Abstract
What is already known about this subject
Dihydropyrimidine dehydrogenase (DPD) deficiency is currently evaluated as a means of identifying patients with a risk of toxicity during 5-fluorouracil (5-FU) treatment.
Therapeutic drug monitoring (TDM) is a complementary tool already shown to be useful for doses of 1000 mg m−2 day−1.
We carried out the first analysis of the concentration – effect relationships of 5-FU administered at a dose of 600 mg m−2 day−1 with concomitant radiotherapy.
What this study adds
No relationship was found between exposure and toxicity for 5-FU administered at a dose of 600 mg m−2 day−1 with concomitant radiotherapy.
The use of TDM to improve tolerance to this treatment protocol is not supported by our data.
This study confirms the existence of an exposure – toxicity relationship for a dose of 1000 mg m−2 day−1 and has developed a simplified sampling strategy to make TDM easier to implement with this dose schedule.
Aims
Toxicity and response are correlated with plasma 5-fluorouracil (5-FU) concentration in patients treated with 5-FU at a dose of 1000 mg m−2 day−1. Head and neck cancer patients are treated with various therapeutic regimens, including chemotherapy with 5-FU at a dose of 600 mg m−2 day−1 with radiotherapy. We investigated the plasma concentration–effect relationship for this regimen, with the aim of developing recommendations for dose adjustment.
Methods
Patients received 5-FU at doses of 600 or 1000 mg m−2 day−1, as a continuous infusion over 4 or 5 days, with or without radiotherapy for the 600 mg m−2 day−1 regimen. The area under the curve (AUC) for 5-FU concentration was estimated, based on a single morning blood sample taken each day during treatment. AUC values were compared between patients with and without toxicity. This simplified method for AUC estimation was compared with the standard two-samples-per-day method in an independent group of 50 patients.
Results
Forty-six patients, corresponding to 115 courses, were included in this prospective study. Considerable interindividual variability in estimated AUC was observed for both doses. Grade 3–4 toxicity occurred in 10 and 21% of patients given doses of 600 and 1000 mg m−2 day−1, respectively. Ths study confirmed the relationship between plasma 5-FU concentration and toxicity previously reported for 1000 mg m−2 day−1, but found no such relationship for the 600 mg m−2 day−1 regimen with concomitant radiotherapy.
Conclusions
Our results do not support the use of therapeutic drug monitoring to improve tolerance for the 600 mg m−2 day−1 regimen with concomitant radiotherapy. A simplified method is proposed for 5-FU monitoring for the 1000 mg m−2 day−1 regimen.
Keywords: 5-fluorouracil, pharmacokinetics, drug monitoring
Introduction
5-Fluorouracil (5-FU) is widely used in systemic chemotherapy to treat colorectal cancer [1], head and neck carcinoma [2] and breast cancer [3]. Its toxicity profile depends on the duration of infusion, and tolerance is better for continuous infusions than for bolus injections [4]. For head and neck carcinoma, 5-FU is administered as a continuous infusion of 600–1000 mg m−2 day−1 over 4 or 5 days. However, outcomes differ considerably among patients treated with the same dose.
A relationship between plasma 5-FU concentration and clinical response was first reported in patients with metastatic colorectal cancer treated for 5 days by continuous infusion without cisplatin [5]. Further studies in head and neck cancer patients treated with cisplatin and 5-FU at a dose of 1000 mg m−2 day−1 over 4 or 5 days showed that both toxicity and response were correlated with plasma 5-FU concentration [6–10]. Prospective studies have demonstrated that pharmacokinetically guided dose adjustment could be used to reduce toxicity without decreasing efficacy [7, 11]. Lower levels of toxicity were observed in patients exposed to no more than 29 000 µg l−1 h−1 than in patients whose dose was calculated according to body surface area. Therapeutic drug monitoring (TDM) is thus recommended in head and neck cancer patients treated with 1000 mg m−2 day−1 over 4 or 5 days without concomitant radiotherapy, the target level being an AUC0−105 h of 30 000 µg l−1 h−1 [12]. Some studies have also reported a relationship between 5-FU exposure and toxicity for a dose of 500 mg m−2 day−1, administered with leucovorin [13], and for a dose of 640 mg m−2 day−1, administered with leucovorin and interferon [9]. Exposure–effect relationships are less clear for a dose of 750 mg m−2 day−1 over 5 days with concomitant radiotherapy [14]. Finally, no data are available concerning the toxicity associated with a dose of 600 mg m−2 day−1 with concomitant radiotherapy. We prospectively evaluated relationships between 5-FU exposure and treatment outcome for this regimen, with the aim of developing recommendations for dose adjustment. We also evaluated a simplified sampling procedure by investigating exposure–effects relationships in patients receiving 1000 mg m−2 day−1, for whom reference AUC values are already available.
Patients and methods
Patients and treatments
We studied patients with squamous cell carcinoma of the upper aerodigestive tract (including the lips, mouth, tongue, nose, throat, vocal cords and part of the oesophagus and windpipe), receiving 5-FU for curative care. Treatment was selected on the basis of tumour site (respiratory tract or upper part of the digestive tract), the extent of the tumour (T), spread to lymph nodes (N), and metastasis (spread to other parts of the body) (M); (TNM stage, American Joint Committee on Cancer. AJCC Cancer Staging Manual, 6th edn. New York, NY: Springer, 2002). 5-FU was administered as a continuous intravenous infusion, using a volumetric pump, at a dose of 600 or 1000 mg m−2 day−1, for 4 or 5 days. Two or three courses of chemotherapy were administered at 21-day intervals, followed by surgical resection of the tumour in some cases. Patients received radiotherapy, 5 days per week, concurrently with chemotherapy, for the 5-FU dose of 600 mg m−2 day−1. The total dose of radiation delivered was 60–70 Gy in 30–37 fractions over 6–7 weeks.
Ethical issues
This pilot study was part of ongoing Phase III studies, funded and monitored by the French Groupe d'Oncologie et de Radiothérapie de la Tête et du Cou (GORTEC group). The protocol and informed consent forms were reviewed and approved by the University Hospital of Tours Research Ethics Committee. All patients provided informed consent before enrolment.
Blood sampling and pharmacokinetic evaluation
Blood samples were drawn from peripheral veins at sites distant from the infusion site. Samples were obtained each morning between 08.00 and 10.00 h, the infusion having begun between 13.00 and 17.00 h on the previous day. This sampling schedule was chosen to limit fluctuations due to chronopharmacokinetics [15]. Blood samples were taken on days 2, 3, 4 and 5 for 4-day treatments and also on day 6 for 5-day infusions. This sampling schedule is simpler than that previously proposed for 5-FU therapeutic monitoring, based on the taking of two samples per day, one in the morning and the other in the afternoon [11]. Blood specimens were placed on ice and centrifuged within 2 h to prevent 5-FU degradation [16]. The supernatant was transferred to polypropylene tubes and stored at −20°C until analysis by high-performance liquid chromatography (HPLC). 5-FU concentrations were measured within a week, using the method described by Gamelin et al. [17]. After precipitation of serum samples (500 µl) with 300 mg ammonium sulphate, 5-FU and internal standard (5-chlorouracil, 25 µl of a 20 µg ml−1 solution) were extracted by vortex mixing with 3 ml ethylacetate-isopropanol (85/15, v/v). The solvent was evaporated to dryness and the residue dissolved in 200 µl mobile phase. The resulting solution (40 µl) was injected into the HPLC system, consisting of an analytical column (Spherisorb ODS1, 250 × 4.6 mm i.d.; Waters, Saint-Quentin en Yvelines, France), the mobile phase (0.01 m potassium dihydrogen sulphate in pure water, adjusted to pH 3 with orthophosphoric acid), delivered at a rate of 1.5 ml mn−1, and a UV detector set at 260 nm. The lower limit of quantification was 20 µg l −1 and the calibration curve used in routine practice extended from 50 to 2500 µg l−1. Quality control specimens were analysed in each analytical run. The within-day coefficient of variation was 7.6% for 100 µg l −1 and 5.7% for 500 µg l−1. Our laboratory also participated, throughout the study period, in the French national quality control programme for anticancer drugs, including 5-FU, organized by the Groupe de Pharmacologie Clinique Oncologique (GPCO) of the Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC; http://www.fnclcc.fr).
The total area under the curve (AUCtotal) was calculated individually from the plasma 5-FU concentrations obtained each morning during treatment (i.e. for 96 h (AUC0−96 h) or 120 h (AUC0−120 h)) using the formula AUC = ΣCi × 24, where Ci is the concentration measured on the ith day of treatment. Previous studies have shown that the concentrations obtained may be erratic, particularly if the infusion rate is not strictly regular, because of the very short half-life of 5-FU [18]. This phenomenon is familiar to clinical pharmacologists carrying out 5-FU TDM on a routine basis. We therefore decided to calculate AUC using all the concentrations available for a patient (4 or 5, according to treatment schedule) only if their coefficient of variation was <50%. In other cases, if only one measurement was markedly different from the others (±100% the mean of the others), it was replaced by the mean of the other values; if two or more values appeared discrepant, the AUC was not calculated.
Previous studies have validated a target value of 30 000 µg l−1 h−1 for 5-FU AUC for the dose of 1000 mg m−2 day−1 [7, 11]. No target AUCs have been defined for other doses and, despite probable dose proportionality, no extrapolation was made for the dose of 600 mg m−2 day−1 due to the presence of associated radiotherapy. Whatever the schedule used in our study, individual dose adjustments for the next course were strictly restricted by the protocols of the main study. Such adjustments were possible, based on postcourse toxicity and, exceptionally, in cases of very high AUC value for the previous course, based on a case-by-case analysis taking into account the patient's status and published exposure–toxicity data [5–7, 11].
The AUC0−48 h was calculated for each patient, from the plasma concentrations obtained on days 2 and 3 (first and second infusion, respectively), for the early identification of patients with slow 5-FU elimination. Dose adjustment for subsequent days of treatment (intracourse dose adjustment) was also strictly restricted, but was possible, taking into account immediate tolerance or high values of AUC0−48 h, using the same strategy described for total AUC.
Comparison of methods for AUC estimation
The simplified sampling schedule used for AUC estimation was designed to facilitate the implementation of a TDM programme in hospitalized patients. However, as different estimation methods may give different values, our AUCs were compared with those estimated with the reference method of Fety et al. [11] in an independent group of 50 patients. Reference AUC0−96 h values were obtained with the trapezoidal method, from measurements taken just before and 2 h after the start of infusion on day 1, then at 08.00 and 17.00 h on days 2, 3, 4 and at 08.00 h and just before the end of the infusion on day 5 [11]. The agreement between the two methods of AUC estimation was analysed, using Bland and Altman's method [19].
Toxicity and response assessments
Toxicity was assessed after each course. A physical examination was carried out to assess nonhaematological toxicity, such as mucositis and digestive toxicity (nausea, vomiting, diarrhoea). Haematological toxicity was assessed on the basis of blood cell counts 7, 14 and 21 days after each course. All types of toxicity were graded according to the National Cancer Institute (NCI) common toxicity criteria classification. The grade of haematological toxicity was determined from the lowest blood cell count observed.
Response was evaluated according to the NCI recommendations by measuring tumour size by clinical, fibroscopic, ultrasound and/or computed tomography, magnetic resonance imaging or positron emission tomography scan examinations. A complete response (CR) was defined as the disappearance of all lesions. A partial response (PR) was defined as at least 50% tumour regression in the absence of new lesions. Stabilization (S) and progression were defined as <50% tumour regression and an increase of at least 25% in tumour mass, respectively.
Data analysis
Description of the data
The interindividual variability of estimated AUCs was analysed for each dose (600 or 1000 mg m−2 day−1), based on all data from individuals, excluding those for whom dose adaptation was carried out. Patients with dose adaptation were also excluded when studying intraindividual variability, which was assessed by means of coefficients of variation, for each dose.
Relationship between AUC and toxicity
Analyses were carried out independently for each dose. All AUCs, including those corresponding to courses with dose adaptation, were included. Courses were then separated into those associated with toxicity and those without toxicity and descriptive statistics (median, range) were estimated for each group. We first considered overall toxicity, whatever its grade, and then toxicity of grade 3 or 4. Haematological toxicity was analysed separately, according to the same stratification. As radiotherapy is known to induce mucositis [20], our analysis of the group of patients treated with 600 mg m−2 day−1 plus concurrent radiotherapy was also based on overall toxicity excluding mucositis.
Logistic regression analysis was then carried out to determine the probability of toxicity for each dose as a function of AUC. A log transformation was applied before data analysis, and analyses were performed in the framework of marginal logistic regression models [21]. Such models make it possible to take into account the natural correlation between different courses in the same patient.
AUCs were stratified and the frequency of toxicity within each range calculated, to define possible cut-off AUC values for toxicity for each dose. This stratification of AUCs was based on published data for the dose of 1000 mg m−2 day−1 and the distribution of observed values for the dose of 600 mg m−2 day−1 [6–8, 11].
Results
Patients
A total of 46 patients (42 men) with a median age of 59 years (range 41–80) were included in the study between February 2003 and March 2004 (Table 1). All patients had an upper aerodigestive tract tumour, which was located in the oesophagus in 14 cases. Fifteen patients received induction chemotherapy at the dose of 1000 mg m−2 day−1 (35 courses) and 31 received concurrent chemotherapy and radiotherapy (80 courses).
Table 1.
Patients' characteristics
| 600 mg m−2 day−1 with radiotherapy | 1000 mg m−2 day−1 | |
|---|---|---|
| Number of patients | 31 | 15 |
| Male/female | 28/3 | 14/1 |
| Median age (range) | 59 (41–71) | 57 (43–80) |
| Median body surface area (range) | 1.77 (1.40–2.46) | 1.63 (1.45–1.88) |
| Upper respiratory/oesophageous localization | 18/13 | 14/1 |
| Number of cycles | 80 | 35 |
| Patients with 1 cycle | 1 | 2 |
| Patients with 2 cycles | 10 | 3 |
| Patients with 3 cycles | 19 | 9 |
| Patients with 4 cycles | 1 | 1 |
| Number of patients with 96-h treatment | 29 | 6 |
| Number of patients with 120-h treatment | 2 | 9 |
Pharmacokinetic analysis
No AUC was calculated for 18 of the 115 courses available, because of missing concentration values or major discrepancies between the values obtained on different days, as previously indicated. AUCs were adjusted in 12 cases (10.4%), in which only one of the four or five concentrations differed markedly from the others. Finally, 68 AUCs from 31 patients and 29 from 15 patients were available for doses of 600 and 1000 mg m−2 day−1, respectively.
The AUCs estimated at each dose differed widely between patients (Figure 1). Dose adjustments were made in four patients (seven courses), involving, in each case, a decrease in dose for the next course (intercourse adaptation). All the patients concerned were treated with 1000 mg m−2 day−1 and the dose adaptation involved stopping treatment 1 day early. In each case, the dose was reduced due to toxicity after the previous course. In those patients whose dose was not adapted, intraindividual variability was moderate (Figure 2), with a median (range) coefficient of variation of 20% (1–72) and 25% (6–41) for doses of 600 and 1000 mg m−2 day−1, respectively.
Figure 1.
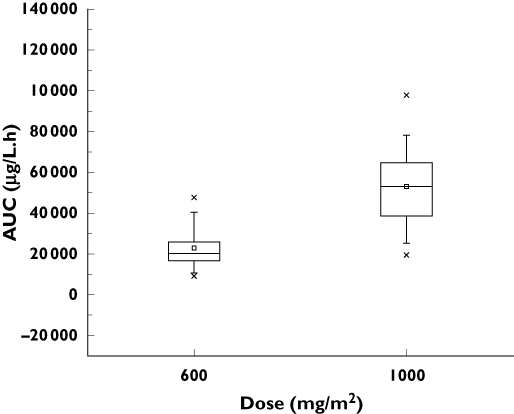
AUCs for 115 courses of chemotherapy in 46 patients treated at doses of 600 or 1000 mg m−2 day−1. Subdivisions of the boxes and the top and bottom lines on the boxes represent median values and the 25th and 75th interquartiles, respectively. The squares (□) represent the mean value, the crosses (×) the 1st and 99th interquartiles, and the dashes (–) the minimum and maximum concentrations observed
Figure 2.
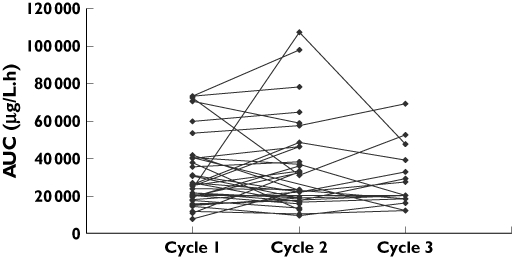
Intraindividual variability in AUCs calculated for each course, whatever the dose used, excluding courses for which the dose was adapted
The median (range) AUC0−48 h was 8880 (2640–38 520) µg l−1 h−1 and 20 640 (5520–46 680) µg l−1 h−1, at doses of 600 and 1000 mg m−2 day−1, respectively. AUC0−48 h and AUC0−96 h were closely correlated (r = 0.82; 95% confidence interval 0.74, 0.88, P < 0.001).
Comparison of methods for AUC estimation
For the independent dataset, the agreement between the AUC0−96 h estimated by the reference method of Fety et al. [11] and by our method, based on the taking of only one sample per day, is illustrated in Figure 3. The mean difference between the two methods was estimated at 3500 µg l−1 h−1. The lower and upper limits of agreement were −15 000 µg l−1 h−1 and 10 000 µg l−1 h−1, respectively, and the discrepancy tended to be greater for higher AUC values.
Figure 3.
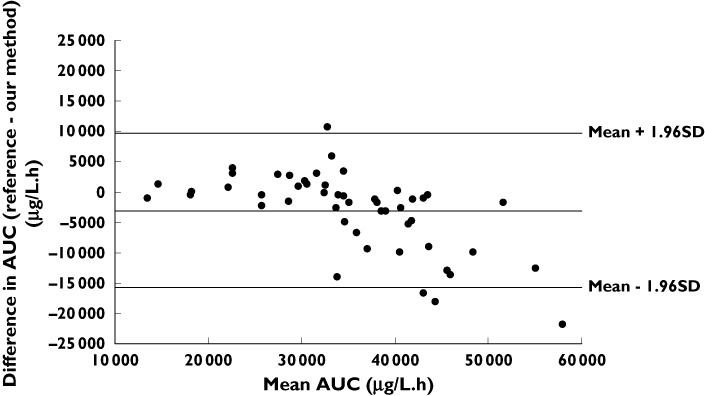
Mean difference in the AUCs measured with reference and simplified methods, plotted against their mean
Treatment side-effects
A total of 115 courses from 46 patients (80 at the dose of 600 mg m−2 day−1 and 35 at the dose of 1000 mg m−2 day−1) were available for the analysis of side-effects. Eighty-seven adverse events were recorded for 66 courses in 36 patients. Overall, only 22% of patients displayed no toxic effect over the entire treatment period, whatever the dose administered. In the remaining patients, haematological toxicity was the most frequent, including a number of grade 3–4 toxicities (Table 2 and Table 3). Twenty-one cases of mucositis were observed, 17 (81%) in patients on concurrent radio- and chemotherapy. For treatments at the dose of 1000 mg m−2 day−1, 62% of courses were associated with toxicity, haematological in most cases. For treatments at the dose of 600 mg m−2 day−1, 47% of courses were associated with toxicity, mostly mucositis or haematological toxicity.
Table 2.
5-FU exposure according to toxicity at each dose level
| Overall toxicity | Haematological toxicity | Toxicity apart from mucositis | ||||
|---|---|---|---|---|---|---|
| No toxicity | Grade 1–4 | Grade 3–4 | Grade 1–4 | Grade 3–4 | Grade 1–4 | |
| 600 mg m−2 day−1 | ||||||
| Number of courses | 36 | 32 | 7 | 28 | 2 | 28 |
| Median AUC (µg l−1 h−1) | 20 880 | 18 840 | 20 640 | 18 720 | 28 200 | 18 720 |
| Minimum AUC (µg l−1 h−1) | 7 800 | 9 480 | 10 320 | 9 480 | 23 040 | 9 480 |
| Maximum AUC (µg l−1 h−1) | 107 520 | 40 560 | 33 360 | 40 560 | 33 360 | 40 560 |
| 1000 mg m−2 day−1 | ||||||
| Number of courses | 9 | 20 | 6 | 19 | 5 | 19 |
| Median AUC (µg l−1 h−1) | 46 080 | 55 320 | 67 380 | 57 360 | 70 680 | 57 360 |
| Minimum AUC (µg l−1 h−1) | 26 040 | 19 560 | 45 794 | 19 560 | 48 480 | 19 560 |
| Maximum AUC (µg l−1 h−1) | 64 920 | 98 040 | 98 040 | 98 040 | 98 040 | 98 040 |
Table 3.
Number of courses associated with toxicity and type of toxicity at each AUC level for doses of 1000 mg m−2 day−1 and 600 mg m−2 day−1
| Overall toxicity | Mucositis | Haematological toxicity | Digestive toxicity | |||||
|---|---|---|---|---|---|---|---|---|
| AUC (µg l−1 h−1) | Number of courses | Grade 1–4 | Grade 3–4 | Grade 1–4 | Grade 3–4 | Grade 1–4 | Grade 3–4 | Grade 1–4 |
| 600 mg m−2 day−1 | ||||||||
| >30 000 | 13 | 6 (46%) | 2 (15%) | 4 | 2 | 5 | 1 | 0 |
| 17–30 000 | 36 | 15 (42%) | 3 (8.3%) | 5 | 2 | 13 | 1 | 0 |
| <17 000 | 19 | 11 (58%) | 2 (10%) | 8 | 2 | 10 | 0 | 0 |
| Total | 68 | 32 (47%) | 7 (10%) | 17 | 6 | 28 | 2 | 0 |
| 1000 mg m−2 day−1 | ||||||||
| >50 000 | 16 | 12 (75%) | 4 (25%) | 3 | 3 | 12 | 4 | 2 |
| 30–50 000 | 10 | 5 (50%) | 2 (20%) | 1 | 2 | 5 | 1 | 1 |
| <30 000 | 3 | 1 (33%) | 0 | 0 | 0 | 2 | 0 | 0 |
| Total | 29 | 18 (62%) | 6 (21%) | 4 | 5 | 19 | 5 | 3 |
Relationship between AUC and toxicity
We were able to use 96 courses from 46 patients for analysis of the relationship between AUC and outcome (Table 2). For the dose of 1000 mg m−2 day−1, median (range) AUCs were 55 320 (19 560–98 040) µg l−1 h−1 and 46 080 (26 040–64 920) µg l−1 h−1 in patients who experienced toxicity of any kind and in those who did not, respectively (P = 0.346). The corresponding values were 18 840 (9480–40 560) µg l−1 h−1 and 20 880 (7800–10 7520) µg l−1 h−1 for the dose of 600 mg m−2 day−1 (P = 0.299). When only grade 3–4 toxicities were considered, AUCs were higher in patients who experienced toxicity of any kind (P = 0.018) or specifically haematological toxicity (P = 0.038), for the dose of 1000 mg m−2 day−1. There was no difference for the dose of 600 mg m−2 day−1.
For the dose of 1000 mg m−2 day−1, AUC was stratified as follows: AUC <30 000 µg l−1 h−1, AUC >50 000 µg l−1 h−1 and AUC between these values. The proportion of side-effects was as high as 75% for patients with AUC values >50 000 µg l−1 h−1 and decreased with decreasing AUC level. Four (66%) of the six patients presenting grade 3–4 toxicity had an AUC >50 000 µg l−1 h−1 and none had an AUC <30 000 µg l−1 h−1 (Table 3). For the dose of 600 mg m−2 day−1, AUCs were classified as <17 000 µg l−1 h−1, >30 000 µg l−1 h−1 or between these values. The rate of toxicity was similar for all AUC levels, whether all grades were considered or just grades 3–4.
Clinical response
Clinical response and AUCav were available for 38 of the 46 patients. Three patients died before the end of treatment and AUCav was not calculated for five patients who did not receive all the scheduled courses of treatment. Twenty-one patients (55%) were classified as responders (CR and PR), of whom 18 (67% of patients) were treated with 600 mg m−2 day−1 and three (27% of patients) were treated with 1000 mg m−2 day−1. These data were not analysed further because of the small number of patients and the heterogeneity of their pathological conditions.
Discussion
Individual dose adaptation based on 5-FU pharmacokinetics is routinely performed in most French Centres Régionaux de Lutte Contre le Cancer (CRLCC; French regional cancer centres), in line with previous collaborative studies showing that plasma exposure to 5-FU is related to treatment response [7, 11, 12]. Each CRLCC is involved in a national quality control programme organized by the FNCLCC (the French Federation of Cancer Centres). Some protocols involve dose reduction according to exposure measured early in infusion, usually based on the AUC0−48 h. Low levels of intraindividual variability of AUCtotal also make it possible to adapt the dose between courses. These methods have been applied only to protocols in which 5-FU is given at a dose of 1000 mg m−2 day−1 over 4 or 5 days, with cisplatin, but without concurrent radiotherapy or other associated chemotherapy. Depending on the site and stage of the tumour, head and neck cancer patients are now treated with various therapeutic regimens, the most common being concurrent radiotherapy and chemotherapy with cisplatin and 5-FU at a dose of 600 mg m−2 day−1. For this regimen, the relationship between exposure and effect remains unknown.
We monitored plasma 5-FU concentrations under two dose regimens, 600 mg m−2 day−1 with radiotherapy and 1000 mg m−2 day−1 without radiotherapy, in patients treated for an upper aerodigestive tract cancer. Great interindividual variability in plasma 5-FU concentration was observed for both doses. Despite the small number of courses analysed for the dose of 1000 mg m−2 day−1 (n = 29), we found that AUC was an independent predictor of grade 3–4 toxicity, thus confirming the link between plasma 5-FU concentration and toxicity already demonstrated for this schedule [11]. In the absence of dose adjustment, this treatment was associated with high exposure to the drug in a large proportion of patients. Based on the target value of 30 000 µg l−1 h−1 proposed by Fety et al., 89% of our patients would have had their dose reduced to prevent toxicity. According to the results obtained in our cohort, and using a simplified sampling schedule, we can set the toxicity threshold at 50 000 µg l−1 h−1, values above this level being associated with significantly higher levels of toxicity.
The analysis of agreement between our method of AUC estimation and that proposed by Fety et al. showed nonnegligible discrepancies. Indeed, AUC estimated by our method may be 15 000 µg l−1 h−1 below or 10 000 µg l−1 h−1 above that estimated using the reference method, particularly for high AUC values. Circadian variations in 5-FU pharmacokinetics are known to occur [22] and may partly explain our results. Indeed, in our independent dataset, median (range) morning concentrations were higher [345 µg l−1 (47–2054)] than afternoon concentrations [295 µg l−1 (20–947)]. The discrepancy between the two methods may have been caused by the use of six afternoon samples and only four morning samples for the standard method, whereas our method used morning samples only. These results do not call into question the reported exposure–toxicity relationships, but show that AUC estimation methods cannot be used interchangeably. For a TDM programme, it is essential to know how the target values were calculated, to ensure that the same method is used throughout, or at least to ensure that the results obtained with another method can be extrapolated. The target value for dose adaptation must therefore be chosen according to the method used for AUC estimation. The actual sampling period used must also be considered. For example, at a dose of 1000 mg m−2 day−1, some published AUC targets refer to the AUC0−105 h [7, 8], whereas others refer to the AUC0−96 h [11]. We determined the AUC0−120 h for 25.7% of patients, giving higher overall AUC values.
In our patients treated with 600 mg m−2 day−1 plus concurrent radiotherapy, median AUCs were similar in patients who experienced toxicity and in those who did not. This result may be explained by the small number of patients studied, resulting in insufficient power to test this hypothesis. Indeed, with 68 observations, the effect size was estimated to 0.275 (after log transformation) and should have been >0.7 to be detected with an 80% power. Several studies have evaluated exposure–toxicity relationships for combinations of 5-FU with pharmacomodulating agents, such as leucovorin [9, 13]. In these treatment schedules, the dose of 5-FU is decreased because of the expected greater toxicity of the combination. Schneider et al. found that mean plasma 5-FU concentration over the course was significantly correlated with haematological toxicity grade or mucositis grade for a dose of 500 mg m−2 day−1 over 5 days [13]. Similarly, Vokes et al. found that afternoon plasma 5-FU concentration on day 3 was correlated with both mucositis grade and the nadir of white blood cell counts, for a dose of 640 mg m−2 day−1 [9]. The situation is less clear for the combination of 5-FU with radiotherapy. Bensadoun et al. [14] evaluated a strategy in which radiotherapy was given concurrently with 750 mg 5-FU m−2 day−1 for 5 days. They observed a high frequency of toxicity in cycle 1 that was not related to 5-FU exposure. During the second cycle, 5-FU was administered at a fixed dose of 750 mg day−1 (i.e. corresponding to 430 mg m−2 for a 1.73 m2 body surface area); AUC was higher in patients with grade 3–4 neutropenia, whereas no relationship between AUC and mucositis was observed. A detailed examination of previously published data revealed a wide overlap in concentrations between patients with and without toxicity. This overlap may provide further insight into the difficulties encountered when trying to detect differences in diverse groups of patients.
Our results confirm the relationship between exposure and toxicity for a dose of 1000 mg m−2 day−1. We were not able to show such an association for the 600 mg m−2 day−1 regimen with concurrent radiotherapy and therefore cannot currently recommend the use of systematic drug monitoring for improving tolerance to this particular treatment protocol. At a dose of 1000 mg m−2 day−1, we have shown that pharmacokinetic monitoring could be simplified by analysing a single sample taken in the morning rather than two samples per day. However, based on our exposure–toxicity relationship data, the target AUC should be revised upwards if this method is used. In our small dataset, values >50 000 µg l−1 h−1 were associated with a higher rate of toxicity, and haematologic toxicity in particular. We thus propose to use this threshold for 5-FU monitoring, using our method of AUC estimation. The good correlation found between AUC0−48 h and AUC0−96 h values indicates that, measured in real time, AUC0−48 h can be used once the treatment has been initiated, to identify patients with unusual pharmacokinetic behaviour. Whatever the method used, if a TDM programme is to be implemented, the infusion rate must be strictly controlled to prevent erratic fluctuations in blood concentrations of the drug.
Acknowledgments
This study was supported by funding from the French Groupe d'Oncologie et de Radiothérapie de la Tête et du Cou (GORTEC group). We thank Valerie Ingremeau for excellent technical assistance and Julie Sappa of Alex Edelman & Associates for correcting the English version of the manuscript.
References
- 1.Poplin E, Baker L. Colon cancer: medical therapy. Gastroenterol Clin North Am. 1988;17:873–86. [PubMed] [Google Scholar]
- 2.Milas L, Mason KA, Liao Z, Ang KK. Chemoradiotherapy: emerging treatment improvement strategies. Head Neck. 2003;25:152–67. doi: 10.1002/hed.10232. [DOI] [PubMed] [Google Scholar]
- 3.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332:901–6. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 4.Lokich JJ, Ahlgren JD, Gullo JJ, Philips JA, Fryer JG. A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: a Mid-Atlantic Oncology Program Study. J Clin Oncol. 1989;7:425–32. doi: 10.1200/JCO.1989.7.4.425. [DOI] [PubMed] [Google Scholar]
- 5.Hillcoat BL, McCulloch PB, Figueredo AT, Ehsan MH, Rosenfeld JM. Clinical response and plasma levels of 5-fluorouracil in patients with colonic cancer treated by drug infusion. Br J Cancer. 1978;38:719–24. doi: 10.1038/bjc.1978.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milano G, Roman P, Khater R, Frenay M, Renee N, Namer M. Dose versus pharmacokinetics for predicting tolerance to 5-day continuous infusion of 5-FU. Int J Cancer. 1988;41:537–41. doi: 10.1002/ijc.2910410411. [DOI] [PubMed] [Google Scholar]
- 7.Santini J, Milano G, Thyss A, Renee N, Viens P, Ayela P, Schneider M, Demard F. 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. Br J Cancer. 1989;59:287–90. doi: 10.1038/bjc.1989.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thyss A, Milano G, Renee N, Vallicioni J, Schneider M, Demard F. Clinical pharmacokinetic study of 5-FU in continuous 5-day infusions for head and neck cancer. Cancer Chemother Pharmacol. 1986;16:64–6. doi: 10.1007/BF00255288. [DOI] [PubMed] [Google Scholar]
- 9.Vokes EE, Mick R, Kies MS, Dolan ME, Malone D, Athanasiadis I, Haraf DJ, Kozloff M, Weichselbaum RR, Ratain MJ. Pharmacodynamics of fluorouracil-based induction chemotherapy in advanced head and neck cancer. J Clin Oncol. 1996;14:1663–71. doi: 10.1200/JCO.1996.14.5.1663. [DOI] [PubMed] [Google Scholar]
- 10.Wendt TG, Grabenbauer GG, Rodel CM, Thiel HJ, Aydin H, Rohloff R, Wustrow TP, Iro H, Popella C, Schalhorn A. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol. 1998;16:1318–24. doi: 10.1200/JCO.1998.16.4.1318. [DOI] [PubMed] [Google Scholar]
- 11.Fety R, Rolland F, Barberi-Heyob M, Hardouin A, Campion L, Conroy T, Merlin JL, Riviere A, Perrocheau G, Etienne MC, Milano G. Clinical impact of pharmacokinetically-guided dose adaptation of 5-fluorouracil: results from a multicentric randomized trial in patients with locally advanced head and neck carcinomas. Clin Cancer Res. 1998;4:2039–45. [PubMed] [Google Scholar]
- 12.Young AM, Daryanani S, Kerr DJ. Can pharmacokinetic monitoring improve clinical use of fluorouracil? Clin Pharmacokinet. 1999;36:391–8. doi: 10.2165/00003088-199936060-00001. [DOI] [PubMed] [Google Scholar]
- 13.Schneider M, Etienne MC, Milano G, Thyss A, Otto J, Dassonville O, Mobayen H, Saudes L, Guillot T, Demard F. Phase II trial of cisplatin, fluorouracil, and pure folinic acid for locally advanced head and neck cancer: a pharmacokinetic and clinical survey. J Clin Oncol. 1995;13:1656–62. doi: 10.1200/JCO.1995.13.7.1656. [DOI] [PubMed] [Google Scholar]
- 14.Bensadoun RJ, Etienne MC, Dassonville O, Chauvel P, Pivot X, Marcy PY, Prevost B, Coche-Dequeant B, Bourdin S, Vallicioni J, Poissonnet G, Courdi A, Teissier E, Lagrange JL, Thyss A, Santini J, Demard F, Schneider M, Milano G. Concomitant b.i.d. radiotherapy and chemotherapy with cisplatin and 5-fluorouracil in unresectable squamous-cell carcinoma of the pharynx: clinical and pharmacological data of a French multicenter phase II study. Int J Radiat Oncol Biol Phys. 1998;42:237–45. doi: 10.1016/s0360-3016(98)00235-1. [DOI] [PubMed] [Google Scholar]
- 15.Milano G, Chamorey AL. Clinical pharmacokinetics of 5-fluorouracil with consideration of chronopharmacokinetics. Chronobiol Int. 2002;19:177–89. doi: 10.1081/cbi-120002597. [DOI] [PubMed] [Google Scholar]
- 16.Murphy RF, Balis FM, Poplack DG. Stability of 5-fluorouracil in whole blood and plasma. Clin Chem. 1987;33:2299–300. [PubMed] [Google Scholar]
- 17.Gamelin E, Boisdron-Celle M, Turcant A, Larra F, Allain P, Robert J. Rapid and sensitive high-performance liquid chromatographic analysis of halogenopyrimidines in plasma. J Chromatogr B Biomed Sci Appl. 1997;695:409–16. doi: 10.1016/s0378-4347(97)00211-9. [DOI] [PubMed] [Google Scholar]
- 18.Adjei AA, Reid JM, Diasio RB, Sloan JA, Smith DA, Rubin J, Pitot HC, Alberts SR, Goldberg RM, Hanson LJ, Atherton P, Ames MM, Erlichman C. Comparative pharmacokinetic study of continuous venous infusion fluorouracil and oral fluorouracil with eniluracil in patients with advanced solid tumors. J Clin Oncol. 2002;20:1683–91. doi: 10.1200/JCO.2002.20.6.1683. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 20.Scully C, Epstein J, Sonis S. Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy. Head Neck. 2004;26:77–84. doi: 10.1002/hed.10326. Part 2: diagnosis and management of mucositis. [DOI] [PubMed] [Google Scholar]
- 21.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 22.Harris BE, Song R, Soong SJ, Diasio RB. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res. 1990;50:197–201. [PubMed] [Google Scholar]


