Abstract
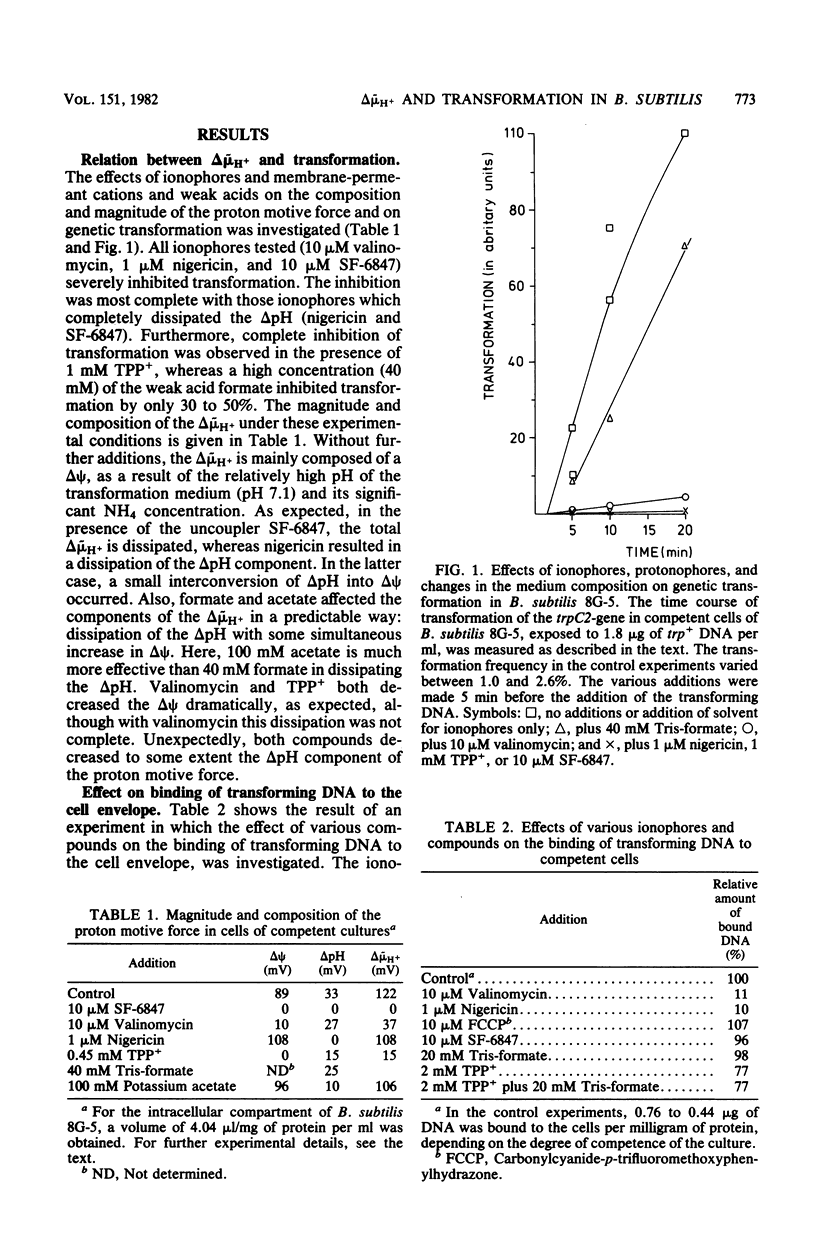
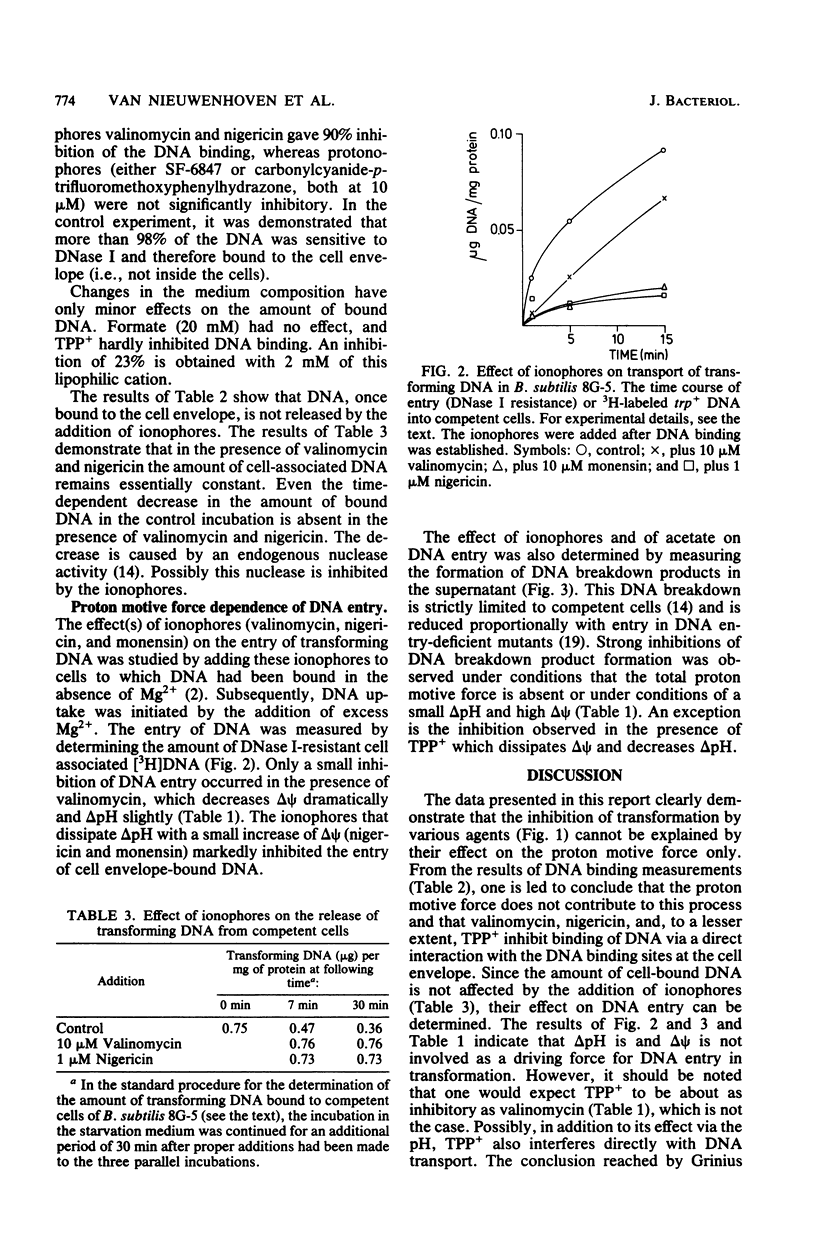
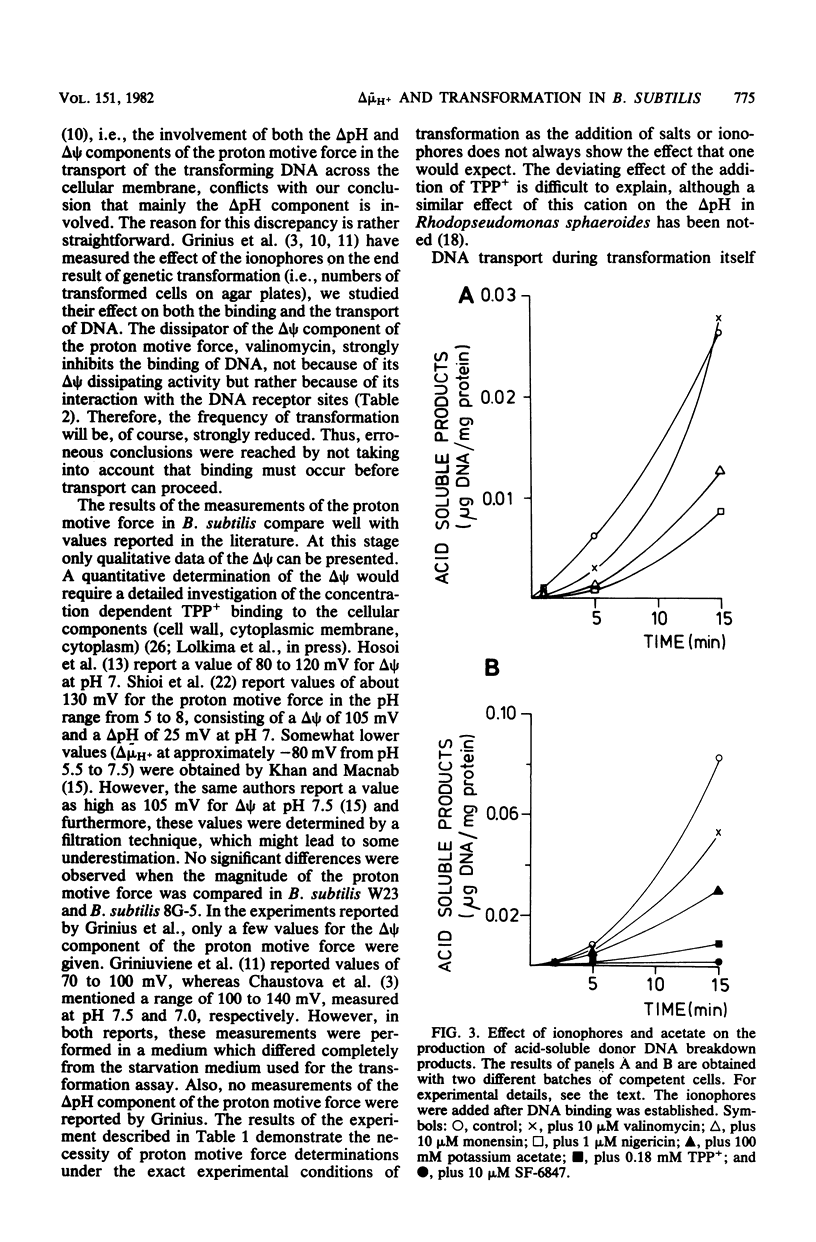
This study explored the role of the proton motive force in the processes of DNA binding and DNA transport of genetic transformation of Bacillus subtilis 168 strain 8G-5 (trpC2). Transformation was severely inhibited by the ionophores valinomycin, nigericin, and 3,5-di-tert-4-hydroxybenzylidenemalononitrite (SF-6847) and by tetraphenylphosphonium. The ionophores valinomycin and nigericin also severely inhibited binding of transforming DNA to the cell envelope, whereas SF-6847 and carbonylcyanide-p-trifluoromethoxyphenylhydrazone hardly affected binding. The proton motive force, therefore, does not contribute to the process of DNA binding, and valinomycin and nigericin interact directly with the DNA binding sites at the cell envelope. The effects of ionophores, weak acids, and tetraphenylphosphonium on the components of the proton motive force and on the entry of transforming DNA after binding to the cell envelope was investigated. DNA entry, as measured by the amount of DNase I-resistant cell-associated [3H]DNA and by the formation of DNA breakdown products, was severely inhibited under conditions of a small proton motive force and also under conditions of a small delta pH and a high electrical potential. These results suggest that the proton motive force and especially the delta pH component functions as a driving force for DNA uptake in transformation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayad S. R., Barker G. R. The integration of donor and recipient deoxyribonucleic acid during transformation of Bacillus subtilis. Biochem J. 1969 Jun;113(1):167–174. doi: 10.1042/bj1130167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitenwerf J., Venema G. Transformation in bacillus subtilis: fate of transforming DNA in transformation deficient mutants. Mol Gen Genet. 1977 Mar 7;151(2):203–213. doi: 10.1007/BF00338696. [DOI] [PubMed] [Google Scholar]
- Chaustova L. P., Grinius L. L., Griniuviene B. B., Jasaitis A. A., Kadziauskas J. P., Kiausinyte R. J. Studies on energy supply for genetic processes. Involvement of membrane potential in genetic transformation of Bacillus subtilis. Eur J Biochem. 1980 Jan;103(2):349–357. doi: 10.1111/j.1432-1033.1980.tb04321.x. [DOI] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Conditions affecting the isolation from transformed cells of Bacillus subtilis of high-molecular-weight single-stranded deoxyribonucleic acid of donor origin. J Bacteriol. 1973 Oct;116(1):146–153. doi: 10.1128/jb.116.1.146-153.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Kinetic analysis of the products of donor deoxyribonucleate in transformed cells of Bacillus subtilis. J Bacteriol. 1973 Oct;116(1):154–162. doi: 10.1128/jb.116.1.154-162.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972 Feb 28;64(1):9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. IV. The endwise attachment and uptake of transforming DNA. J Mol Biol. 1972 Feb 28;64(1):31–46. doi: 10.1016/0022-2836(72)90319-1. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: size and distribution of the integrated donor segments. J Bacteriol. 1972 Aug;111(2):488–494. doi: 10.1128/jb.111.2.488-494.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornili S. L., Fox M. S. Electron microscope visualization of the products of Bacillus subtilis transformation. J Mol Biol. 1977 Jun 15;113(1):181–191. doi: 10.1016/0022-2836(77)90048-1. [DOI] [PubMed] [Google Scholar]
- Grinius L. Nucleic acid transport driven by ion gradient across cell membrane. FEBS Lett. 1980 Apr 21;113(1):1–10. doi: 10.1016/0014-5793(80)80482-0. [DOI] [PubMed] [Google Scholar]
- Harris E. J., van Dam K. Changes of total water and sucrose space accompanying induced ion uptake or phosphate swelling of rat liver mitochondria. Biochem J. 1968 Feb;106(3):759–766. doi: 10.1042/bj1060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi S., Mochizuki N., Hayashi S., Kasai M. Control of membrane potential by external H+ concentration in Bacillus subtilis as determined by an ion-selective electrode. Biochim Biophys Acta. 1980 Aug 14;600(3):844–852. doi: 10.1016/0005-2736(80)90487-3. [DOI] [PubMed] [Google Scholar]
- Joenje H., Venema G. Different nuclease activities in competent and noncompetent Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):25–33. doi: 10.1128/jb.122.1.25-33.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. Proton chemical potential, proton electrical potential and bacterial motility. J Mol Biol. 1980 Apr 15;138(3):599–614. doi: 10.1016/s0022-2836(80)80019-2. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. The steady-state counterclockwise/clockwise ratio of bacterial flagellar motors is regulated by protonmotive force. J Mol Biol. 1980 Apr 15;138(3):563–597. doi: 10.1016/s0022-2836(80)80018-0. [DOI] [PubMed] [Google Scholar]
- Piechowska M., Fox M. S. Fate of transforming deoxyribonucleate in Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):680–689. doi: 10.1128/jb.108.2.680-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelnikov A. G., Domaradsky I. V. Effect of metabolic inhibitors on entry of exogenous deoxyribonucleic acid into Ca2+-treated Escherichia coli cells. J Bacteriol. 1981 May;146(2):435–443. doi: 10.1128/jb.146.2.435-443.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Kaback H. R. Involvement of the proton electrochemical gradient in genetic transformation in Escherichia coli. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1153–1160. doi: 10.1016/0006-291x(81)90739-7. [DOI] [PubMed] [Google Scholar]
- Shioi J. I., Matsuura S., Imae Y. Quantitative measurements of proton motive force and motility in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):891–897. doi: 10.1128/jb.144.3.891-897.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENEMA G., PRITCHARD R. H., VENEMA-SCHROEDER T. FATE OF TRANSFORMING DEOXYRIBONUCLEIC ACID IN BACILLUS SUBTILIS. J Bacteriol. 1965 May;89:1250–1255. doi: 10.1128/jb.89.5.1250-1255.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Competence in Bacillus subtilis transformation system. Nature. 1967 Feb 25;213(5078):773–775. doi: 10.1038/213773a0. [DOI] [PubMed] [Google Scholar]
- Zaritsky A., Kihara M., Macnab R. M. Measurement of membrane potential in Bacillus subtilis: a comparison of lipophilic cations, rubidium ion, and a cyanine dye as probes. J Membr Biol. 1981;63(3):215–231. doi: 10.1007/BF01870983. [DOI] [PubMed] [Google Scholar]



