Abstract
What is already known about this subject
Capsaicin rapidly produces local neurogenic inflammation (characterized by oedema and erythema) when locally administered to the human skin by binding to the TRPV1 receptor present on dermal sensory nerve endings.
In nonhuman primates, a pharmacodynamic assay has been described and validated using capsaicin-induced dermal vasodilation measured by laser Doppler perfusion imaging to assess calcitonin gene-related peptide antagonist activity.
Laser Doppler perfusion imaging has also been shown to be a reliable method for characterizing microvascular changes in the human skin.
What this study adds
Capsaicin induces a reproducible within-subject arm-to-arm increase in dermal blood flow (DBF) when applied to the human skin.
This is the first study to describe a non-invasive pharmacodynamic model in humans using capsaicin-induced neurogenic inflammation and allowing repeated, reproducible measurements of DBF to be performed.
This model might therefore be utilized in the early clinical evaluation of antagonists of putative mediators involved in capsaicin-induced dermal vasodilation.
Aims
Part I: to establish the dose and appropriate application site of capsaicin on the human forearm in order to produce a robust and reproducible dermal blood flow (DBF) response. Part II: to evaluate the within-subject arm-to-arm and period-to-period reproducibility.
Methods
Both parts consisted of two study visits. In part I, placebo and 100, 300 and 1000 µg capsaicin were applied at four predefined sites on the volar surface of both forearms. Placebo and capsaicin doses were randomized and balanced by site between subjects. Changes in DBF were assessed by laser Doppler perfusion imaging up to 60 min after capsaicin application. In part II, only 1000 µg capsaicin was applied on the proximal forearm and changes in DBF assessed up to 30 min (t30). DBF response was expressed as percent change from baseline ± SD and the corresponding AUC0−30. Reproducibility assessment included calculation of the concordance correlation coefficient (CCC).
Results
Part I (n = 12 subjects): compared with placebo, 300 and 1000 µg capsaicin increased DBF (P < 0.05) at all time points except at 10 min. This increase was reproducible at the two most proximal sites from the 30-min time point onwards when compared between arms (CCC ≥ 0.8, i.e. substantial to almost perfect reproducibility). In part II (n = 11), t30 averaged 390 ± 120% and arm-to-arm reproducibility was almost perfect (CCC = 0.91) for AUC0−30.
Conclusions
Capsaicin induces a reproducible within-subject arm-to-arm increase in DBF. We provide a non-invasive pharmacodynamic model in humans to test antagonists of mediators involved in capsaicin-induced dermal vasodilation, including calcitonin gene-related peptide antagonists.
Keywords: calcitonin gene-related peptide, capsaicin, migraine therapy, reproducibility, vasodilation
Introduction
Capsaicin is a vanilloid responsible for the pungent taste of hot peppers. Högyes was the first to state that the pungent and irritant nature of capsaicin is mediated by sensory nerves [1, 2]. This was confirmed in the second half of the 20th century mainly by the work of Hungarian scientists [3]. More recently, it has emerged that the selectivity of capsaicin for the thin afferent neurons of mammalian species is due to the existence of the transient receptor potential vanilloid type 1 receptor (TRPV1) [4–8]. This receptor is expressed on a subpopulation of primary sensory neurons consisting of Aδ- and C-fibre nociceptors. Binding of capsaicin to the TRPV1 receptor provokes an inward nonselective cationic current depolarizing the neuron [5, 9]. This activation results in the release of bioactive substances, which in turn act on target cells in the surrounding tissue including mast cells, immune cells and vascular smooth muscle cells. The resulting response is characterized by redness and warmth (secondary to vasodilation), swelling (secondary to plasma extravasation) and hypersensitivity (secondary to alterations in the excitability of primary sensory neurons). Collectively, this response is referred to as ‘neurogenic inflammation’ [10].
Capsaicin has been used extensively in human pain models to induce experimental pain [11–13]. When capsaicin is locally administered to the human skin, by topical application or intradermal injection, it rapidly produces local neurogenic inflammation by binding to the TRPV1 receptor present on sensory nerve endings in the skin [10, 14]. Most evidence indicates that the release of calcitonin gene-related peptide (CGRP) is a major initiator of this response [15, 16]. Other putative bioactive mediators are substance P (SP), neurokinin (NK) A, somatostatin, nitric oxide (NO), histamine and prostaglandins, although their role in capsaicin-induced neurogenic inflammation in the normal human skin is less well established [17–21]
Capsaicin-induced neurogenic inflammation shows striking similarities with the neurogenic inflammation of the trigeminovascular system, which is thought to be of central importance during a migraine attack [22]. The cranial release of CGRP, for example, is suggested to be pivotal in the pathogenesis of migraine, as confirmed by the fact that the CGRP receptor antagonist BIBN4096BS is efficacious in the treatment of migraine [23]. It is tempting to speculate that capsaicin-induced dermal neurogenic inflammation might serve as a model for neurogenic inflammation elsewhere in the body. Therefore, a better knowledge of the cascade of antidromic effects of dermal sensory neuron stimulation may improve our understanding of the pathophysiology of migraine and the ways to prevent and/or abort a migraine attack.
Helme et al. extensively studied the dermal neurogenic inflammation that occurs after the topical application of capsaicin onto the human skin. They assessed both the size of the flare response (i.e. erythema due to an increase in blood flow) and allodynia (i.e. hypersensitivity to heat and touch) [10]. The advent of an X-Y scanning laser probe now allows a more reliable method for characterizing the microvascular changes leading to the flare reaction [24]. Laser Doppler perfusion imaging has been used to study the influence of topically applied capsaicin on blood flow responses in the human skin [25]. Furthermore, Hershey et al. used topical application of capsaicin on the skin of nonhuman primates and rats to stimulate CGRP-mediated vasodilation, which was measured by laser Doppler perfusion imaging [26]. They have also demonstrated that this capsaicin-induced vasodilation is inhibited by a novel potent and selective CGRP antagonist, thus validating a non-invasive pharmacodynamic assay in animals that provides a rapid evaluation of CGRP antagonists.
The overall aim of the present study was to evaluate whether this pharmacodynamic model could be translated in healthy volunteers which would allow rapid and objective readouts of CGRP antagonist activity directly in the human species itself. In order to develop a useful model for human studies, we first needed to address two major issues, for which we designed a two-part study.
In the first part, we identified the dose of capsaicin needed to produce a robust and reproducible dermal blood flow (DBF) response after topical application to the human forearm. Simultaneously, the influence of the forearm location on the capsaicin response was assessed.
In the second part, the within-subject arm-to-arm and period-to-period reproducibility of the forearm DBF response to capsaicin were evaluated using the optimal dose of capsaicin and the most appropriate forearm location as assessed in part I. In addition, different ways of expressing the DBF response were compared and sample size calculations performed.
Methods
Subjects
After approval by the ethics committee of the University Hospital, written informed consent was obtained from all subjects during a screening visit. Twelve men were recruited for participation in the first part of the study; 21 in the second part. All subjects were caucasian, nonsmoking, healthy men between 18 and 45 years of age.
Study design
Subjects were instructed to abstain from any drugs during 3 days and from chocolate-, alcohol- and caffeine-containing beverages and food during 12 h preceding each study period. Both study parts (part I and part II) were open-label and consisted of two periods separated by at least 1 week. Subjects fasted for at least 4 h before each study period. All measurements were performed while the subjects rested in a semirecumbent position on a comfortable bed in a quiet, temperature-controlled room (ambient temperature of 24 ± 1°C).
During each period, 10-mm rubber O-rings (McMaster-Carr, New Brunswick, NJ, USA; 8 mm inner diameter) were placed onto the skin at four equally spaced sites on the volar surface of both forearms. The rings were positioned so that their distal edges were 10, 14, 18 and 22 cm proximal to the wrist crease, within approximately 1 cm of the midline and avoiding visible veins. The proximal ring (i.e. closest to the antecubital crease) is referred to as site 1, the distal ring (i.e. closest to the wrist crease) as site 4. After placement of the O-rings, a laser Doppler imager was used to obtain baseline scans of the DBF in the areas defined by the rings. Subsequently, these O-rings served as reservoirs to contain the topically applied 20-µl capsaicin or placebo solutions.
Capsaicin powder was obtained from Sigma-Aldrich N.V. (Bornem, Belgium) and was dissolved in a 3 : 3 : 4 mixture of ethanol 100%, Tween-20 and distilled water. Capsaicin was diluted so that 20 µl of the mixture contained 100, 300 or 1000 µg capsaicin. The placebo solution corresponded to the same 3 : 3 : 4 mixture of ethanol 100%, Tween-20 and distilled water without capsaicin.
Part I: dose finding and assessment of the influence of the forearm location
After obtaining baseline scans, subjects received single topical doses of 100, 300 and 1000 µg capsaicin per 20 µl vehicle and placebo (i.e. 20 µl of vehicle) in the four rings at each forearm. Applications were randomized and balanced by site between the different subjects. Each subject received the same treatment on the left and right forearms and during both study periods. The time course of the blood flow response to each dose of capsaicin/placebo was measured by performing laser Doppler scans of the area within each ring at 10, 20, 30, 45 and 60 min postcapsaicin application.
Part II: within-subject arm-to-arm and period-to-period reproducibility
After obtaining baseline scans, subjects received on both forearms a topical dose of 1000 µg capsaicin per 20 µl vehicle in the two proximal O-rings; placebo in the two distal O-rings. Subsequently, laser Doppler scans were performed at 10, 20 and 30 min postcapsaicin application. Only subjects with an increase in forearm blood flow from baseline of ≥100% in both proximal sites of both arms and during both study periods were allowed to participate. If this DBF increase threshold was not attained, subjects were excluded from the study.
Assessment of forearm DBF response to capsaicin
The skin perfusion of the test sites, i.e. the region delimited by the rubber O-rings, was mapped using a High Resolution Laser Doppler Perfusion Imager (HR-LDPI system, PeriScan PIM II®; Perimed, Järfälla, Sweden). With the X-Y scanning laser probe, laser Doppler perfusion imaging allows two-dimensional mapping of the blood flow variability over an extended skin surface. The method is noncontact, the laser beam being controlled by the computer-controlled rotation of a mirror about two perpendicular axes. The red light of a 633-nm helium-neon laser penetrates the skin variably to a depth of about 0.6 mm. The vasculature within these layers comprises vessels of variable size, orientation and function. For this reason, it is not meaningful to express the cutaneous flow in absolute terms, but rather as arbitrary ‘perfusion units’. The estimation of the average skin perfusion at the test sites was based on the measurement of perfusion values of approximately 60 individual sampling sites. These calculations were performed using the built-in statistical function of the HR-LDPI system (LDPIwin; Perimed). The methodology is fully described by Fullerton et al. [24].
Data analysis and statistics
The change in DBF in response to capsaicin was expressed as the percent change from baseline. At each time point the mean of observations with the 90% two-sided confidence interval (CI) is given. In addition, a paired Student's t-test was performed to compare the DBF percent change from baseline to placebo.
In part I of the study, we first calculated the means of the DBF responses in both arms and during both periods for every dose of capsaicin or placebo, at each time point regardless of the site of application on the forearm. A second analysis was made in which the means of the increases in DBF in both arms and periods were assessed at each site tested for each dose of capsaicin or placebo. For each subject, the DBF percent change from baseline was then compared between both arms (i.e. arm-to-arm reproducibility) and between both study periods (i.e. period-to-period reproducibility). To assess the test–retest reproducibility of the DBF increase, the Bradley–Blackwood procedure, which corresponds to the graphical method of Altman and Bland, was used [27, 28]. Practically, the individual means of the increases of DBF for each dose, at each time point and test site were plotted vs. the individual differences in DBF response between the right and left forearm (arm-to-arm reproducibility) or first and second study period (period-to-period reproducibility). For the arm-to-arm comparison, the observations from the first and second study periods were used, whereas for the period-to-period comparison, the observations from both arms were included in the analysis. Using the Bradley–Blackwood procedure, we then tested site-by-site whether at any given time point the regression coefficient of this regression analysis was significantly different from zero. A regression coefficient significantly different from zero suggests that the test–retest reproducibility or model assumptions may be violated. The Bradley–Blackwood test was performed at the 0.05 significance level. As an exploratory assessment for test–retest reproducibility, the concordance correlation coefficient (CCC) was calculated for each time point and each site [29–31]. A CCC value closer to 1 indicates a more reproducible response.
In part II of the study, only subjects with an increase in forearm blood flow from baseline of ≥100% (i.e. responders) in both proximal sites of both arms and during both study periods were included. The means of the DBF percent change from baseline were calculated for the two proximal test sites (i.e. the rings containing the 1000 µg capsaicin dose) and for the two distal test sites (i.e. the rings containing placebo). As in part I, the observations from the first and second study periods were used for the arm-to-arm comparison, whereas for the period-to-period comparison the observations from both arms were included in the analysis. In addition, the area under the curve of the percent change from baseline up to 30 min after capsaicin application (AUC0−30) was calculated as a summary measure. To assess test–retest reproducibility, the mean difference and the repeatability coefficient (RC), i.e. 1.96 times the SD of the differences, were calculated according to Bland and Altman [32]. Second, the mean within-subject coefficient of variation (WCV) was calculated by dividing the within-subject SD by the mean and expressing it as a percentage. The within-subject SD was calculated as the square root of the residual mean square using two-way analysis of variance (anova) [33]. A two-sided 95% CI was calculated for the mean difference and WCV. Finally, the CCC was calculated.
Sample size calculations for a paired study design with continuous response measures were performed using the mean response of periods 1 and 2 and the SD of the difference in response between period 1 and period 2 and between the dominant and nondominant arm [34]. Sample sizes required to detect a predetermined difference of 10, 20 and 50% in DBF response between treatment periods and arms given a type I error probability (α) of 0.05 and a power of 80% were calculated. P < 0.05 was considered statistically significant.
Results
Capsaicin application was well tolerated by all subjects in both study parts and no adverse events of note were reported. In most subjects, capsaicin provoked a local flare and stinging sensation that disappeared within 2–6 h after application. Two subjects experienced a stinging sensation at the exact spot of capsaicin application whilst asleep, whereas another healthy volunteer noted an aggravation of the residual stinging whilst taking a hot bath.
Part I: dose finding and assessment of the influence of the forearm location
The study was completed successfully by all 12 subjects. Mean ± SD (range) for age, weight and height was 24 ± 3 years (20–30), 81 ± 12 kg (67–115) and 184 ± 7 cm (169–196), respectively.
When calculating the means of the DBF responses in both arms and during both periods for each dose of capsaicin or placebo, at each time point regardless of the site of application on the forearm, a gradual increase of the DBF after both the 300 µg and 1000 µg capsaicin applications became apparent (Figure 1A). This increase was statistically significant compared with placebo at all time points except at 10 min postdose. The 100 µg dose failed to increase DBF significantly at any time point.
Figure 1.
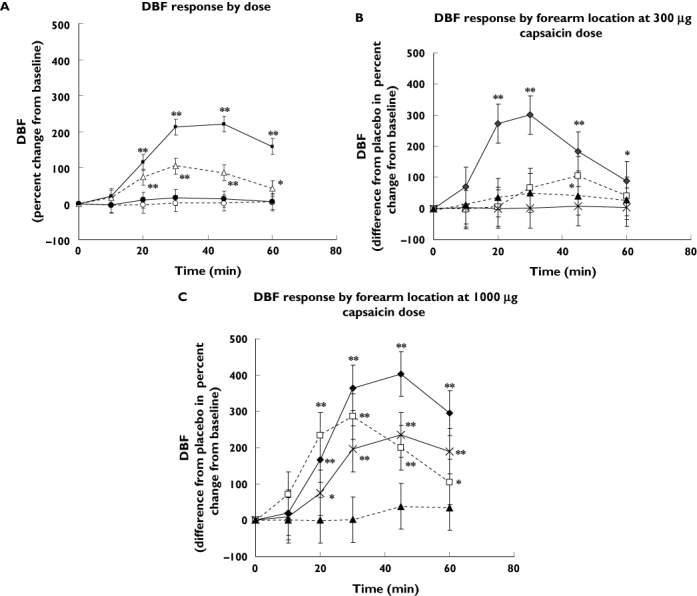
(A) Dermal blood flow (DBF) response (percent change from baseline) after application of placebo (○), 100 µg (•), 300 µg (▵) and 1000 µg (▪) capsaicin. The mean of the DBF responses in both arms and during both periods for each dose of capsaicin or placebo was calculated for each subject. Number of subjects = 12, number of observations per dose/placebo = 48. *P < 0.05; **P < 0.001 (paired Student's t-test comparing the percent change from baseline to placebo). Data are mean of observations ± 90% confidence interval (CI). (B) DBF response (difference from placebo in the percent change from baseline) after application of 300 µg capsaicin. The mean of the DBF responses in both arms and during both periods was calculated for each subject. Site 1 is most proximal; site 4 most distal. Number of subjects receiving 300 µg capsaicin at given site = 3, number of observations per site = 12. *P < 0.05; **P < 0.001 (paired Student's t-test comparing the percent change from baseline to placebo). Data are mean of observations ± 90% CI. (C) DBF response (difference from placebo in the percent change from baseline) after application of 1000 µg capsaicin. The mean of the DBF responses in both arms and during both periods was calculated for each subject. Site 1 is most proximal; site 4 most distal. Number of subjects receiving 1000 µg capsaicin at given site = 3, number of observations per site = 12. *P < 0.05; **P < 0.001 (paired Student's t-test comparing the percent change from baseline to placebo). Data are mean of observations ± 90% CI. Site 1 (♦); site 2 (□); site 3 (▴); site 4 ( )
)
Data were also analysed as a function of the forearm location for each dose of capsaicin or placebo. At all time points and at all sites, the 100 µg dose failed to raise DBF compared with placebo (data not shown). At the 300 µg dose, only site 1 (i.e. most proximal) demonstrated a significant increase compared with placebo at every time point except the 10-min time point (Figure 1B). At the 1000 µg dose, sites 1, 2 and 4 showed significant increases of DBF compared with placebo, again at all time points except at 10 min (Figure 1C).
Test–retest reproducibility was assessed by site from arm-to-arm and from period-to-period using the Bradley–Blackwood test (Table 1). Apart from site 4, no sites violated arm-to-arm or period-to-period test–retest reproducibility from 30 min onwards. One exception was site 2, for which the period-to-period reproducibility was violated at 60 min postdose.
Table 1.
Test–retest reproducibility for dermal blood flow response by site
| Arm-to-arm reproducibility | Period-to-period reproducibility | ||||
|---|---|---|---|---|---|
| Site | Time, min | P-value* | CCC | P-value | CCC |
| 1 | 10 | < 0.001 | 0.269 | 0.0005 | 0.190 |
| 20 | 0.0360 | 0.766 | 0.7684 | 0.697 | |
| 30 | 0.2117 | 0.804 | 0.8034 | 0.780 | |
| 45 | 0.4483 | 0.790 | 0.7481 | 0.859 | |
| 60 | 0.9618 | 0.760 | 0.4384 | 0.715 | |
| 2 | 10 | 0.0692 | 0.355 | 0.0244 | 0.215 |
| 20 | 0.2384 | 0.951 | 0.0009 | 0.836 | |
| 30 | 0.2479 | 0.887 | 0.1039 | 0.714 | |
| 45 | 0.4876 | 0.932 | 0.4859 | 0.376 | |
| 60 | 0.1515 | 0.797 | 0.0431 | 0.344 | |
| 3 | 10 | 0.4623 | 0.163 | 0.4421 | 0.423 |
| 20 | 0.0025 | 0.325 | 0.0006 | 0.163 | |
| 30 | 0.1137 | 0.513 | 0.3259 | 0.117 | |
| 45 | 0.0552 | 0.372 | 0.5679 | 0.408 | |
| 60 | 0.1548 | 0.490 | 0.1614 | 0.245 | |
| 4 | 10 | 0.0448 | 0.236 | 0.1160 | 0.050 |
| 20 | 0.0056 | 0.073 | 0.0001 | 0.367 | |
| 30 | 0.0028 | 0.623 | 0.0220 | 0.644 | |
| 45 | 0.0048 | 0.808 | 0.8164 | 0.720 | |
| 60 | 0.4062 | 0.951 | 0.3193 | 0.788 | |
Number of subjects = 12, number of observations = 48. The proximal ring (i.e. closest to the antecubital crease) is referred to as site 1, the distal ring (i.e. closest to the wrist crease) as site 4.
Based on Bradley–Blackwood test: P < 0.05 indicates evidence of unequal means or unequal variances from right arm to left arm or from period 1 to period 2. CCC, Concordance correlation coefficient indicating strength of agreement; CCC > 0.9, almost perfect; CCC 0.8–0.9, substantial; CCC 0.65–0.8, moderate; CCC < 0.65, poor.
The CCC showed substantial to almost perfect strength-of-agreement for test–retest arm-to-arm DBF measurements at the 30-min time point at sites 1 and 2. The test–retest period-to-period reproducibility was moderate for the same sites at the same time point (Table 1).
Part II: within-subject arm-to-arm and period-to-period reproducibility
Of the 21 subjects enrolled in part II, 10 nonresponders were excluded from further analysis (i.e. the increase in forearm blood flow was <100% in one of the proximal sites during one of the two study periods). The data of the 11 remaining subjects were included in the reproducibility analysis. Mean ± SD (range) for age, weight and height of these 11 subjects was 25 ± 5 years (20–37), 81 ± 7 kg (69–91) and 182 ± 5 cm (174–192), respectively.
The gradual increase in the DBF after 1000 µg capsaicin application is shown by arm pooled over periods (Figure 2A) and by period pooled over arms (Figure 2B). This increase was statistically significant compared with placebo at all time points. However, the increase in the DBF was not significantly different when comparing the same time points between arms or periods.
Figure 2.
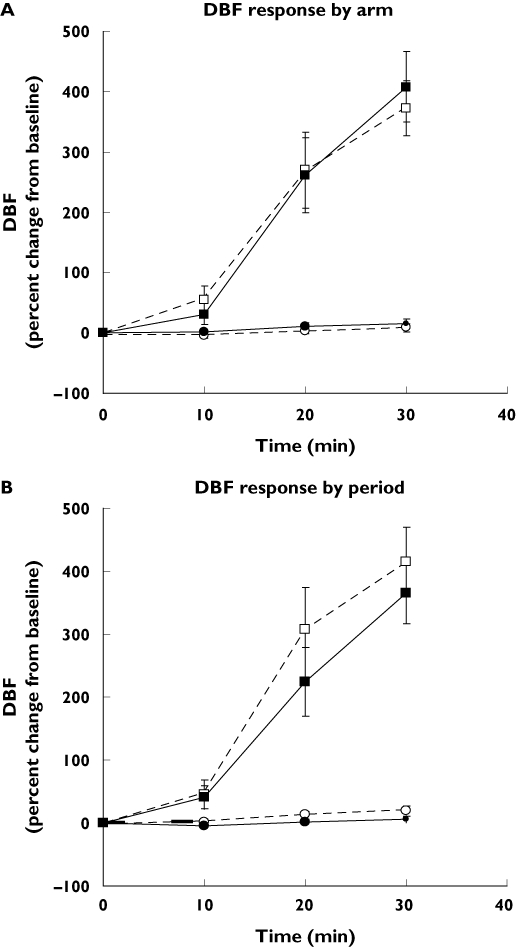
(A) Dermal blood flow (DBF) response (percent change from baseline) after application of placebo and 1000 µg capsaicin. The mean of the DBF responses at the capsaicin or placebo application sites during both study periods was calculated. Number of subjects = 11, number of observations = 44. D, Dominant arm; ND, nondominant arm. Data are mean of observations ± 90% confidence interval (CI). Placebo D (○); Placebo ND (•); 1000 µg capsaicin D (□); 1000 µg capsaicin ND (▪). (B) DBF response (percent change from baseline) after application of placebo and 1000 µg capsaicin. The mean of the DBF responses at the capsaicin or placebo application sites in both arms was calculated. Number of subjects = 11, number of observations = 44. P1, Period 1; P2, period 2. Data are mean of observations ± 90% CI. Placebo P1 (○); Placebo P2 (•); 1000 µg capsaicin P1 (□); 1000 µg capsaicin P2 (▪)
Results for the reproducibility analyses of DBF responses are given in Table 2. The test–retest reproducibility was better from arm-to-arm than when compared from period-to-period. This is reflected by the CCC for arm-to-arm test–retest reproducibility, which varies from moderate for DBF expressed at t30 to almost perfect for DBF expressed as AUC0−30. As a consequence, a smaller number of subjects was needed to detect a predetermined shift in DBF response between arms than between periods.
Table 2.
Test–retest reproducibility of dermal blood flow (DBF) response and sample size calculations
| DBF response | Test–retest reproducibility | Mean difference (95% CI) | RC | WCV (%) (95% CI) | CCC | Sample size 10% shift | Sample size 20% shift | Sample size 50% shift |
|---|---|---|---|---|---|---|---|---|
| t30 (%) | Period-to-period | 49 (−19.6, 118.0) | 307 | 28 (19, 38) | 0.40 | 129 | 34 | 7 |
| Arm-to-arm | 36 (−85.4, 14.4) | 223 | 21 (14, 27) | 0.68 | 69 | 19 | 5 | |
| AUC0−30 (% min−1) | Period-to-period | 1119 (−239, 2476) | 6058 | 43 (30, 57) | 0.33 | 297 | 76 | 14 |
| Arm-to-arm | 139 (−340, 619) | 2140 | 15 (11, 20) | 0.91 | 39 | 11 | 4 |
Number of subjects = 11, number of observations = 44, the mean DBF response in the two proximal rings was used in the test–retest analysis. Test–retest period-to-period and arm-to-arm reproducibility data for DBF response expressed as percent change from baseline at 30 min (t30) and as the area under the curve of the percent change from baseline (AUC0−30). RC, Repeatability coefficient; WCV, within-subject coefficient of variation; CCC, concordance correlation coefficient; 95% CI, 95% two-sided confidence interval.
Discussion
The present study convincingly demonstrates that the increase in DBF following capsaicin application is adequately reproducible at the proximal forearm when the DBF response is sufficiently robust (i.e. defined as ≥100% increase from baseline). The procedure was well tolerated and no adverse events of note were reported.
Exploration of the skin microcirculation by laser Doppler technology has often been considered poorly reproducible [35]. However, the introduction of laser Doppler perfusion imagers that allow the quasi-simultaneous measurement of skin blood flow at a very large number of points, thus allowing the ‘averaging out’ of spatial variations encountered within the explored area, has improved the reproducibility of this technique considerably [24, 36, 37]. Clear guidelines for the measurement of DBF by laser Doppler perfusion imaging are now available and it has become a regularly used technique for assessing mean cutaneous blood flow of both normal and irritated forearm skin [24].
Data from the first part of this study revealed a dose-dependent capsaicin-induced increase in DBF. At the 1000 µg capsaicin dose, a robust and reproducible response was present in both proximal sites at the 30-min time point. The maximum response occurred between 30 and 45 min after capsaicin application, with a clear decline at the 60-min time point. The transient nature of the capsaicin-induced vasodilation has also been seen in rats, rabbits and rhesus monkeys and may be attributed to the half-life and depletion of the endogenous mediators and/or the desensitization of the TRPV1 receptor [26, 38, 39].
Data from the first part of this study clearly indicated that, compared with the more distal sites, the two proximal sites of the human forearm showed the most reproducible and robust responses to capsaicin. This regional variation in response has also been seen in a study in humans using transdermal iontophoretic application of acetylcholine to induce vasodilation in the forearm skin [36]. The reasons for this regional variation remain uncertain. Heterogeneity in the density and function of the capsaicin-sensitive nociceptive nerve endings as well as of the dermal microcirculation seem the most plausible explanations. In addition, the transdermal transfer of the capsaicin-containing solution might differ between the proximal and the distal forearm due to differences in skin thickness. Whatever the explanation, the results indicate the importance of clearly defining application sites and of using the same sites on different occasions if one wishes to optimize the test–retest reproducibility of the DBF response.
From the first part of the study, it became apparent that although overall DBF increased significantly after both the 300 µg and 1000 µg capsaicin applications, there were large interindividual differences.
In a large percentage of the volunteers, DBF failed to increase by >100% when compared with baseline, even at the 1000 µg capsaicin dose. Helme et al. have extensively investigated the wide variation in size and intensity of the capsaicin-induced flare response. They found that the major factors in flare response were body site and age, though they also emphasized that one must be guarded in the interpretation because of the large number of variables involved [10]. Gazerani et al. recently found the capsaicin-induced sensory and vasomotor responses were also gender specific [40]. As our aim in the present study was to produce a robust and reproducible DBF response, we chose to include males only and decided, in part II of the study, to select only men with a DBF increase from baseline of ≥100% following capsaicin application. This decision was based on our findings in part I, which suggested that in this way reproducibility would further increase.
In part II, we chose to report the mean difference and RC [32], the WCV [33] and the CCC [29–31] to characterize the test–retest reproducibility of the DBF response to capsaicin application. Comparing reproducibility data between studies is often a daunting experience, as various measures of reproducibility are reported in medical literature and measurement protocols often differ between studies. The majority of studies that used laser Doppler perfusion imaging and for which reproducibility data are available assessed the DBF response to acetylcholine and sodium nitroprusside [41–43]. These studies differ strongly in design, population and even drug administration, which makes comparison of reproducibility with our current study rather meaningless. Furthermore, there is general lack of detailed information on the exact conditions under which reproducibility was tested in most of these studies.
In our study we used the AUC0−30 as a summary measure. The use of summary measures to analyse serial measurements is a useful and simple tool in medical research [44]. Summary measurements are considered to be more clinically relevant than data obtained on discrete points in time and allow the presentation of a response over time as a single value. It is also believed that the use of summary measures may reduce variability and thus increase reproducibility. In the present study, reproducibility of the summary response AUC0−30 was not consistently better compared with reproducibility of the single time point measure t30. This is in agreement with previous findings of our group [45]. Here the use of the AUC as a summary measure accurately accentuates the inferiority of period-to-period compared with arm-to-arm reproducibility.
In rats and rabbits, CGRP has been identified as one of the major mediators of capsaicin-induced vasodilation [46, 47]. This was confirmed by Hershey et al., who developed and validated a non-invasive pharmacodynamic model using capsaicin in rats and nonhuman primates to assess CGRP antagonist activity in vivo [26]. They demonstrated that capsaicin induces dose- and time-dependent vasodilation that could be readily detected and quantified by laser Doppler perfusion imaging. They also showed that a CGRP antagonist inhibited the capsaicin-induced increase in DBF, but at doses that were different between rhesus monkeys and rats. This difference in inhibitory dose serves as an illustration of the molecular differences in CGRP receptor pharmacology between species and was one of the prime motives for the translation of this model for CGRP release to the human species itself.
In addition to CGRP, capsaicin-sensitive nociceptors in the skin contain a number of other mediators that might play a role in capsaicin-induced vasodilation. The involvement of SP in capsaicin-induced vasodilation has been proven in several animal species [16, 48]. In the human skin, SP has been shown to induce vasodilation in the absence of neuropeptide-induced activation of nociceptors [49]. However, there is as yet no conclusive evidence for the involvement of SP in capsaicin-induced vasodilation in the human skin [17, 50]. Additionally, there are, to our knowledge, no reports on the influence of NK1 receptor antagonists on capsaicin-induced flare in vivo in humans. The putative role of proinflammatory prostaglandins and NO in capsaicin-induced neurogenic inflammation also remains a much debated issue [21, 51, 52]. Therefore, further characterization and understanding of the (patho) physiological process following dermal capsaicin application is warranted.
The pathophysiology of migraine is complex and incompletely understood, but vasodilation of cranial blood vessels and the activation of the trigeminal vascular system probably play a significant role [53]. Observations in animal and human studies suggest that CGRP, released from perivascular nerve terminals, is a key player involved in the activation of the trigeminovascular system. Therefore, inhibition of CGRP-driven pathophysiological processes may yield a novel therapeutic approach in migraine, as confirmed by the experience with BIBN4096BS, the first highly selective and potent CGRP receptor antagonist proven to be efficacious in the acute treatment of migraine headache [23]. Given the species-dependent affinity of CGRP receptor antagonists for the CGRP receptor [26], different pharmacodynamic assays have been developed in several clinically relevant animal models to predict the efficacy of CGRP receptor antagonists in vivo. One of our primary goals was to develop a non-invasive pharmacodynamic model in healthy volunteers to provide a rapid assessment of CGRP antagonist activity and/or CGRP release inhibition in humans. Nevertheless, depending on the further characterization of the dermal capsaicin response, the model might also be of value when studying drugs that influence SP, prostaglandins or NO release.
Although we are fully aware of the gender-specific response to capsaicin, we used our model solely in male healthy volunteers on account of standardization concerns [40]. Future gender-specific investigations using capsaicin-induced neurogenic inflammation could nevertheless greatly improve our understanding of gender-related (patho) physiological processes, such as migraine-related trigeminal sensitization. Finally, it should be noted that no pharmacodynamic model, including that presented here, completely mimics the pathophysiological processes one wants to study. The effect of a drug on capsaicin-induced dermal neurogenic inflammation is indicative only of its efficacy in treating the neurogenic inflammation within the meningeal vasculature during a migraine attack. Nevertheless, the use of such a model can have great advantages in dose finding and proof of concept studies.
In summary, we have developed a pharmacodynamic model in humans which is non-invasive, technically uncomplicated and has a rapid and objective end-point. This pharmacodynamic model allows for repeated measurements to be performed which we have shown to be adequately reproducible. This model might therefore facilitate the early clinical evaluation of antagonists of mediators involved in neurogenic inflammation, including CGRP, TRPV1 and, possibly, SP antagonists.
Acknowledgments
Competing interests: None declared.
The authors gratefully acknowledge Jo Van Effen and Marc Oeyen for their assistance during the laser Doppler imaging and Ayline Yudhira for her help with the data analysis.
References
- 1.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 2.Högyes A. Beiträge zur physiologischen Wirkung der Bestandteile des Capsicum annuum. Arch Exp Pathol Pharmakol. 1878;9:117–30. [Google Scholar]
- 3.Porszasz J, Jancso N. Studies on the action potentials of sensory nerves in animals desensitized with capsaicine. Acta Physiol Acad Sci Hung. 1959;16:299–306. [PubMed] [Google Scholar]
- 4.Szallasi A, Blumberg PM. Resiniferatoxin and its analogs provide novel insights into the pharmacology of the vanilloid (capsaicin) receptor. Life Sci. 1990;47:1399–408. doi: 10.1016/0024-3205(90)90518-v. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 6.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 7.Nagy I, Rang H. Noxious heat activates all capsaicin-sensitive and also a sub-population of capsaicin-insensitive dorsal root ganglion neurons. Neuroscience. 1999;88:995–7. doi: 10.1016/s0306-4522(98)00535-1. [DOI] [PubMed] [Google Scholar]
- 8.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 9.Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–51. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 10.Helme RD, McKernan S. Neurogenic flare responses following topical application of capsaicin in humans. Ann Neurol. 1985;18:505–9. doi: 10.1002/ana.410180414. [DOI] [PubMed] [Google Scholar]
- 11.LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- 12.LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–64. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumikura H, Andersen OK, Drewes AM, Arendt-Nielsen L. Spatial and temporal profiles of flare and hyperalgesia after intradermal capsaicin. Pain. 2003;105:285–91. doi: 10.1016/s0304-3959(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 14.Hughes SR, Brain SD. A calcitonin gene-related peptide (CGRP) antagonist (CGRP8-37) inhibits microvascular responses induced by CGRP and capsaicin in skin. Br J Pharmacol. 1991;104:738–42. doi: 10.1111/j.1476-5381.1991.tb12497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brain SD, Williams TJ. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br J Pharmacol. 1985;86:855–60. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escott KJ, Connor HE, Brain SD, Beattie DT. The involvement of calcitonin gene-related peptide (CGRP) and substance P in feline pial artery diameter responses evoked by capsaicin. Neuropeptides. 1995;29:129–35. doi: 10.1016/0143-4179(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 17.Petersen LJ, Winge K, Brodin E, Skov PS. No release of histamine and substance P in capsaicin-induced neurogenic inflammation in intact human skin in vivo: a microdialysis study. Clin Exp Allergy. 1997;27:957–65. [PubMed] [Google Scholar]
- 18.Wallengren J. Vasoactive peptides in the skin. J Invest Dermatol Symp Proc. 1997;2:49–55. doi: 10.1038/jidsymp.1997.11. [DOI] [PubMed] [Google Scholar]
- 19.Tafler R, Herbert MK, Schmidt RF, Weis KH. Small reduction of capsaicin-induced neurogenic inflammation in human forearm skin by the glucocorticoid prednicarbate. Agents Actions. 1993;38:C31–4. doi: 10.1007/BF01991128. [DOI] [PubMed] [Google Scholar]
- 20.Huttunen M, Harvima IT, Ackermann L, Harvima RJ, Naukkarinen A, Horsmanheimo M. Neuropeptide- and capsaicin-induced histamine release in skin monitored with the microdialysis technique. Acta Derm Venereol. 1996;76:205–9. doi: 10.2340/0001555576205209. [DOI] [PubMed] [Google Scholar]
- 21.Herbert MK, Tafler R, Schmidt RF, Weis KH. Cyclooxygenase inhibitors acetylsalicylic acid and indomethacin do not affect capsaicin-induced neurogenic inflammation in human skin. Agents Actions. 1993;38:C25–7. doi: 10.1007/BF01991126. [DOI] [PubMed] [Google Scholar]
- 22.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 23.Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 24.Fullerton A, Stucker M, Wilhelm KP, Wardell K, Anderson C, Fischer T, Nilsson GE, Serup J. Guidelines for visualization of cutaneous blood flow by laser Doppler perfusion imaging. A report from the Standardization Group of the European Society of Contact Dermatitis based upon the HIRELADO European community project. Contact Dermatitis. 2002;46:129–40. doi: 10.1034/j.1600-0536.2002.460301.x. [DOI] [PubMed] [Google Scholar]
- 25.Del Bianco E, Geppetti P, Zippi P, Isolani D, Magini B, Cappugi P. The effects of repeated dermal application of capsaicin to the human skin on pain and vasodilatation induced by intradermal injection of acid and hypertonic solutions. Br J Clin Pharmacol. 1996;41:1–6. doi: 10.1111/j.1365-2125.1996.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 26.Hershey JC, Corcoran HA, Baskin EP, Salvatore CA, Mosser S, Williams TM, Koblan KS, Hargreaves RJ, Kane SA. Investigation of the species selectivity of a nonpeptide CGRP receptor antagonist using a novel pharmacodynamic assay. Regul Pept. 2005;127:71–7. doi: 10.1016/j.regpep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez MM, Binkowitz BS. Guidelines for measurement validation in clinical trial design. J Biopharm Stat. 1999;9:417–38. doi: 10.1081/BIP-100101185. [DOI] [PubMed] [Google Scholar]
- 28.Bradley E, Blackwood L. Comparing Paired Data: a simultaneous test of means and variances. Am Stat. 1981;43:234–35. [Google Scholar]
- 29.Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials. 1991;12(4 Suppl.):142S–158S. doi: 10.1016/s0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 30.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68. [PubMed] [Google Scholar]
- 31.Quiroz J. Assessment of equivalence using a concordance correlation coefficient in a repeated measurements design. J Biopharm Stat. 2005;15:913–28. doi: 10.1080/10543400500265652. [DOI] [PubMed] [Google Scholar]
- 32.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Measurement error. BMJ. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–28. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 35.Schabauer AM, Rooke TW. Cutaneous laser Doppler flowmetry: applications and findings. Mayo Clin Proc. 1994;69:564–74. doi: 10.1016/s0025-6196(12)62249-6. [DOI] [PubMed] [Google Scholar]
- 36.Kubli S, Waeber B, Dalle-Ave A, Feihl F. Reproducibility of laser Doppler imaging of skin blood flow as a tool to assess endothelial function. J Cardiovasc Pharmacol. 2000;36:640–8. doi: 10.1097/00005344-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Fullerton A, Rode B, Serup J. Studies of cutaneous blood flow of normal forearm skin and irritated forearm skin based on high-resolution laser Doppler perfusion imaging (HR-LDPI) Skin Res Technol. 2002;8:32–40. doi: 10.1046/j.0909-752x.2001.10327.x. [DOI] [PubMed] [Google Scholar]
- 38.Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431:828–37. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Simon SA. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J Neurophysiol. 1996;75:1503–14. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- 40.Gazerani P, Andersen OK, Arendt-Nielsen L. A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain. 2005;118:155–63. doi: 10.1016/j.pain.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Morris SJ, Shore AC, Tooke JE. Responses of the skin microcirculation to acetylcholine and sodium nitroprusside in patients with NIDDM. Diabetologia. 1995;38:1337–44. doi: 10.1007/BF00401767. [DOI] [PubMed] [Google Scholar]
- 42.Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol. 1996;496:531–42. doi: 10.1113/jphysiol.1996.sp021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veves A, Saouaf R, Donaghue VM, Mullooly CA, Kistler JA, Giurini JM, Horton ES, Fielding RA. Aerobic exercise capacity remains normal despite impaired endothelial function in the micro- and macrocirculation of physically active IDDM patients. Diabetes. 1997;46:1846–52. doi: 10.2337/diab.46.11.1846. [DOI] [PubMed] [Google Scholar]
- 44.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–5. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanmolkot FH, de Hoon JN. Reproducibility of forearm vasodilator response to intra-arterial infusion of calcitonin gene-related peptide assessed by venous occlusion plethysmography. Br J Clin Pharmacol. 2005;59:387–97. doi: 10.1111/j.1365-2125.2005.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akerman S, Kaube H, Goadsby PJ. Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br J Pharmacol. 2003;140:718–24. doi: 10.1038/sj.bjp.0705486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newbold P, Brain SD. An investigation into the mechanism of capsaicin-induced oedema in rabbit skin. Br J Pharmacol. 1995;114:570–7. doi: 10.1111/j.1476-5381.1995.tb17177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moussaoui SM, Philippe L, Le Prado N, Garret C. Inhibition of neurogenic inflammation in the meninges by a non-peptide NK1 receptor antagonist, RP 67580. Eur J Pharmacol. 1993;238:421–4. doi: 10.1016/0014-2999(93)90879-m. [DOI] [PubMed] [Google Scholar]
- 49.Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS, Petersen LJ, Schmelz M. Acute effects of substance P and calcitonin gene-related peptide in human skin – a microdialysis study. J Invest Dermatol. 2000;115:1015–20. doi: 10.1046/j.1523-1747.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 50.Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:175–83. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- 51.Hughes SR, Brain SD. Nitric oxide-dependent release of vasodilator quantities of calcitonin gene-related peptide from capsaicin-sensitive nerves in rabbit skin. Br J Pharmacol. 1994;111:425–30. doi: 10.1111/j.1476-5381.1994.tb14752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldsmith PC, Leslie TA, Hayes NA, Levell NJ, Dowd PM, Foreman JC. Inhibitors of nitric oxide synthase in human skin. J Invest Dermatol. 1996;106:113–8. doi: 10.1111/1523-1747.ep12328204. [DOI] [PubMed] [Google Scholar]
- 53.Goadsby PJ, Lipton RB, Ferrari MD. Migraine – current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]


