Abstract
What is already known about this subject
• Rivaroxaban is a novel anticoagulant with predictable, dose-proportional pharmacokinetics and pharmacodynamics in healthy subjects.
• It is in clinical development for the prevention of thromboembolic disorders in patients undergoing major orthopaedic surgery.
• Nonsteroidal anti-inflammatory drugs (NSAIDs), such as naproxen, are widely used for pain relief and treatment of inflammation, particularly in patients who have undergone orthopaedic surgery. As it is likely that patients receiving rivaroxaban after orthopaedic surgery will also receive NSAIDs, this study was performed to determine whether there was any mechanistic interaction between rivaroxaban and naproxen.
What this study adds
• This study demonstrated that there was no mechanistic interaction between rivaroxaban and naproxen in healthy subjects.
• This finding was used to design large-scale clinical trials of rivaroxaban, in which patients were also allowed to use NSAIDs.
• These ongoing trials will provide definitive data regarding the interaction between rivaroxaban and NSAIDs.
Aims
Rivaroxaban (BAY 59-7939) is in advanced clinical development for the prevention and treatment of thromboembolic disorders. Frequent co-medications in the patient populations likely to receive rivaroxaban include NSAIDs. This randomized, two-way crossover study, with a naproxen run-in period, was performed to determine whether naproxen influences the tolerability, pharmacodynamics and pharmacokinetics of rivaroxaban.
Methods
Eleven healthy, young males received naproxen 500 mg on two consecutive days, a single dose of rivaroxaban 15 mg, or both.
Results
Treatments were well tolerated: adverse events (eight in total), reported by three subjects, were mild and not drug related. Rivaroxaban inhibited Factor Xa activity by 35% and prolonged prothrombin time [by 1.4 times baseline (tb)], activated partial thromboplastin time (1.3 tb) and the HepTest (1.9 tb). Naproxen had no influence on these measures and the combination of rivaroxaban and naproxen did not affect platelet aggregation. Rivaroxaban and naproxen given together significantly increased bleeding time compared with rivaroxaban alone (P = 0.017). However, this difference was small compared with the effect of naproxen given alone, except in one subject. Least squares-means ratios for the AUC and Cmax of rivaroxaban after administration alone and with naproxen were 1.125 [90% confidence interval (CI) 0.995, 1.271] and 1.095 (90% CI 0.905, 1.325), respectively.
Conclusions
There appeared to be no clinically relevant interaction between rivaroxaban and naproxen in healthy subjects, although some individuals may be more sensitive to the combination. Large-scale Phase III clinical studies will be required to confirm whether there is an increased risk of bleeding during treatment with rivaroxaban and concomitant NSAIDs.
Keywords: BAY 59-7939, Factor Xa inhibitor, NSAID, oral anticoagulant, rivaroxaban
Introduction
Anticoagulants are commonly prescribed for a wide range of medical conditions, including the prevention of stroke in atrial fibrillation (AF) and the prevention of recurrent thromboembolic events in patients with acute coronary syndromes (ACS), such as non-ST-segment elevated myocardial infarction. Another important indication for anticoagulants is for the prevention and treatment of venous thromboembolism (VTE), such as deep vein thrombosis and pulmonary embolism, following surgery. In patients not given adequate anticoagulation, major orthopaedic surgery – such as total knee or hip replacement – carries a particularly high risk of VTE [1]. The currently available anticoagulants, though effective, have limitations [2]. Thus, unfractionated heparin and the low-molecular-weight heparins (LMWHs) require parenteral administration, making their long-term use outside hospital inconvenient. Vitamin K antagonists, such as warfarin, have a slow onset of action, a narrow therapeutic window and numerous drug and food interactions, and therefore require frequent monitoring.
Efforts are being made to develop new, oral anticoagulants with a wide therapeutic window and no requirement for monitoring [2]. Recently, selective inhibition of single clotting factors within the coagulation pathway, such as Factor Xa (FXa), has emerged as an attractive antithrombotic strategy, instead of the multitargeted anticoagulant effects of the heparins and vitamin K antagonists [3].
Rivaroxaban (BAY 59-7939) is a novel, oral, direct FXa inhibitor in advanced clinical development for the prevention and treatment of thromboembolic disorders. Rivaroxaban effectively inhibits thrombogenesis via selective inhibition of FXa, without the requirement for cofactors such as antithrombin [4]. In healthy subjects, the drug has been shown to be well tolerated, with predictable pharmacokinetics and predictable anticoagulant effects over a 5–80 mg dose range [5, 6]. Phase II clinical studies of rivaroxaban for the prevention of VTE after total hip or knee replacement have shown that it has a wide therapeutic window, with similar efficacy and safety to the LMWH enoxaparin at total daily doses of up to 20 mg (with twice-daily dosing) [7, 8]. These findings were confirmed in a further study for the prevention of VTE after total hip replacement, which established that rivaroxaban 10 mg given once daily is the optimum dose, in this indication [9].
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used for the relief of pain and inflammation, in both the short and long term, and as over-the-counter preparations or on prescription. NSAIDs are effective, although they are associated with side-effects, including the risk of gastrointestinal bleeding [10]. Chronic pain relief in patients with osteoarthritis (OA) or rheumatoid arthritis (RA) is a particularly important indication for which NSAIDs are commonly prescribed. These patients are also candidates for elective joint replacement surgery and are likely to be prescribed NSAIDs after surgery for both their analgesic and anti-inflammatory properties [11]. In accordance with current guidelines, most of these patients will also be prescribed an anticoagulant for VTE prevention [12]. Because of the likelihood of concomitant use of NSAIDs and anticoagulants, and because both types of drugs can affect haemostasis, interaction studies between new anticoagulants and NSAIDs are necessary. Accordingly, the present exploratory study was performed to determine whether there was any mechanistic interaction between the NSAID naproxen (Naprosyn®/Proxen®; Roche, Grenzach-Wyhlen, Germany) and rivaroxaban in healthy subjects.
Methods
Study design, subjects and treatments
This was a randomized, nonblinded, single-centre, two-way crossover study, with a naproxen run-in period, and was conducted in healthy, male White subjects aged 18–45 years with a body mass index of 18–32 kg m−2. Subjects attended a screening visit to determine if they were eligible for enrolment and were excluded if they had any of the following conditions that might have influenced the study results: known coagulation disorders (e.g. von Willebrand's disease, haemophilias); known disorders with increased risk of bleeding; known sensitivity to common types of bleeding (e.g. nasal); sensitivity to naproxen, rivaroxaban or any of the tablet ingredients; hypersensitivity to anti-inflammatory drugs, antirheumatics or analgesics; a history of gastrointestinal disease, signs or symptoms of gastrointestinal ulcers or inflammation of the intestine. Subjects provided written, informed consent before participating in the study, which was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, German drug law and with approval from the Ethics Committee of the North-Rhine Medical Council.
After enrolment, all subjects received two doses of naproxen 500 mg alone on consecutive days (naproxen run-in period). Subjects were then randomized to receive rivaroxaban alone or with naproxen. Rivaroxaban was administered as a single 15-mg dose on the second study day. The combination treatment consisted of naproxen 500 mg on the first study day, then coadministration of naproxen 500 mg and rivaroxaban 15 mg on the second day. After the first randomization period, subjects crossed over to receive the other treatment. There were 14-day wash-out periods between treatments to allow recovery of platelet function. Naproxen is an irreversible platelet inhibitor and its effect therefore subsides after complete turnover of the platelets. Subjects returned for a final examination 1 week after the last treatment.
The naproxen dose of 500 mg used in this study was selected because this dose is indicated in rheumatic diseases as well as for pain relief [13]. A slightly lower dose of naproxen (440 mg per day) is the maximum recommended by the Food and Drug Administration for over-the-counter treatment of minor ailments and pain relief. Rivaroxaban given at 15 mg has been shown to have relevant pharmacodynamic effects [5, 6] and is within the range of clinically effective doses (5–20 mg total daily dose) observed in Phase II studies for the prevention of VTE after major orthopaedic surgery [7–9].
Tolerability and adverse events
Adverse events were documented following questioning, or spontaneous reporting. They were classified according to the degree of severity. Heart rate, blood pressure and electrocardiograms (ECG), and the results of laboratory tests, namely haematology and clinical chemistry, were monitored.
Pharmacodynamic measurements
The anticoagulant effects of rivaroxaban and naproxen were determined in blood samples taken before, and at 0.5, 1, 2, 3, 4, 6, 8, 12, 15, 24, 36, 48 and 72 h after drug administration. Plasma was obtained by centrifugation and frozen until analysis. Factor X in plasma was activated to FXa using Russell's viper venom in the presence of calcium ions. The chromogenic substrate ZD-Arg-Gly-Arg-pNA (S-2765™; Chromogenix, Milan, Italy) was hydrolysed by FXa, releasing pNA, which was quantified by spectrophotometry at 405 nm. Standards and controls were prepared from the 3rd International Standard Coagulation Factors II and X Concentrate, Human 98/590 (NIBSC, Potters Bar, UK). Prothrombin time (PT) is a method of analysing clotting activated by the extrinsic coagulation pathway, and utilizes thromboplastin (tissue factor). PT was measured using freeze-dried thromboplastin from rabbit brain (Neoplastin® Plus; Roche Diagnostics, Mannheim, Germany). Activated partial thromboplastin time (aPTT) is a measure of clotting activated by the intrinsic coagulation pathway and was determined using a kaolin-activated test (Roche Diagnostics). The HepTest, an assay devised to monitor the anticoagulant effects of LMWHs by measuring anti-FXa activity, was assessed using a kit from Haemachem (St Louis, MO, USA). All three coagulation tests were carried out using a ball coagulometer KC 10 (Amelung, Germany), according to the manufacturer's instructions.
Platelet aggregation was determined quantitatively before, and 4 h after drug administration, using the Born method of turbidimetry [14]. Platelet-enriched plasma was prepared by low-speed centrifugation of whole blood and aggregation was induced by the addition of collagen over a range of concentrations (0.025–1 µg ml−1) (Nycomed, Unterschleissheim, Germany). The extent of inhibition of platelet aggregation was determined by titration with collagen, with the first concentration to cause maximum aggregation before drug administration being the 100% aggregation baseline.
Bleeding time was measured before, and 4 h after drug administration, and before discharge, using the Surgicutt® device (ITC, Milan, Italy) according to the Mielke template [15].
Pharmacokinetic measurements
Blood samples for characterization of the pharmacokinetics of rivaroxaban were taken at the time of drug administration, and after 0.5, 1, 2, 3, 4, 6, 8, 12, 15 and 24 h.
Rivaroxaban plasma concentrations were determined using a high-performance liquid chromatography (HPLC) system coupled to a tandem mass spectrometer in selected reaction monitoring mode with a turbo ion spray (Hewlett-Packard system 1100 coupled with MS/MS API 3000; MDS Sciex, Concord, ON, Canada). A close chemical analogue of rivaroxaban was used as the internal standard (BAY 60-4758; 5-chloro-N-({3- [3,5-dimethyl-4-(3-oxomorpholin-4-yl)phenyl]-2-oxo-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide; Bayer HealthCare AG, Wuppertal, Germany). Stock solutions of rivaroxaban and the internal standard were made up in acetonitrile. Before HPLC analysis, rivaroxaban and the internal standard were extracted from the matrix by solid-phase extraction using C18 cartridges. Both rivaroxaban and the internal standard eluted from the HPLC column with retention times of approximately 3.0 min. The ions monitored were 436→145 (rivaroxaban) and 464→145 (internal standard). The method was validated by assaying quality control samples of blank plasma spiked with known concentrations of rivaroxaban (n = 18). Rivaroxaban concentrations above the lower limit of quantification (0.5 µg l−1) were determined with a precision of 4.25–5.68% and an accuracy of 102–109%.
The following pharmacokinetic parameters were determined: area under the plasma concentration–time curve (AUC), AUC normalized to dose and body weight (AUCnorm), maximum plasma concentration (Cmax), Cmax normalized to dose and body weight (Cmax,norm), terminal elimination half-life (t1/2), and time to reach maximum drug concentration in plasma (tmax).
Statistical analysis
The bleeding times and collagen-activated platelet aggregation results for each subject 4 h after drug administration were analysed using descriptive statistics. Student's paired t-test was used to compare these pharmacodynamic parameters and 95% confidence intervals (95% CIs) on the differences were calculated. The pharmacokinetic parameters AUC and Cmax were analysed assuming log-normally distributed data. anova was used to compare the effects of rivaroxaban alone with the combination of rivaroxaban and naproxen, with sequence, subject, period and treatment effects. Point estimates (least squares-means) and 90% CIs were calculated.
Results
Subjects
Thirteen subjects took part in the study. Their mean age was 32.5 years (range 25–42 years), mean weight 81.4 kg, mean height 181.4 cm and mean body mass index 24.7 kg m−2 (range 19.4–28.3 kg m−2). Two subjects withdrew from the study after taking rivaroxaban alone but before receiving the combination of rivaroxaban and naproxen, one because of an unrelated adverse event (impetigo contagiosa), and one withdrew consent. As a result, 13 subjects received rivaroxaban and were included in the assessment of adverse events, whereas pharmacodynamic and pharmacokinetic data were obtained in 11 subjects.
Tolerability and adverse events
In total, eight adverse events were reported by three subjects. All were categorized as mild, none was considered to be drug related, and all resolved by the end of the study. One subject experienced a cough following naproxen alone, and immediately before administration of rivaroxaban and naproxen. Another subject experienced a headache after receiving rivaroxaban alone, and then had an episode of impetigo contagiosa that warranted withdrawal from the trial. A third subject had elevations in aspartate transaminase, alanine transaminase (ALT) and glutamate dehydrogenase, throat irritation and heartburn only after administration of the combination of rivaroxaban and naproxen. The elevations in liver enzymes were low, the highest being 2.5 times the upper limit of normal for ALT, and returned to normal within 19 days. The subject admitted to drinking alcohol the previous weekend, during the wash-out period. No clinically relevant changes in heart rate, ECG or blood pressure were observed.
Pharmacodynamics
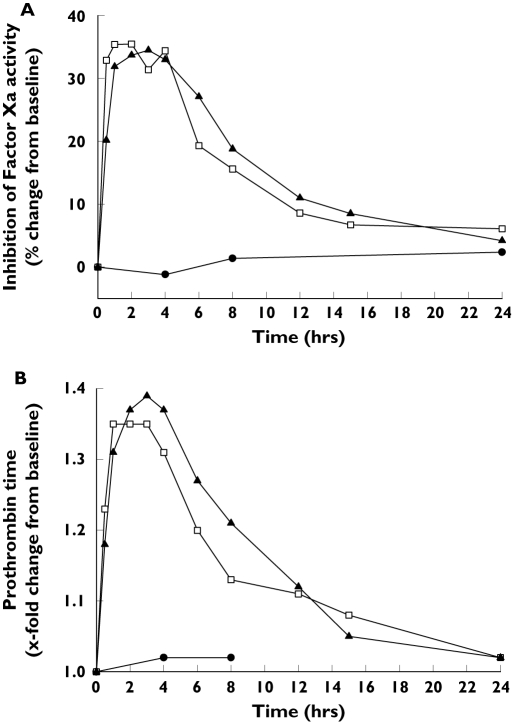
The median maximum inhibition of FXa activity (35.5%) occurred 2 h after administration of rivaroxaban alone. FXa activity was unaffected by naproxen given alone. Coadministration of rivaroxaban and naproxen resulted in a median maximum inhibition of FXa activity of 34.5% after 3 h (Figure 1A). Rivaroxaban prolonged PT, aPTT and the HepTest to similar extents when administered alone and in combination with naproxen, whereas monotherapy with the latter had no effect on these clotting tests (Figure 1B; data not shown for aPTT and the HepTest). The maximum PT prolongation was 1.35 times baseline (tb) 1 h after rivaroxaban alone and 1.39 tb 3 h after the combination. The median maximum prolongation of aPTT was 1.31 tb with both rivaroxaban alone (after 1 h) and with the combination (after 3 h). The HepTest was prolonged by 1.92 tb 1 h after rivaroxaban alone, and by 1.88 tb 2 h after administration of the combination.
Figure 1.
Effect of naproxen (500 mg), rivaroxaban (15 mg) and the combination of rivaroxaban (15 mg) and naproxen (500 mg) on median inhibition of Factor Xa activity (A) and prothrombin time (B) in healthy male subjects (n = 11). Naproxen (•), rivaroxaban (□), rivaroxaban + naproxen (▴)
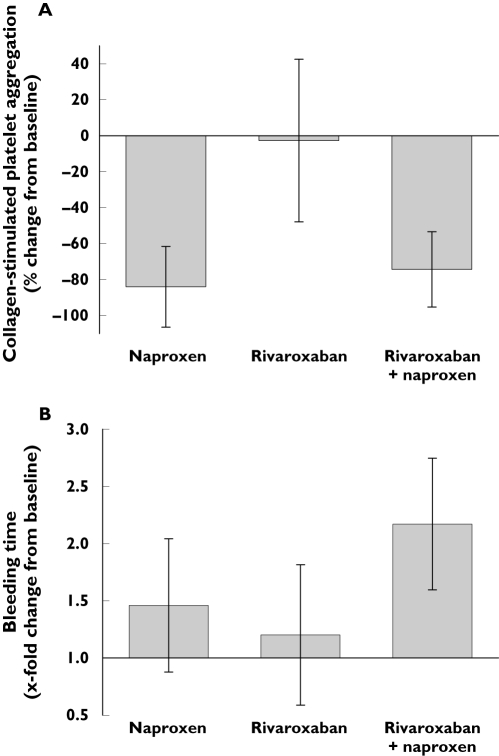
Rivaroxaban administered alone did not inhibit collagen-stimulated platelet aggregation (mean 1.5% inhibition of aggregation; Figure 2A). Naproxen alone and in combination with rivaroxaban markedly inhibited platelet aggregation (by 85.4% and 80.4%, respectively). There was no statistically significant difference in the extent of inhibition of platelet aggregation after either naproxen alone or the combination of rivaroxaban and naproxen (P = 0.64; Table 1).
Figure 2.
Effect of naproxen (500 mg), rivaroxaban (15 mg) and the combination of rivaroxaban (15 mg) and naproxen (500 mg) (mean ± SD) on (A) collagen-stimulated platelet aggregation (percentage change from baseline) and (B) bleeding time (x-fold change from baseline) in healthy male subjects (n = 11) 4 h after administration of medication
Table 1.
Maximum collagen-stimulated platelet aggregation and bleeding time in healthy male subjects 4 h after administration of naproxen 500 mg alone, rivaroxaban 15 mg alone, or rivaroxaban 15 mg and naproxen 500 mg given together (n = 11)
| Parameter | Rivaroxaban minus naproxen | Rivaroxaban + naproxen minus naproxen | Rivaroxaban minus rivaroxaban + naproxen |
|---|---|---|---|
| Collagen-stimulated platelet aggregation,% | 67.4* (40.0, 94.8) | 4.72ns (−17.2, 26.6) | 62.7* (35.6, 89.8) |
| Bleeding time, min | −1.81ns (−3.91, 0.30) | 3.43** (2.36, 4.50) | −5.24* (−7.73, −2.75) |
Values are means (95% confidence interval). For differences between groups: ns, not significant;
P < 0.001;
P < 0.0001.
Mean (±SD) bleeding times (relative changes from baseline) 4 h after administration of study drug were 1.46 ± 0.58, 1.20 ± 0.61 and 2.17 ± 0.58 after naproxen alone, rivaroxaban alone and the combination of rivaroxaban and naproxen, respectively (Figure 2B). One subject had an increase in bleeding time of 3.27 tb 4 h after coadministration of rivaroxaban and naproxen. The combination of rivaroxaban and naproxen significantly increased bleeding time compared with rivaroxaban alone (P = 0.017). Furthermore, comparison of individual differences showed that bleeding time was significantly increased after treatment with rivaroxaban and naproxen, compared with naproxen alone (P < 0.0001; Table 1).
Pharmacokinetics
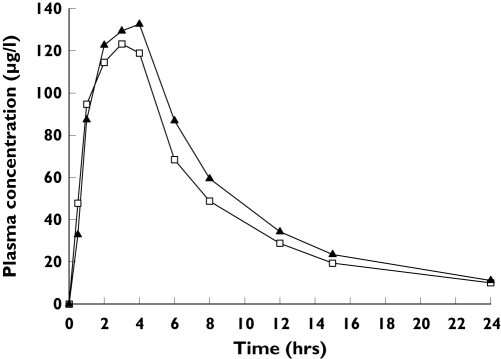
Coadministration of rivaroxaban and naproxen increased the peak plasma concentration of rivaroxaban slightly compared with rivaroxaban alone (Figure 3), with a corresponding increase in the AUC and Cmax of rivaroxaban of approximately 10% (Table 2). The ratios of the point estimates for the combination of rivaroxaban and naproxen vs. rivaroxaban alone were 1.125 (90% CI 0.995, 1.271) for AUC, and 1.095 (90% CI 0.905, 1.325) for Cmax. The tmax of rivaroxaban was increased slightly and its t1/2 decreased slightly in combination with naproxen.
Figure 3.
Mean plasma concentrations of rivaroxaban in healthy male subjects (n = 11) given alone (15 mg) and with naproxen (500 mg). Rivaroxaban (□), rivaroxaban + naproxen (▴)
Table 2.
Pharmacokinetics of rivaroxaban (15 mg) given alone and with naproxen (500 mg) to 11 healthy subjects
| Parameter | Rivaroxaban | Rivaroxaban + naproxen |
|---|---|---|
| AUC, µg·h l−1 | 1250 (28.6) | 1396 (26.3) |
| AUCnorm, g·h l−1 | 6740 (35.8) | 7526 (31.7) |
| Cmax, µg l−1 | 152.9 (31.5) | 165.3 (27.7) |
| Cmax,norm, g l−1 | 824.5 (36.5) | 891.0 (33.9) |
| t1/2, h | 8.59 (29.0) | 7.85 (24.6) |
| tmax*, h | 1.0 (1.0–3.0) | 2.0 (0.5–4.0) |
Median value (range). Values are means (coefficient of variation).
Discussion
In this study in healthy males, rivaroxaban and naproxen given alone and the two drugs taken in combination were well tolerated, with no drug-related adverse events being reported. Adverse events were mild in severity, transient in nature and occurred in only three of the 13 subjects. Elevations in liver enzymes were observed in one subject after the combination of rivaroxaban and naproxen. The subject admitted drinking alcohol during a wash-out period and the investigator concluded that the increases in liver enzymes were not related to the study medication. Rivaroxaban has shown no signs of liver toxicity in short-term studies for the prevention of VTE after major orthopaedic surgery [7, 8]. However, large Phase III studies will be required to confirm this. These results confirm previous findings on the tolerability of rivaroxaban in healthy subjects [5, 6] and show that coadministration of rivaroxaban with naproxen, at these doses, does not lead to an increase in the incidence of adverse events.
Rivaroxaban, given alone and in combination with naproxen, substantially inhibited FXa activity and prolonged PT, aPTT and the HepTest. In general, coadministration of rivaroxaban with naproxen slightly increased the effect of rivaroxaban on these clotting tests, and slightly delayed the time taken to reach the peak effect. However, the increase in the effect of rivaroxaban was very small and was not considered clinically relevant. These findings suggest that when naproxen and rivaroxaban are given together, the antithrombotic effect of rivaroxaban will not be affected.
Rivaroxaban had no effect on platelet aggregation, and did not influence the effect of naproxen on platelets. Furthermore, there was no additive effect on inhibition of platelet aggregation when the two drugs were taken together.
Rivaroxaban alone did not significantly influence bleeding time, which has been observed previously [5, 6]. As expected, naproxen prolonged bleeding time, and the two drugs taken together prolonged bleeding to a greater extent than naproxen alone. The observed prolongation of bleeding time (mean increase 3.43 min) in the absence of effects on platelet aggregation, FXa activity and clotting tests is not easily interpreted. Furthermore, the increase in bleeding time in one patient observed approximately 3–4 h after coadministration of rivaroxaban and naproxen could either be a random finding, or indicate that some individuals may be more sensitive to the combination of these drugs.
Bleeding time varies between subjects [16], and an increase alone does not necessarily predict risk of haemorrhage without confirmation from other diagnostic tests, such as platelet aggregation [17]. Even pronounced prolongations of bleeding time have been shown not to be predictive of haemorrhage during surgery [18]. Bleeding time was used as a surrogate measure for bleeding events in this study, as they are unlikely to be observed in healthy subjects receiving rivaroxaban [5, 6]. However, analysis of the incidence of bleeding events in patients taking part in large-scale Phase III clinical studies of rivaroxaban will be required for a full interpretation of these results.
A post hoc analysis of concomitant use of NSAIDs in patients receiving rivaroxaban in Phase II clinical studies has shown that there was no increased risk of bleeding with NSAID use with doses of rivaroxaban up to 10 mg twice daily [7, 8]. Combined bleeding rates in the pooled populations of Phase II study patients not taking NSAIDs and receiving rivaroxaban (2.5, 5 and 10 mg twice daily) were 1.1%, 3.9% and 5.2%, respectively. In patients who did receive postoperative NSAIDs as well as the same doses of rivaroxaban, the rates were 2.9%, 4.8% and 4.3%. This subgroup analysis allowed Phase III studies of rivaroxaban for the prevention of VTE after major orthopaedic surgery to proceed, using 10 mg rivaroxaban once daily, without anticipating the need to adjust the dose in patients also taking NSAIDs. Detailed analyses of the effect of concomitant medications on bleeding will be carried out in these studies.
Naproxen increased the bioavailability of rivaroxaban by approximately 10%. The 90% CI on the ratios of AUC and Cmax for rivaroxaban given alone and in combination with naproxen exceeded 1.25, the limit that is generally accepted as indicating no interaction between two drugs [19]. The pharmacokinetics and pharmacodynamics of rivaroxaban correlate closely [6] and the time courses for inhibition of FXa activity and prolongation of PT in this study were very similar to the plasma concentration–time curve for the drug. However, the small increase in the bioavailability of rivaroxaban did not translate into similar changes in the pharmacodynamic parameters. The increase in bioavailability observed here is well within the variability observed for the pharmacokinetics of rivaroxaban in patients undergoing orthopaedic surgery in Phase II studies [20]. It is unlikely to be of clinical relevance and may be a chance finding.
The absence of a clinically relevant interaction between rivaroxaban and naproxen observed in this study suggests that it may be possible to coadminister rivaroxaban, for the prevention of VTE, and NSAIDs, for their anti-inflammatory properties. Other potential indications for rivaroxaban include the prevention of stroke in patients with AF and secondary prevention of myocardial infarction in patients with ACS, which would require long-term treatment with the drug. Co-morbidities in these patients, who are more likely to be elderly, include OA or RA, for which they also may take NSAIDs.
The present study has shown that concomitant administration of rivaroxaban and naproxen was well tolerated in healthy subjects. The pharmacodynamics and pharmacokinetics of rivaroxaban were not affected in a clinically relevant manner by coadministration with naproxen. Bleeding times were slightly increased by the combination and an increase in the bleeding time in one subject suggested that some individuals may be more sensitive than others in this respect. However, Phase II clinical studies showed that, with total daily rivaroxaban doses up to 20 mg, there was no increased risk of bleeding in patients also receiving NSAIDs. Large-scale Phase III studies will be required to confirm this finding. In conclusion, a clinically relevant interaction between rivaroxaban and naproxen was not detected in healthy subjects, suggesting that the two drugs could be given together.
Acknowledgments
All authors are employees of Bayer HealthCare AG.
References
- 1.Paiement GD, Mendelsohn C. The risk of venous thromboembolism in the orthopedic patient: epidemiological and physiological data. Orthopedics. 1997;20(Suppl.):7–9. [PubMed] [Google Scholar]
- 2.Ansell J, Bergqvist D. Current options in the prevention of thromboembolic disease. Drugs. 2004;64:1–5. doi: 10.2165/00003495-200464001-00002. [DOI] [PubMed] [Google Scholar]
- 3.Weitz JI, Bates SM. New anticoagulants. J Thromb Haemost. 2005;3:1843–53. doi: 10.1111/j.1538-7836.2005.01374.x. [DOI] [PubMed] [Google Scholar]
- 4.Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, Straub A. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005;3:514–21. doi: 10.1111/j.1538-7836.2005.01166.x. [DOI] [PubMed] [Google Scholar]
- 5.Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–21. doi: 10.1016/j.clpt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 – an oral, direct Factor Xa inhibitor – after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005;61:873–80. doi: 10.1007/s00228-005-0043-5. [DOI] [PubMed] [Google Scholar]
- 7.Turpie AG, Fisher WD, Bauer KA, Kwong LM, Irwin MW, Kalebo P, Misselwitz F, Gent M. BAY 59-7939: an oral, direct Factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose-ranging study. J Thromb Haemost. 2005;3:2479–86. doi: 10.1111/j.1538-7836.2005.01602.x. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson BI, Borris L, Dahl OE, Haas S, Huisman MV, Kakkar AK, Misselwitz F, Kalebo P. Oral, direct Factor Xa inhibition with BAY 59-7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost. 2006;4:121–8. doi: 10.1111/j.1538-7836.2005.01657.x. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, Muehlhofer E, Dierig C, Misselwitz F, Kalebo P. A once-daily, oral, direct Factor Xa inhibitor – rivaroxaban (BAY 59-7939) – for thromboprophylaxis after total hip replacement. Circulation. 2006 doi: 10.1161/CIRCULATIONAHA.106.642074. in press. [DOI] [PubMed] [Google Scholar]
- 10.Akarca US. Gastrointestinal effects of selective and non-selective non-steroidal anti-inflammatory drugs. Curr Pharm Des. 2005;11:1779–93. doi: 10.2174/1381612053764904. [DOI] [PubMed] [Google Scholar]
- 11.Moote C. Efficacy of nonsteroidal anti-inflammatory drugs in the management of postoperative pain. Drugs. 1992;44(Suppl. 5):14–29. doi: 10.2165/00003495-199200445-00004. [DOI] [PubMed] [Google Scholar]
- 12.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman La Roche AG. Proxen® Enteric Coated Tablets. Drug information. 2003.
- 14.Born GV, Cross MJ. The aggregation of blood platelets. J Physiol. 1963;168:178–95. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mielke CH. Measurement of the bleeding time. Thromb Haemost. 1984;52:210–1. [PubMed] [Google Scholar]
- 16.Rodgers RP, Levin J. A critical reappraisal of the bleeding time. Semin Thromb Hemost. 1990;16:1–20. doi: 10.1055/s-2007-1002658. [DOI] [PubMed] [Google Scholar]
- 17.International Technidyne Corporation. Surgicutt Bleeding Time Determination. Device manufacturer's instructions. 2000.
- 18.Lind SE. The bleeding time does not predict surgical bleeding. Blood. 1991;77:2547–52. [PubMed] [Google Scholar]
- 19.Committee for Proprietary Medicinal Products (CPMP) and EMEA. Note for Guidance on the Investigation of Bioavailability and Bioequivalence. [2006 October 2]. Available at http://www.emea.eu.int/pdfs/human/ewp/140198en.pdf.
- 20.Turpie AGG, Eriksson BI, Mueck W, Bauer KA, Borris LC, Dahl OE, Fisher WD, Gent M, Haas S, Huisman MV, Kakkar AK, Kalebo P, Kwong LM, Misselwitz F. Pharmacokinetic and pharmacodynamic analyses of rivaroxaban in patients undergoing orthopaedic surgery. Pathophysiol Haemost Thromb. 2006;35:1182. A2 Abstract. [Google Scholar]