj = B. These two conditions are only approximately true due to mixing within the urethra and the intermediate fractions represent a progression between these two extreme conditions (Figures 1 and 2). The graph of PSA vs. fructose should therefore be linear with negative slope and axis intercepts indicate concentrations in cognate glands of origin.
This relationship is used to calculate the drug concentration in the seminal vesicle and prostate in two steps. In step 1, PSA and fructose concentrations in the glands of origin (A and B, respectively) are estimated by regression of PSA on fructose and vice versa; the axis intercepts of the resulting lines estimate the fructose concentration in the seminal vesicle (where PSA is zero) and the concentration of PSA in the prostate (where fructose is zero). Each regression equation (y = mx + b) could provide an estimate for both A and B by using both the y-intercept (b) and x-intercept (–b/m). However, because the estimate using the x-intercept can result in overestimation due to attenuation of slope estimates, as previously described by Fuller [21], we used two regression equations, fructose on PSA and PSA on fructose, using only the y-intercept from each to estimate A and B, hereafter referred to as the ‘y-intercept’ regression method (recommended by Winsor [22]) in contrast to the ‘x-intercept’ regression. In step 2, one estimates the linear equations describing the relationship between the drug and glandular markers (PSA or fructose) into which one substitutes the value of the glandular marker in its gland of origin (A or B from step 1) to estimate the drug concentration in each of the glands. Our validation of the final steps for calculating glandular drug concentration is reported in a separate study [23].
Contribution of glands to semen volume
Consistent with previously listed methodologic assumptions, the fraction of whole ejaculate volume contributed to by the seminal vesicle (SV fraction) and prostate (PR fraction), is calculated as the ratio of whole ejaculate PSA and fructose concentrations, as and bs, respectively (subscript s for whole semen in place of fraction j), to the estimated marker concentration in each gland of origin, A and B, respectively (PR Fraction = as/A and SV fraction = bs/B). The whole ejaculate marker concentrations (as, bs) are calculated as the sum of the products of fraction weight (wj with units converted to volume) and fraction marker concentration, as = Σwj × aj, where j represents individual fractions from a single ejaculate.
Fructose-PSA residual analysis
To check for systematic deviation in the fructose-PSA linear regressions, possibly due to unmeasured testicular-epididymal fluid, we calculated the percent of the residual deviation for each observation (actual value minus fitted value divided by fitted value) for both fructose and PSA. For these residuals percents, the linear equation was used in which the marker being evaluated was the dependent variable of the regression. Each fraction's location among the other fractions in the ejaculate was quantified by the proportion of the total ejaculate volume that had been expelled up to and including the midpoint of a given fraction volume. Volume for each fraction was measured experimentally as weight (wj), assuming a specific gravity of 1.0 which is consistent with our own observations. To calculate the relative location of each successive fraction, first, the proportion of total weight for each discrete fraction (Fj) is calculated (Fj = wj/Σw1,2, … ,n, where Σw1,2, … ,n is the total ejaculate volume constituted by n discrete fractions for each individual). Second, the midpoint of each fraction's proportional weight (Fj/2) was added to the sum of the proportions of the preceding ejaculate fractions (ΣFj−1) to indicate the accumulated percent of the whole ejaculate volume up to the midpoint of the fraction of interest (ΣFj−1 + Fj/2)). Data for all subjects' fractions were then pooled.
Influence of fraction number
The sensitivity of the Lundquist and Regression methods to fraction number were evaluated by using the A and B estimates for all subjects from whom five fraction ejaculates were collected as reference values. By systematically dropping one or more of these subjects' five fractions, we recalculated A and B using all possible two, three, and four fraction combinations. The deviation of these estimates from the original five fraction values were expressed as a ratio of the recalculated estimates relative to the original five fraction estimate.
Descriptive statistics and comparisons
Normally distributed variables are summarized as mean with 95% confidence interval (95% CI) and geometric means for ratios. Variables which do not appear to be normally distributed are summarized by median and interquartile range. Correlation between variables is described with the square of the Pearson correlation coefficient (r2). Differences among the collection devices were assessed with anova with post hoc Dunnett's t-test for pairwise comparisons. Two-sided P values smaller than or equal to 0.05 were considered statistically significant. SPSS statistical software was used for all descriptive and comparative statistics (Version 9.0.1, SPSS, Inc., Chicago, IL, USA).
Results
Compartmental markers and semen volume
Sixty-eight men, including 30 previously tested and known to be HIV-infected, attempted to contribute split ejaculate semen samples. Four individuals participated in multiple cohorts and their repeat measures were treated as if separate individuals. Sixty-five percent of subjects (44 of 68) successfully provided split ejaculate samples with the three or more fractions desired, though this rose to >90% as our collection methods evolved. Forty-one of these 44 subjects had complete fructose and PSA results. These subjects provided a mean of 2.0 ml (95% CI 1.6, 2.3) of semen per ejaculate.
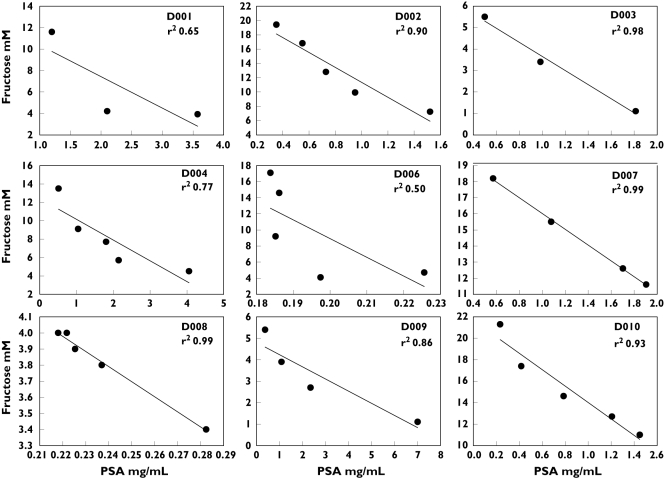
Fructose vs. PSA relationship
A linear relationship between fructose and PSA was demonstrated for most subjects, consistent with our expectations that fructose concentrations would rise and PSA concentrations would fall from first to final fraction of ejaculate (Figure 4). The strength of this inverse relationship varied among the collection devices and was highest and most consistent for cohort D (Table 1).
Figure 4.
Fructose (y-axis) plotted against PSA (x-axis) for all subjects in cohort D. Each plot represents a single subject; each point represents a single fraction from one split ejaculate. Fitted ordinary least squares regression lines and the square of the correlation coefficient (r2) is shown for each plot
Table 1.
Comparison of collection methods
| Variable | Cohort A eight-chambera (4 × 2) | Cohort B Troughb | Cohort C 18-Chamberb(6 × 3) | Cohort D five-chamber (5 × 1) |
|---|---|---|---|---|
| Total attempted (n) | 36 | 10 | 12 | 10 |
| Fractions ≥3 | 17 (47%) | 10 (100%) | 8 (67%) | 9 (90%) |
| Fractions ≥5 | – | 7 (70%) | 3 (25%) | 5 (50%) |
| Slope negative | 10 (71%) | 6 (60%) | 5 (63%) | 9 (100%) |
| CV fructose | 0.4 (0.26, 0.54) | 0.29 (0.17, 0.41) | 0.31 (0.13, 0.48) | 0.42 (0.26, 0.59) |
| CV PSA | 0.37 (0.24, 0.50) | 0.48 (0.30, 0.66) | 0.42 (0.32, 0.52) | 0.53 (0.30, 0.77) |
| Fructose highest/lowest | 2.87 (1.50, 4.23) | 1.69 (1.02, 2.37) | 2.79 (0.19, 5.39) | 2.45 (1.29, 3.61) |
| PSA highest/lowest | 2.40 (1.74, 3.06) | 3.81 (2.17, 5.45) | 2.86 (1.57, 4.16) | 5.50 (1.36, 9.65) |
| r2 (fructose vs. PSA)c | 0.71 (0.53, 0.88) | 0.35 (0.13, 0.57)d | 0.35 (0.08, 0.62)e | 0.84 (0.71, 0.98)de |
Values are number (percent) for categorical variables and mean (95% confidence interval) for continuous variables. Calculations for fructose-PSA relationships (slope, coefficient of variation, r2) were only calculated on subjects with at least three fractions and complete fructose-PSA data; fructose-PSA data incomplete in three of 17 in Cohort A.
three fractions (maximum) were formed from all existing fractions for Cohort A so five fractions were not possible;
three or more fractions were formed in order to achieve adequate sample volume for at least three and up to five fractions.
anova P < 0.001 among all Cohorts.
Dunnett's t posthoc pairwise comparison, P < 0.002 for Cohort D vs. both Cohort B and C; Other comparisons (Fisher's exact for categorical variables, anova for continuous variables) were not significant.
Dunnett's t posthoc pairwise comparison, P < 0.002 for Cohort D vs. both Cohort B and C; Other comparisons (Fisher's exact for categorical variables, anova for continuous variables) were not significant.
Comparison of collection methods
We noted two types of practical difficulty working with several of our devices: first, some fractions were quite difficult to withdraw from the collection device, especially the trough and the 18-compartment device, due to incomplete liquefaction; second, pooling the contents from the smaller chambered 8- and 18-compartment devices (to achieve adequate fraction volume for assays to be performed) required assessments of fraction sequence which was not always clear and aspiration of very small fraction volumes. The five-compartment device had neither of these practical difficulties.
The trough, followed by the five-chamber device, had the highest success rate for three or more fractions and for five fractions collected, though not statisticallysignificant (Table 1). Measures of fraction heterogeneity (marker coefficient of variation and high : low ratios) were similar and not statistically significant among the collection methods. The best linearity of fructose with PSA, hence the narrowest point estimates for gland of origin marker concentrations, was observed with the five-chamber device (mean r2 = 0.84 [95% CI 0.71, 0.98]). Based on these results, cohort D was used to compare analytical methods in the remainder of this paper.
Comparison of modified Lundquist and Linear Regression methods
The estimates of PSA in the prostate (A) and fructose in the seminal vesicle (B) were calculated using both the modified Lundquist and the Regression methods. Among the 41 subjects providing three or more fractions, the Lundquist method gave biologically implausible negative values for either seminal vesicle fructose or prostatic PSA concentrations in 7 of 41 subjects (17%), although all cohort B and cohort D estimates were positive. Using the y-intercept Regression method (separate regression for A and B each using y-intercept) returned negative values for either seminal vesicle fructose or prostatic PSA for 6 of 41 subjects (15%); again, all cohort B and cohort D subjects had positive values. Using the x-intercept (–b/m) Regression method, 25% of subjects had implausible negative values for either fructose or PSA, with only cohort D having all positive values.
For all subjects, the mean whole ejaculate PSA concentration was 1.24 mg ml−1 (95% CI 0.89, 1.56) while the fructose concentration was 13.5 mm (95% CI 10.8, 16.2). For the four subjects who participated in more than one cohort, the intraindividual variability as measured by the ratio of the upper quartile to lower quartile was 1.1, 1.3, 1.9, and 3.3 for fructose and 1.1, 1.7, 4.2, and 3.7 for PSA. For cohort D, the estimated concentrations of PSA in the prostate (A) as estimated by either Lundquist (geometric mean 2.1 mg ml−1) or Regression methods (2.1 and 2.2 mg ml−1) were greater than the concentration in the whole ejaculate (1.0 mg ml−1) by at least a factor of 2.1 (Table 2). This concentration difference is consistent with prostatic fluid constituting 37–44% of the whole ejaculate (Table 3). For fructose, the geometric mean seminal vesicle concentration was estimated to fall between 13.8 and 15.6 mm, depending on method, representing a 1.9- to 2.2-fold increase above the whole ejaculate value (7.2 mm), consistent with seminal vesicular fluid constituting 55–61% of the whole ejaculate (Tables 2 and 3).
Table 2.
Fructose and PSA concentrations in their glands of origin as estimated by the Modified Lundquist and Regression methods
| Whole ejaculate | Lundquist | PSA (mg ml−1) Prostate (A) Regression y-intercept (b) | Lundquist | Whole ejaculate | Regression) x-intercept (–b/m) | Fructose (mm) Seminal vesicle (B) Regression y-intercept | Regression x-intercept (–b/m) | |
|---|---|---|---|---|---|---|---|---|
| D001 | 2.1 | 4.8 | 3.8 | 4.5 | 6.9 | 15.5 | 13.3 | 16.9 |
| D002 | 0.9 | 1.8 | 2.0 | 2.1 | 12.4 | 23.5 | 21.8 | 22.7 |
| D003 | 1.1 | 2.1 | 2.1 | 2.1 | 3.4 | 7.2 | 6.9 | 7.0 |
| D004 | 2.1 | 4.9 | 4.7 | 5.5 | 7.5 | 13.6 | 12.4 | 13.7 |
| D006 | 0.2 | 0.2 | 0.2 | 0.2 | 9.5 | 79.1 | 55.5 | 101.6 |
| D007 | 1.2 | 4.2 | 4.3 | 4.3 | 15.0 | 21.0 | 20.9 | 20.9 |
| D008 | 0.2 | 0.6 | 0.6 | 0.6 | 3.9 | 6.1 | 6.0 | 6.1 |
| D009 | 2.5 | 7.0 | 7.7 | 8.5 | 3.0 | 5.3 | 4.8 | 5.1 |
| D010 | 0.9 | 2.9 | 2.7 | 2.8 | 14.8 | 20.9 | 21.7 | 22.1 |
| Geometric mean | 1.0 | 2.1 | 2.1 | 2.2 | 7.2 | 15.1 | 13.8 | 15.6 |
| 95% CI | 0.5, 2.2 | 0.9, 5.2 | 0.9, 5.0 | 0.9, 5.5 | 4.5, 11.7 | 7.9, 28.7 | 7.6, 24.0 | 7.7, 31.4 |
Table 3.
Proportion of the whole ejaculate contributed by the seminal vesicle and prostate according to each of three estimation methods for cohort D
| Parameter | Method | Median (25%ile −75%ile) |
|---|---|---|
| Fraction from seminal vesicle | Modified Lundquist | 0.56 (0.46–0.67) |
| Regression y-intercept (b) | 0.61 (0.50–0.66) | |
| Regression x-intercept (–b/m) | 0.55 (0.45–0.65) | |
| Fraction from prostate | Modified Lundquist | 0.42 (0.33–0.51) |
| Regression y-intercept (b) | 0.44 (0.33–0.54) | |
| Regression x-intercept (–b/m) | 0.37 (0.31–0.49) | |
| Sum of fractions | Modified Lundquist | 1.00 (0.96–1.03) |
| Regression y-intercept (b) | 1.01 (1.00–1.05) | |
| Regression x-intercept (–b/m) | 0.98 (0.90–0.99) |
Subject D006 was different from the others in the cohort in several ways: 1) PSA transition across fractions was very flat, with a maximum: minimum ratio of 1.23 which was below the lower 25th quartile for any cohort, 2) all of his PSA concentrations, ranging from 0.18 to 0.23 mg ml−1, were below the 95% confidence interval for all subjects and 3) fructose concentrations, while individually in the range of other subjects values, displayed a large maximum : minimum ratio, 4.1, which was above the upper 75th quartile for any cohort. These differences were in spite of a nearly perfect monotonic decrease in PSA and increase in fructose with increasing fraction number and adequate fraction volumes (0.1–1.1 µl). Explanations for the flat PSA and steep fructose progression include inhomogeneity of either marker within their gland of origin and a large unmeasured testicular-epididymal fraction that has a significant dilutional effect primarily on the early PSA fractions. Though not measured, sperm counts could have supported this latter explanation.
Considering together all cohorts, the Lundquist and Regression estimates for seminal vesicle fructose concentration (excluding biologically implausible negative values) were highly correlated when using the y-intercept (b) Regression method (r2 = 0.961; P < 0.001; n = 39); correlation with the Lundquist method results were less impressive when using the x-intercept (–b/m) Regression method (r2 = 0.363; P = 0.03; n = 35). Similarly, Lundquist and Regression estimates for prostatic PSA concentration were highly correlated when using the y-intercepts (b) Regression method (r2 = 0.683; P < 0.001; n = 37), but not correlated when using the x-intercept (–b/m) method (r2 = 0.230; P = 0.20; n = 33). There was a statistically significant correlation between y-intercept and x-intercept Regression methods for seminal vesicle fructose concentration (r2 = 0.510; P = 0.002; n = 35), but not for PSA (r2 = 0.208; P = 0.25; n = 33), demonstrating a substantial sensitivity to the regression strategy used when the data are variable, such as in some of the non-D cohorts.
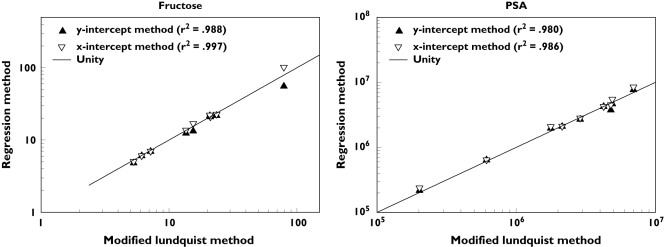
For cohort D, there was a very high correlation between the Lundquist and Regression estimates (by either method) for both seminal vesicle fructose and prostatic PSA (r2 ≥ 0.98; P < 0.001; n = 9) as well as between Regression methods, y-intercept and x-intercept, for both fructose and PSA (r2 ≥ 0.98; P < 0.001; n = 9) (Figure 5). As expected [21], the Regression method estimates provided by the x-intercept (–b/m) method were higher than those of the y-intercept (b) method for each cohort D subject, but this difference was small for both PSA (median 6%, interquartile range (IQR) 1%−11%) and fructose (median 4%, IQR 1%−11%).
Figure 5.
Correlation of Modified Lundquist method estimates (x-axis) for seminal vesicle fructose concentration (left panel) and prostatic PSA concentration (right panel) with corresponding Regression method estimates (y-axis) using both y-intercept (closed symbol) and x-intercept methods (open symbol) methods. The lines of unity are shown
These estimates of the proportion of whole ejaculate contributed by the seminal vesicle ranged from 55% to 61% and the prostate contribution ranged from 37% to 44%, having fairly narrow interquartile ranges with strong agreement among methods (Table 3). When summing these fractions for both glands for each subject, the median of the resulting totals were very close to unity (98%−101%) with IQRs all within 10%.
Fraction number sensitivity
When A and B were recalculated after removing one or more fractions from those originally with five, the resulting estimates for seminal vesicle fructose and prostatic PSA were positive for all possible combinations of either three or four fractions using both the Lundquist and Regression (y-intercept) methods (Table 4). The estimates for both four and three fraction sampling were within a similar a three-fold range of the five fraction estimates using either the Lundquist or Regression method. Fructose values ranged somewhat more widely than PSA values. When only two fractions were used, yielding identical estimates for the Lundquist and Regression methods, negative solutions were common and the estimates were very wide ranging. Estimates for all non-D cohorts demonstrated wider and more often negative parameter estimates than for cohort D (data not shown).
Table 4.
Sensitivity to fraction number. For all five cohort D subjects with five fractions, all possible combinations of four fractions (five combinations), three fractions (10 combinations), and two fraction (10 combinations) were re-calculated to simulate the range of possible estimates for fructose and PSA obtained when fewer than five fractions were collected. The numbers are the range of values (minimum–maximum) for the recalculated two, three, and four fraction estimates expressed as a ratio of the original five fraction estimate for fructose and PSA. Negative values are in parentheses
| Four fractions | Three fractions | Two fractions | |||
|---|---|---|---|---|---|
| Marker | Lundquist | Regression | Lundquist | Regression | Both |
| Fructose | 0.58–2.41 | 0.76–2.87 | 0.38–2.64 | 0.43–4.71 | (12.52)−13.32 |
| PSA | 0.70–1.32 | 0.71–1.31 | 0.60–1.47 | 0.67–1.50 | (24840.00)−2.29 |
Testicular-epididymal fraction
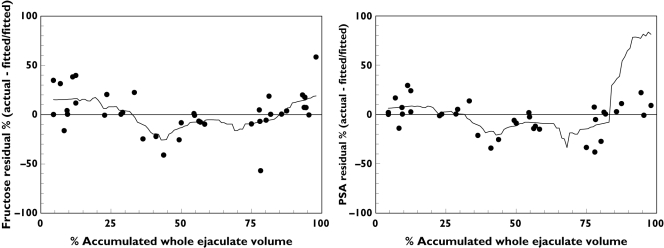
Since sperm count was measured in only four of nine cohort D subjects, no formal estimation for testicular-epididymal fraction was done. The peak sperm count occurred in the middle fractions in three of four subjects. Expressing the location of the peak as the percent of total volume (at the midpoint of the fraction) these subjects had peak sperm counts at 44%, 50% and 58% of accumulated ejaculate volume. The fourth subject had an early sperm count peak (11% of accumulated ejaculate volume) and a late peak (93% accumulated ejaculate volume) which would violate assumptions inherent to both the Lundquist and Regression methods. In all cases, the range of sperm counts across fractions was very flat with high : low ratios of 1.04, 1.42, 1.53, and 2.17, values outside or similar to the lower quartile values for both PSA and fructose (Table 1). Indirect evidence of the unmeasured testicular-epididymal volume was assessed by evaluating the residuals of the fructose-PSA fitted lines (Figure 6). For both fructose and PSA, the percent deviation from fitted values (actual minus fitted value divided by fitted value) showed a pattern of negative residual percent in the middle fractions of the split ejaculate, consistent with the location of the sperm peaks in three of four subjects assessed.
Figure 6.
Residual plots of fructose-PSA linear regression for cohort D. The residual percent is defined as the residual value (actual – fitted value) as a percent of the fitted value. The residuals and fitted values are taken from separate fructose vs. PSA linear regressions where each marker was the dependent variable. The x-axis values are the midpoints of the percent of accumulated whole ejaculate volume represented by each of the fractions. Trend lines are smoothed using the inverse square method with 20% sampling
Discussion
Knowledge of drug kinetics in each of the major glands of the male genital tract is essential to the understanding of drug action in these compartments. In the many prior studies assessing drug concentrations in semen, even in split fractions, no quantitative estimates of drug concentration in the fluids from individual glands have been reported. At best, only early fraction or late fraction predominance has been suggested and used to infer relative concentration differences between fluids from the major glands. Our methods, for the first time, can be used to estimate quantitative drug concentrations in the seminal vesicle and prostate to determine compartmental pharmacokinetics in these compartments. Building on the previously described, but rarely used, split ejaculate method only partially developed by Lundquist, we explored several modifications of this simple and noninvasive method to quantify better drug concentration in the seminal vesicle and prostate. In these developmental studies, we established 1) the superiority of a five-chamber collection device among three other options, 2) superiority of three–five ejaculate fractions relative to fewer (unstable estimates) and more (sample handling difficulty, 3) a consistent inverse relationship between fructose and PSA concentrations in split ejaculate fractions consistent with the methodologic assumptions, and 4) a simpler, novel Regression method for estimating fructose and PSA in their glands of origin, an essential step toward demonstrating the feasibility of quantitative determination of drug concentrations in the seminal vesicle and prostate. One need only assay for the drug of interest in each fraction to estimate noninvasively and quantitatively the drug concentration in the seminal vesicle and prostate.
Collection and analytical method selection
After initial use of the eight-compartment device, we explored the 18-compartment device and the trough to increase the number of fractions to improve our linear regression estimates. However, the multicompartment devices with two rows occasionally added confusion as to the correct sequence of fractions and led to tiny fractions too small to analyze. These devices also more frequently had nonliquefied coagulum making for difficult and partial extraction of fluid. Liquefaction of semen results from PSA-mediated proteolysis of coagulum proteins, primarily semenogelin I which arises exclusively from the seminal vesicle [24, 25]. Apparently, we achieved our goal of maximizing fraction heterogeneity so well that seminal vesicle fluid mixing with prostatic fluid was sufficiently incomplete that liquefaction did not occur. Leonardi et al. reported similar problems when separating semen into 10–15 fractions along a turning wheel, resulting in persistent coagulum in PSA-poor late fractions [26].
Interestingly, the collection of just three–five fractions usually resulted in adequate liquefaction, excellent linear fructose-PSA relationships, and biologically plausible seminal vesicle fructose and prostatic PSA estimates. However, dropping one or two fractions from those who had five and recalculating resulted in a range of estimates three-fold higher or lower relative to using all five fractions. Having just two fractions resulted in wildly fluctuating and biologically implausible negative results, even in our best behaved cohort. Altogether, the five compartment device used by cohort D represented the best balance between simplicity of sample collection, adequate fraction number, sufficient fraction volume for multiple assays, and enough fraction heterogeneity to result in both liquefaction and consistent fructose-PSA linearity. The concordance of Modified Lundquist and Regression estimates of marker concentrations in their glands of origin was greatest when subjects used the five-chamber device. We have used the five-compartment device in subsequent studies with 100% success of achieving three or more fractions, most typically five [23].
Our observations of fructose concentrations in semen, 12.5 mm, are near the mean value (15 mm) and within the range (8–35} of many reports in the literature recently summarized by Owen & Katz [27]. Unfortunately, there is no standard available for fructose or PSA concentrations in seminal vesicle or prostatic fluid, so we simply compared the Lundquist method with the Regression method and found them very closely correlated for our selected cohort D. When all other cohorts were considered with their more frequently wide ranging estimates, the correlation was typically excellent between methods as long as the y-intercept Regression was used. There was either poor or no correlation with the x-intercept Regression method, perhaps due to a combination of an exaggerated attenuation effect of both fructose and PSA using x-intercept estimates and also because this method involves using two variables, each with inherent estimation error, from the fitted line (slope and intercept), rather than simply one estimated variable (y-intercept).
The algebraic equations used to provide the Lundquist estimates are essentially the same as simple two-point linear regression equations, so, comparing the two methods is essentially selecting the median y-intercept from a series of lines defined by all possible fructose-PSA pairs (Lundquist) and comparing these to a full set of fructose-PSA fractions through which a single line is fitted to estimate the y-intercept. In practical terms, software is easily accessible to fit simple linear regressions, so the Regression method is likely to be more accessible for those who do not wish to set up the formulae to calculate the Lundquist estimates.
Methodologic Improvements
Non-invasive quantitative estimates of fructose and PSA concentrations in their glands of origin has only previously been reported by Lundquist [10] and there are no reports that apply his method to the quantitative assessment of drug concentration in the accessory glands. Lundquist, however, did use the method to determine calcium concentration among the accessory glands by determining the linear equation for calcium vs. citrate (his prostatic marker) and then using the calcium axis intercept, where citrate equalled zero, as the estimate of calcium concentration in the seminal vesicle, similar to our more fully developed one step Regression method [10].
Most investigators using the split ejaculate to assess accessory gland drug distribution have not measured glandular markers, but only measured drug in the first and second or, rarely, later fraction [15, 16, 28–31]. A few authors include one or more glandular markers (most often zinc for prostatic, sometimes fructose for seminal vesicular, and, rarely, sperm for testicular-epididymal) and describe higher marker concentration in an ejaculate fraction as supportive evidence of that fraction's association with one of the glands [12, 17, 32–34]. Some authors simply conclude a qualitatively higher drug concentration in the prostate if drug is higher in the first fraction, as was observed for pivampicillin [34], doxycycline [12], and erythromycin [32], and higher drug concentration in the seminal vesicle for higher drug concentration in the second fraction. There is one quantitative use of the split ejaculate method, by Rui et al. [14], where the percent of total ejaculate contributed by each gland to the whole was calculated (PR 18%, TE 2%, and SV 80%) as was done by Lundquist [6, 10].
We believe these qualitative methods, without explicit calculation of marker concentrations in the glands of origin and subsequent calculation of drug concentration, are insensitive to clinically significant drug concentration differences that might exist between glands. In our studies, the median fructose and PSA concentration gradients across three–five fractions were only 1.8- and 3.6-fold differences, respectively, even when prior to ejaculation there was complete absence of each marker in one of the glands indicating substantial mixing of fluid from each gland. Drug concentration gradients across glands cannot be greater than fructose and PSA and very likely will be smaller. Either vary large sample sizes or quantitative use of glandular markers would be needed to improve the sensitivity of such methods in detecting inter gland drug concentration differences of clinical relevance.
Limitations of the method
Potential limitations of the new method we propose concern violation of methodologic assumptions and statistical issues. Regarding the first three methodologic assumptions, namely the unique glandular markers, inhomogeneity of markers across fractions, and within gland homogeneity, there is ample evidence in the literature and our own data that these assumptions are generally true [6, 10, 35–39]. The assumption of trivial pre-ejaculate volume is reasonable since it is a relatively minor component of ejaculate, pre-ejaculate was removed prior to ejaculation, and there was no evidence of early fraction dilutional effects. Similarly, ignoring the testicular-epididymal contribution appeared reasonable since it ranges from 2% to 5% of the ejaculate volume [6, 10]. Our data also suggest that any dilutional effect of the testicular-epididymal fluid is small based on our residual analysis We attempted to use sperm counts to quantify directly the testicular-epididymal contribution, but it behaved poorly as a compartmental marker as has been reported by others [10, 17]. This is due, perhaps, to the very small volume relative to the whole ejaculate, variability of other compartment markers which dominates the estimation method, highly variable timing of testiculo-epididymal fluid release (coincident with or following prostatic release) within and among studies, and heterogeneous sperm concentration within the testicular-epididymal fluid due, in part, to sperm residing largely within the ampulla as well as in transit from the epididymus [10, 35, 38, 40, 41].
We improved several limitations of the classic Lundquist method. First, Lundquist used sample volumes in his equations which introduced substantial measurement error given the very small fraction volumes and often tenacious fractions. We derived a set of equations that avoided the use of volume. Second, Lundquist used only two fractions at a time to estimate glandular marker concentrations and then subjectively selecting one or a few fraction pairs to represent the individual, thus ignoring a substantial amount of information in the other fractions. He also made no proposal to calculate a single point estimate for each individual. We modified the classic method by calculating A and B using all pairwise fraction combinations and then summarizing with the median to avoid subjective filtering of data. We took this further with the Regression approach which we developed to make use of all data simultaneously (rather than only pairwise) with well established, easily employed methods for which error estimates could be determined. The y-intercept Regression method most closely correlated with the modified Lundquist method and less often yielded biologically implausible negative estimates, so we have selected it for future use. Still, given the small number of fractions, the uncertainty inherent in intercept and slope estimates is large and accounting for error in multiple discrete regression steps is problematic. We have subsequently developed a statistical method which combines information across subjects and which is relatively insensitive to outliers due to experimental error or individual exceptions to assumptions of the split ejaculate method [23].
Alternative methods and validation
Other methods considered for assessment of seminal vesicle and prostate drug concentrations include biopsy, seminal vesicle cannulation, and prostatic massage. Biopsy is highly invasive and associated with more than minimal risk. Even if the biopsy is taken from a convenience sample of tissue removed for medical reasons repeated sampling over time within an individual is not possible, thus requiring large sample sizes and sparse sampling analytical methods. Direct seminal vesicle cannulation for sperm evaluation has been successfully performed by one investigator [9]. While this may be useful for further validation of our current work (see below), this method is more resource intensive, adds risk, and willing volunteers will be less common than for our noninvasive split ejaculate methods.
While prostatic massage has been used by some investigators to assess drug concentrations in the prostate [7, 8, 13, 42], there are several reasons for caution with this approach. First, in one study, drug concentrations in prostatic massage fluid are six-fold lower than the first of two split ejaculate fractions, a difference too large to be explained by seminal vesicle fluid dilutional effects, suggesting a real physiologic difference between fluid expressed by prostatic massage compared with ejaculation that is worthy of further investigation [13]. Second, seminal vesicular fluid contamination of prostatic massage fluid has been reported, which is not unexpected given the close proximity of the two glands [6], though adjustments for seminal vesicular contamination could easily be made by employing fructose measurement as in our split ejaculate estimates. It has also been reported that fluid produced by prostatic massage is heterogeneous and varies in its acid phosphatase content from early to late fractions [43]. Finally, producing sufficient volume for analysis via prostatic massage, even in the best hands, is commonly unsuccessful; Naber et al. reported prostatic massage yielding adequate volume for analysis in 10 of 16 and 4 of 24 patients in two of their studies [7, 8]. Much larger sample sizes or more limited assay volume requirements would be necessary to compensate for these sampling inefficiencies. By contrast, we and others were successful collecting multiple split ejaculate fractions from nearly all subjects in volumes sufficient for analysis of both compartmental markers and drug or other biochemical markers of interest [6, 10–12, 14, 26, 37–39, 44–46].
Our split ejaculate method has now been used to demonstrate the ability to provide quantitative estimates of seminal vesicular and prostatic drug concentrations. Probe drugs were chosen based on their likelihood of achieving different concentrations in the seminal vesicle and prostate so that the ability of the split ejaculate method to detect such differences could be demonstrated [23]. Despite the limitations of biopsy mentioned above, a confirmatory study with single point in time observations of tissue biopsy samples could be done using the same probe drugs in a convenience sample of patients planning to undergo surgical extirpation of the prostate and seminal vesicles.
Applications
The same methods used to assess gland specific drug concentrations should be feasible for quantitative estimation of native biochemicals of interest, cells, and pathogens. For example, these split ejaculate methods could contribute to our understanding of antiretroviral drug, susceptible and infected CD4 cells, and free HIV pharmacodynamic relationships within individual glands of the male genital tract. Microbiologic and immunologic parameters of interest could be quantitatively evaluated in the setting of prostatitis. Male fertility studies may also benefit from noninvasive quantification and localization of hormones and other endogenous chemicals of interest. Prostate specific drug concentration of oncology treatments could be noninvasively assessed. Beyond these specific therapeutic applications, these methods can be used, along with selected probe drugs, to investigate the individual glands of the male genital tract to define better local physiologic factors (e.g. pH, drug binding proteins, and membrane transporters) which control drug movement into these compartments to enable the building of predictive models for future drugs developed for use in these glands.
In conclusion, we successfully demonstrated the feasibility of quantitatively and noninvasively determining the contribution of the prostate and seminal vesicle to the ejaculate through the calculation of fructose and PSA concentrations in their glands of origin using a five-compartment device, three–five split ejaculate fractions, and readily accessible linear regression methods. This work significantly extends existing knowledge in its careful selection of an optimal collection device, determination of optimal fraction number, avoidance of volume assessment, employment of improved statistical methods for split ejaculate calculations, and quantitative assessment of glandular contribution to ejaculate through fructose and PSA assessment in their glands of origin. Armed with these methods, quantitative estimation of drug concentration in the seminal vesicles and prostate is possible. Improved means of accounting for the testicular-epididymal contribution to the ejaculate and measuring uncertainty in the statistical methods would be welcome improvements. The ability to quantify drugs, endogenous chemicals, cells, and pathogens in the accessory glands of the male genital tract opens the way to formal assessment of gland-specific pharmacokinetic and pharmacodynamic studies to inform the rational selection of drugs and their regimens for numerous conditions where the male genital tract is a relevant site of action.
Acknowledgments
This work was supported in part by a Mid-Career Investigator Award in Patient-Oriented Research (NIH K24 AI01825), General Clinical Research Center (NIH M01 RR002719, M01RR000052-430919, 5M01RR000052-430852), Johns Hopkins University Center for AIDS Research (NIH P30 AI042855), & HIV Prevention Trials Network Central Laboratory (NIH U01 AI46745).
The authors wish to thank the following individuals for their excellent and sustained technical contributions to these studies without which none of this work would have been accomplished: Anita Guidos, R.N., Jared Christopher, BSN, Jin Lee, MS, James Johnson, and Jane Scocca, PhD.
Conflict of interest
None of the authors identify a conflict of interest relevant to this manuscript.
Appendix 1:Nomenclature and derivation of Modified Lundquist equations
Nomenclature
A concentration of PSA in prostatic secretions (calculated)
aj concentration of PSA in fraction j (measured)
B concentration of fructose in seminal vesicle secretions (calculated)
bj concentration of fructose in fraction j (measured)
pj proportion of prostatic fluid in secretion fraction j (calculated)
vj proportion of seminal vesicle secretion in fraction j (calculated)
Cp concentration of substance of interest in prostatic secretions (calculated)
Cv concentration of substance of interest in seminal vesicle secretions (calculated)
cj concentration of substance of interest in the fraction j (measured)
Derivation
If one assumes, for the sake of simplicity, that only the prostate and seminal vesicle contribute to the whole ejaculate, then each fraction of a split ejaculate contains a proportion form the seminal vesicle, vj, and a proportion from the prostate, pj, such that within any given fraction:
| (1) |
In any discrete fraction j of ejaculate, which is the mixture of a contribution from two separate glands (prostate and seminal vesicle), the concentration of a biochemical marker originating uniquely from only one of the contributing glands (aj, PSA unique to prostate or bj, fructose unique to seminal vesicle) is proportional to the concentration of the marker in the originating gland (A, PSA concentration in prostate and B, fructose in seminal vesicle) times the proportion of the fraction originating from that gland (pj and vj):
| (2) |
or
| (3) |
If the sum of the proportions within each fraction j which originates from each gland equals 1 (equation 1), then the sum for all fractions of the ratio of the glandular marker concentration relative to its concentration in its gland of origin (aj/A and bj/B) also equals 1:
| (4) |
If there are two fractions of the ejaculate, one can determine A and B by solving a series of equations. Solving first for B, exclusive of a term for A (equation 5) yields:
| (5a) |
or
| (5b) |
where a1, a2, b1, and b2 are the marker concentrations in fraction 1 and 2. Then, substituting the solution for B back into our previous equation 4 and solving for A using the marker concentrations for fraction 1 and 2 yields:
| (6a) |
or
| (6b) |
A and B are now expressed solely in terms of measurable quantities, aj and bj, the marker concentrations in each fraction. Equation 6a and 6b yield identical solutions for each unique fraction pair. Solving first for A then B, by substituting bjs for ajs, also yields identical solutions. Each unique pair of fractions yields discrete solutions.
Now, the amount of prostate and seminal vesicular contribution in each fraction, pj and vj, can be calculated in terms of two known quantities, the measured marker concentration in the fraction, aj or bj, and the calculated value of the marker concentration in its gland of origin, A or B, respectively, using equations 4.
One can now calculate the concentration of the unknown drug in the originating compartments (Cp and Cv). The concentration of the drug of interest in each fraction (cj) is the sum of the concentrations in the originating glands (Cp or Cv) times the proportion contributed by that gland to the specific fraction (pj or vj), expressed as follows:
| (8) |
With at least two fractions, one can determine Cp and Cv by solving another series of equations based on equation 8. First, using equation 8, solve for Cv, exclusive of the Cp term (equation 9); then, using equation 8, use this result for Cv to solve for the single remaining unknown, Cp, in the previous expression using either c1 or c2. Rearrangements yield the values of Cv and Cp as follows:
| (9) |
| (10) |
Solutions for Cv achieved by reversing c1 with c2 and p1 with p2 yields identical solutions and solutions for Cp, using either c1 or c2, yield identical solutions. Likewise, solving first for Cp in equation 9 by making the appropriate substitution of pjs for vjs, also yields identical solutions. Since each unique pair of fractions yields discrete solutions for Cv and Cp, we reported the solution as the median of all pairwise combinations of fractions.
References
- 1.Ndovi TT, Parsons TL, Hendrix CW. FL: Estimating seminal vesicle (SV) and prostate (PR) gland concentrations of drugs using linear regression of gland-specific biochemical markers in split ejaculate fractions. Paper presented at: American Society for Clinical Pharmacology and Therapeutics Annual Meeting, Orlando. [Google Scholar]
- 2.Ndovi TT. Baltimore: Graduate Training Program in Clinical Investigation, Johns Hopkins University; Compartmental kinetics of antiretroviral drugs in the human male genital tract. PhD thesis. [Google Scholar]
- 3.Choi L. Baltimore: Biostatistics, Johns Hopkins University; Modelling biomedical data and the foundations of bioequivalence. PhD thesis. [Google Scholar]
- 4.Taylor S, Boffito M, Vernazza PL. Antiretroviral therapy to reduce the sexual transmission of HIV. J HIV Ther. 2003;8:55–66. [PubMed] [Google Scholar]
- 5.Kepler TB, Perelson AS. Drug concentration heterogeneity facilitates the evolution of drug resistance. Proc Natl Acad Sci USA. 1998;95:11514–9. doi: 10.1073/pnas.95.20.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rui H, Mevag B, Purvis K. Two-dimensional electrophoresis of proteins in various fractions of the human split ejaculate. Int J Androl. 1984;7:509–20. doi: 10.1111/j.1365-2605.1984.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 7.Naber KG, Kinzig M, Adam D, Sorgel F, Bajorski AH, Kiehn R. Concentrations of cefpodoxime in plasma, ejaculate and in prostatic fluid and adenoma tissue. Infection. 1991;19:30–5. doi: 10.1007/BF01643755. [DOI] [PubMed] [Google Scholar]
- 8.Naber KG, Sorgel F, Kinzig M, Weigel DM. Penetration of ciprofloxacin into prostatic fluid, ejaculate and seminal fluid in volunteers after an oral dose of 750 mg. J Urol. 1993;150:1718–21. doi: 10.1016/s0022-5347(17)35877-9. [DOI] [PubMed] [Google Scholar]
- 9.Jarow JP. Seminal vesicle aspiration of fertile men. J Urol. 1996;156:1005–7. [see comments] [PubMed] [Google Scholar]
- 10.Lundquist F. Aspects of the biochemistry of human semen. Acta Physiologica Scandinavica. 19:1–95. doi: 10.1111/j.1748-1716.1949.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 11.Lindholmer C, Carlstrom A, Eliasson R. Occurrence and origin of proteins in human seminal plasma with special reference to albumin. Andrologia. 1974;6:181–96. doi: 10.1111/j.1439-0272.1974.tb01619.x. [DOI] [PubMed] [Google Scholar]
- 12.Eliasson R, Malmborg AS. Concentrations of doxycycline in human seminal plasma. Scand J Infect Dis Suppl. 1976;9:32–6. [PubMed] [Google Scholar]
- 13.Naber KG, Sorgel F, Kees F, Jaehde U, Schumacher H. Pharmacokinetics of ciprofloxacin in young (healthy volunteers) and elderly patients, and concentrations in prostatic fluid, seminal fluid, and prostatic adenoma tissue following intravenous administration. Am J Med. 1989;87:57S–59S. doi: 10.1016/0002-9343(89)90023-5. [DOI] [PubMed] [Google Scholar]
- 14.Rui H, Torjesen PA, Jacobsen H, Purvis K. Testicular and glandular contributions to the prolactin pool in human semen. Arch Androl. 1985;15:129–36. doi: 10.3109/01485018508986902. [DOI] [PubMed] [Google Scholar]
- 15.Schramm P. Ofloxacin: concentration in human ejaculate and influence on sperm motility. Infection. 1986;14(Suppl 4):S274–5. doi: 10.1007/BF01661292. [DOI] [PubMed] [Google Scholar]
- 16.Comhaire F. Concentration of pefloxacine in split ejaculates of patients with chronic male accessory gland infection. J Urol. 1987;138:828–30. doi: 10.1016/s0022-5347(17)43386-6. [DOI] [PubMed] [Google Scholar]
- 17.van Praag RM, Repping S, de Vries JW, Lange JM, Hoetelmans RM, Prins JM. Pharmacokinetic profiles of nevirapine and indinavir in various fractions of seminal plasma. Antimicrob Agents Chemother. 2001;45:2902–7. doi: 10.1128/AAC.45.10.2902-2907.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson RA, Reddy JM, Oswald C, Zaneveld JD. Enzymic determination of fructose in seminal plasma by initial rate analysis. Clin Chem. 1979;25:1780–2. [PubMed] [Google Scholar]
- 19.Beuter HO. Methods of enzymatic analysis. 3. Vol. 6. Weiheim, FRG: VCH; 1985. [Google Scholar]
- 20.Laffin RJ, Chan DW, Tanasijevic MJ, Fischer GA, Markus W, Miller J, Matarrese P, Sokoll LJ, Bruzek DJ, Eneman J, Nelson J, Bray KR, Huang J, Loveland KG. Hybritech total and free prostate-specific antigen assays developed for the Beckman Coulter access automated chemiluminescent immunoassay system: a multicenter evaluation of analytical performance. Clin Chem. 2001;47:129–32. [PubMed] [Google Scholar]
- 21.Fuller WA. Measurement error models. New York: Wiley; 1987. [Google Scholar]
- 22.Winsor CP. Which regression? Biometrics. 1946;2:101–9. [PubMed] [Google Scholar]
- 23.Ndovi TT, Choi L, Caffo B, Parsons T, Baker S, Zhao M, Rohde C, Hendrix CW. Quantitative assessment of seminal vesicle and prostate drug concentrations by use of a noninvasive method. Clin Pharmacol Ther. 2006;80:146–58. doi: 10.1016/j.clpt.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Robert M, Gagnon C, Semenogelin I. A coagulum forming, multifunctional seminal vesicle protein. Cell Mol Life Sci. 1999;55:944–60. doi: 10.1007/s000180050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert M, Gibbs BF, Jacobson E, Gagnon C. Characterization of prostate-specific antigen proteolytic activity on its major physiological substrate, the sperm motility inhibitor precursor/semenogelin I. Biochemistry. 1997;36:3811–9. doi: 10.1021/bi9626158. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi D, Colpi GM, Campana A, Balerna M. Protein characterization of multi-fraction split-ejaculates. Some physicochemical properties of prostatic and vesicular proteins. Acta Eur Fertil. 1983;14:181–9. [PubMed] [Google Scholar]
- 27.Owen DH, Katz DF. A Review of the Physical and Chemical Properties of Human Semen and the Formulation of a Semen Simulant. J Androl. 2005;26:459–69. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 28.Berger SA, Yavetz H, Paz G, Gorea A, Homonnai Z. Concentration of ofloxacin and ciprofloxacin in human semen following a single oral dose. J Urol. 1990;144:683–4. doi: 10.1016/s0022-5347(17)39554-x. [DOI] [PubMed] [Google Scholar]
- 29.Naber KG, Sorgel F, Sigl G, Schumacher H, Metz R. Penetration of temafloxacin into prostatic and seminal fluid in volunteers. In: Rubinstein E, editor. Proceedings of 3rd International Symposium on New Quinolones; Vancouver. pp. 29–30. [Google Scholar]
- 30.Schramm P, Schopf RE, Wildfeuer A. Josamycin concentration in human ejaculate and its influence on sperm motility – a contribution to antibiotic therapy in andrological patients. Andrologia. 1988;20:521–5. [PubMed] [Google Scholar]
- 31.Naber KG. Enoxacin concentration in seminal fluid, in prostate secretions and in prostatic adenoma tissue following oral administration or intravenous infusion. Infection. 1989;17(Suppl. 1):S30–36. doi: 10.1007/BF01643634. [DOI] [PubMed] [Google Scholar]
- 32.Eliasson R, Malmborg AS, Dornbusch K, Kvist U. Secretion of erythromycin into human semen – methodological, experimental and clinical spects. Curr Med Res Opin. 1978;5(Suppl 2) [Google Scholar]
- 33.Eliasson R, Dornbusch K. Levels of trimethoprim and sulphamethoxazole in human seminal plasma. Androl. 1977;9:195–202. doi: 10.1111/j.1439-0272.1977.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 34.Malmborg AS, Dornbusch K, Eliasson R, Lindholmer C. Concentrations of various antibacterials in human seminal plasmaGenital Infections and their complications. Stockholm: Almqvist and Wiksell International; 1975. pp. 307–12. [Google Scholar]
- 35.Broesike G. Ueber die Entleerung und Beschaffenheit der menschlichen Samenflussigheit. Arch F Mikr Anat. 1911;78:128. [Google Scholar]
- 36.McLeod J, Hotchkiss RS. Distribution of certain chemical constituents in human ejaculate. J Urol. 1942;48:225. [Google Scholar]
- 37.Gerozissis K, Jouannet P, Soufir JC, Dray F. Origin of prostaglandins in human semen. J Reprod Fertil. 1982;65:401–4. doi: 10.1530/jrf.0.0650401. [DOI] [PubMed] [Google Scholar]
- 38.Tauber PF, Zaneveld LJ, Propping D, Schumacher GF. Components of human split ejaculates. I. Spermatozoa, fructose, immunoglobulins, albumin, lactoferrin, transferrin and other plasma proteins. J Reprod Fertil. 1975;43:249–67. doi: 10.1530/jrf.0.0430249. [DOI] [PubMed] [Google Scholar]
- 39.Simbini T, Umapathy E, Jacobus E, Tendaupenyu G, Mbizvo MT. Study on the origin of seminal leucocytes using split ejaculate technique and the effect of leucocytospermia on sperm characteristics. Urol Int. 1998;61:95–100. doi: 10.1159/000030296. [DOI] [PubMed] [Google Scholar]
- 40.Simbini T. Study on the origin of seminal leucocytes using split ejaculate technique and the effect of leucocytospermia on sperm characteristics. Urol Int. 1998;61:95–100. doi: 10.1159/000030296. [DOI] [PubMed] [Google Scholar]
- 41.van Praag RM, van Heeswijk RP, Jurriaans S, Lange JM, Hoetelmans RM, Prins JM. Penetration of the nucleoside analogue abacavir into the genital tract of men infected with human immunodeficiency virus type 1. Clin Infect Dis. 2001;33:e91–92. doi: 10.1086/322682. [DOI] [PubMed] [Google Scholar]
- 42.Naber KG, Sorgel F, Kees F, Schumacher H, Metz R, Grobecker H. In-vitro activity of fleroxacin against isolates causing complicated urinary tract infections and concentrations in seminal and prostatic fluid and in prostatic adenoma tissue. J Antimicrob Chemother. 1988;22(Suppl D):199–207. doi: 10.1093/jac/22.supplement_d.199. [DOI] [PubMed] [Google Scholar]
- 43.Hansen PF. Determination of the ‘acid’ prostatic phosphatase as a new method for medicolegal demonstration of sperm spots. Acta Path Microbiol. 1946;23:187. [Google Scholar]
- 44.Tauber PF, Zaneveld LJ, Propping D, Schumacher GF. Components of human split ejaculates. II. Enzymes and proteinase inhibitors. J Reprod Fertil. 1976;46:165–71. doi: 10.1530/jrf.0.0460165. [DOI] [PubMed] [Google Scholar]
- 45.Stegmayr B, Ronquist G, Kollberg H, Brody I. Analysis of ejaculates from patients with cystic fibrosis. Ultrastruct Pathol. 1981;2:357–63. doi: 10.3109/01913128109081983. [DOI] [PubMed] [Google Scholar]
- 46.Bjorndahl L, Kjellberg S, Kvist U. Ejaculatory sequence in men with low sperm chromatin-zinc. Int J Androl. 1991;14:174–8. doi: 10.1111/j.1365-2605.1991.tb01079.x. [DOI] [PubMed] [Google Scholar]