Abstract
What is already known about this subject
Tenofovir disoproxil fumarate and some of the HIV protease inhibitors show drug–drug interactions that cannot be predicted based on their metabolic profiles.
Tenofovir disoproxil fumarate and HIV protease inhibitors are often combined as part of antiretroviral therapy.
What this study adds
TMC114 (darunavir) is the latest HIV protease inhibitor approved by the US Food and Drug Administration and is used in combination with low-dose ritonavir.
This study shows for the first time the extent of the drug–drug interaction between tenofovir disoproxil fumarate and TMC114 combined with low-dose ritonavir and compares the interaction observed with other HIV protease inhibitors.
Aim
TMC114 is a new HIV protease inhibitor, used in combination with low-dose ritonavir (TMC114/r) as a pharmacokinetic enhancer. Tenofovir disoproxil fumarate (TDF) is a nucleotide reverse transcriptase inhibitor. Both antiretrovirals show activity against wild-type and resistant HIV. An open-label crossover study was conducted in HIV – healthy volunteers to investigate the potential for a pharmacokinetic interaction between TMC114/r and tenofovir.
Methods
Two groups, each of six volunteers, were evaluated in two consecutive sessions. In session 1, volunteers received TMC114/r (300/100 mg bid) for 7 days, followed by a wash-out period of at least 6 days. In session 2, volunteers received TMC114/r (300/100 mg bid) plus TDF (300 mg qd).
Results
When TMC114/r and TDF were coadministered, tenofovir plasma concentrations (Cmin and Cmax), and area under the curve (AUC24 h) increased by 37%, 24% and 22%, respectively. When TDF and ritonavir were coadministered, TMC114 plasma Cmin, Cmax and AUC12 h increased by 24%, 16% and 21%, respectively. There were no changes in the urinary excretion of unchanged tenofovir or TMC114 during coadministration. Administration of TMC114/r in HIV– healthy volunteers with or without TDF was well tolerated.
Conclusions
The interaction between TMC114/r and tenofovir is not clinically relevant and no dose adjustments are required when these drugs are coadministered.
Keywords: darunavir, interaction, ritonavir, tenofovir disoproxil fumarate, TMC114
Introduction
TMC114 (Prezista™) is a new protease inhibitor (PI), highly active against both wild-type and resistant HIV-1 strains in vitro [1]. TMC114 is approved in the USA in combination with ritonavir for the treatment of HIV infection in treatment-experienced adults [2]. TMC114 with low doses of ritonavir (TMC114/r) results in improved pharmacokinetic characteristics compared with TMC114 alone [3]. Ongoing randomized, controlled trials are designed to evaluate the efficacy and safety of TMC114/r in HIV-infected patients. Results of the 24-week primary analyses of studies in the treatment-experienced have shown significant benefits in efficacy outcomes when TMC114/r is compared with investigator-selected control PIs in combination with an optimized background regimen (OBR) [4, 5]. In addition, TMC114/r is generally safe and well tolerated.
Tenofovir disoproxil fumarate (TDF) is a nucleotide reverse transcriptase inhibitor (NtRTI) with efficacy in treatment-naive and -experienced patients [6, 7]. Tenofovir has been shown to interact with some PIs and nucleoside analogues; an unexpected finding, as tenofovir does not induce or inhibit cytochrome P450 (CYP) 3A4 metabolism; most of these interactions are deemed not clinically relevant [8]. As TMC114/r will be used in combination with other antiretroviral agents, a pharmacokinetic interaction trial of TMC114/r and TDF was performed in HIV– healthy volunteers.
Methods
Study design
This was an open-label, randomized, two-session crossover trial to determine the extent of pharmacokinetic interaction between TMC114/r and TDF. The study participants were HIV– healthy volunteers, aged 18–55 years. During session 1, volunteers were randomized into group 1 (n = 7) or group 2 (n = 6). In session 1, both groups received TMC114/r 300/100 mg bid for 6 days with an additional dose in the morning of day 7. After a wash-out period of 6 days, both groups were administered TDF 300 mg qd for 14 days (session 2). In addition, volunteers in group 1 received TMC114/r 300/100 mg bid from day 8 to day 14, whereas volunteers in group 2 received TMC114/r from day 1 to day 7. No formal sample size calculation was performed. A total of 13 subjects were enrolled to allow for relevant conclusions. A regimen of TMC114/r 300/100 mg bid has been found to be well tolerated in healthy volunteers [3]. This dose is lower than the recommended 600/100 mg bid dose of TMC114/r. TDF is licensed at a dosing regimen of 300 mg qd [8], which was the recommended dose for this study. A wash-out period of at least 6 days between the two sessions was considered sufficient to avoid carry-over effects from one session to the next.
All study medication was taken with food. TMC114 was formulated as an oral aqueous solution of 20 mg ml−1 containing vitamin E-d-α-tocopheryl polyethylene glycol 1000 succinate and polyethylene glycol 400 as main solubilizing agents, while ritonavir and TDF were obtained as commercially available capsules and tablets, respectively.
Full pharmacokinetic profiles of TMC114 and ritonavir were determined on day 7 of session 1 and on day 14 (group 1) or day 7 (group 2) of session 2. Full pharmacokinetic profiles of tenofovir were determined on day 7 and day 14 of session 2 for both groups. On days when samples were taken for pharmacokinetic analysis, TMC114, ritonavir and/or TDF were ingested within 15 min after a standard breakfast. Volunteers fasted for 8 h before breakfast and resumption of normal water and food intake was allowed 2 and 4 h after breakfast, respectively.
The study protocol was reviewed and approved by the appropriate institutional ethics committee(s) and health authorities, and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all volunteers.
Pharmacokinetic assessments
TMC114 and ritonavir in plasma and urine were determined simultaneously using a validated LC-MS/MS method. The lower limit of quantification in plasma was 10.0 ng ml−1 for TMC114, 5.00 ng ml−1 for RTV and 20.0 ng ml−1 for tenofovir. The lower limit of quantification in urine was 20.0 ng ml−1 for TMC114 and 1.00 µg ml−1 for tenofovir. The precision and accuracy for the TMC114 (40, 400 and 8000 ng ml−1) and ritonavir (20, 200, 4000 ng ml−1) quality control (QC) samples in plasma and urine were <12% and 16%, respectively, and met the predefined criteria of <20% for the low QC and 15% for the medium and high QC samples, respectively [9]. Concentrations of tenofovir in plasma and in urine were determined using a high-performance liquid chromatography method with fluorescence detection. The precision and accuracy for the tenofovir QC (75, 750 and 15 000 ng ml−1) samples in plasma and urine were <11% and met the predefined criteria.
During session 1, predose plasma concentrations of TMC114 and ritonavir were assessed on days 4 and 6. For pharmacokinetic analysis of TMC114 and ritonavir, day 7 blood samples were taken predose and 0.5, 1, 1.5, 2, 3, 4, 6, 9 and 12 h postdose. During session 2, predose plasma concentrations of TMC114, ritonavir and tenofovir were assessed on days 11 and 13 (group 1) or days 4 and 6 (group 2). For pharmacokinetic analysis of TMC114 and ritonavir, blood samples were taken on day 14 (group 1) or day 7 (group 2), predose and 0.5, 1, 1.5, 2, 3, 4, 6, 9 and 12 h postdose. On day 7 and day 14 of session 2, tenofovir concentrations were determined in blood samples taken predose and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 9, 12 and 24 h postdose for both groups.
Predose urine samples were taken on day 1 of session 1 and days 1 and 8 of session 2. For pharmacokinetic analysis of urine during session 1, output was collected on day 7 over a 12-h period following study drug dosing. During session 2, urine output was collected on day 14 (group 1) or day 7 (group 2) over a 24-h period after dosing.
Safety assessments
Volunteers were monitored regularly throughout the study for cardiovascular parameters, blood pressure and biochemical characteristics of blood and urine. Adverse events (AEs) were recorded. All safety evaluations were graded according to the AIDS Clinical Trials Group/World Health Organization grading scales.
Statistical methods
Descriptive statistics were calculated for the plasma concentrations of TMC114, ritonavir and tenofovir, and the urinary concentrations of TMC114 and tenofovir. Pharmacokinetic data for TMC114, ritonavir and tenofovir were analysed by noncompartmental methods using WinNonlin Professional™ software (version 3.3; Pharsight Corp., Mountain View, CA, USA). Analyses included area under the curve from time of administration to 12 h postdosing (AUC12 h) or 24 h postdosing (AUC24 h), predose plasma concentrations (C0 h), minimum (Cmin) and maximum (Cmax) plasma concentrations, average steady-state plasma concentrations (Css,av) and time to reach Cmax (tmax). The percentage of administered drug excreted in urine (Durine) from 0 to 12 h, 12–24 h and 0–24 h after dosing was also analysed by noncompartmental methods, using Microsoft Excel® software (version 2000; Microsoft, Redmond, WA, USA).
To assess the impact of coadministration with TDF on TMC114 and ritonavir pharmacokinetic characteristics, treatment with tenofovir (session 2) was used as the test and treatment without tenofovir (session 1) was used as the reference. Similarly, to assess the impact of coadministration of TMC114/r on the pharmacokinetic parameters of tenofovir, treatment with TMC114/r was used as the test and treatment without TMC114/r was used as the reference. Data for both test and reference in this case were from session 2. The primary plasma pharmacokinetic variables were C0 h, Cmin, Cmax, as well as AUC12 h (for TMC114 and ritonavir) or AUC24 h (for tenofovir) on a logarithmic scale. The primary urine pharmacokinetic parameters were Durine,0−12 h for TMC114 and Durine,total for tenofovir. Only paired observations for test and reference were included in the statistical analyses.
The least square (LS) means of the AUC, Cmin, Cmax and C0 h for each treatment group were estimated using a linear, mixed-effects model. The model controlled for period and randomization group as fixed effects and volunteer (nested in randomization group) as a random effect. Period effects were considered significant at the 5% level and sequence effects were considered significant at the 10% level. Where period and sequence effects were not significant, they were not retained in the model. A 90% confidence interval (CI) was constructed around the difference between the LS means of test and reference. Tmax was analysed by the nonparametric Koch test, using the bioequivalence module of WinNonlin Professional™ software (version 3.3; Pharsight Corp.).
Results
Pharmacokinetic data
Data from 12 HIV– healthy volunteers were available for analysis of pharmacokinetic parameters. When TDF and ritonavir were coadministered, TMC114 mean plasma C0 h, Cmin, Cmax and AUC12 h of TMC114 increased by 12%, 24%, 16% and 21%, respectively, as shown in Figure 1 and Table 1. Urinary excretion of unchanged TMC114 was not significantly altered. The LS means of Durine,0−12 h for TMC114 were 6.3% and 6.8% with and without tenofovir, respectively.
Figure 1.

Profile of mean plasma concentration of TMC114 over time, in the presence or absence of a steady-state concentration of tenofovir. With Tenofovir, (•—•); Without Tenofovir, (○—○)
Table 1.
Pharmacokinetic results of TMC114 administered with low-dose ritonavir (TMC114/r), with or without TDF
| Pharmacokinetic parameter | Without TDF | With TDF | LS mean ratio (90% CI) | P-value |
|---|---|---|---|---|
| Mean ± SD | ||||
| n | 12 | 12 | – | – |
| C0 h, ng ml−1 | 2262 ± 908 | 2473 ± 991 | 1.12 (0.85, 1.47) | 0.50 |
| Cmin, ng ml−1 | 1845 ± 842 | 2220 ± 1051 | 1.24 (0.90, 1.69) | 0.26 |
| Cmax, ng ml−1 | 3971 ± 1385 | 4448 ± 1008 | 1.16 (0.94, 1.42) | 0.24 |
| AUC12 h, ng h−1 ml−1 | 29837 ± 10345 | 35755 ± 12582 | 1.21 (0.95, 1.54) | 0.19 |
| Median (range) | ||||
| Tmax, h | 0.5 (0.5–4.0) | 0.5 (0.5–0.5) | – | 0.09 |
CI, Confidence interval; LS, least square; n, number of volunteers; SD, standard deviation; TDF, tenofovir disoproxil fumarate.
When TMC114/r was coadministered, the C0 h, Cmin, Cmax and AUC24 h for tenofovir increased by 36%, 37%, 24% and 22%, respectively, as shown in Figure 2 and Table 2. Coadministration of TMC114/r had no effect on the urinary excretion of tenofovir. The LS means of Durine,total for tenofovir were 34.9% and 33.6% with or without TMC114/r, respectively.
Figure 2.
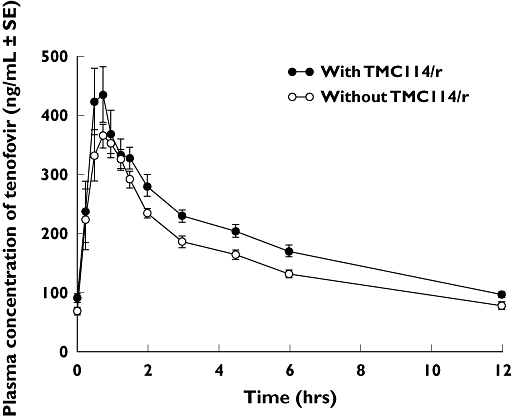
Profile of mean plasma concentration of tenofovir over time, in the presence or absence of a steady-state concentration of TMC114 and ritonavir. With TMC114/r, (•—•); Without TMC114/r, (○—○)
Table 2.
Pharmacokinetic results of tenofovir, with or without coadministration of TMC114 and ritonavir (TMC114/r)
| Pharmacokinetic parameter | Without TMC114/r | With TMC114/r | LS mean ratio (90% CI) | P-value |
|---|---|---|---|---|
| Mean ± SD | ||||
| n | 12 | 12* | – | – |
| C0 h, ng ml−1 | 67.4 ± 14.3 | 88.9 ± 17.0 | 1.36 (1.17, 1.58) | 0.003 |
| Cmin, ng ml−1 | 65.6 ± 13.4 | 86.8 ± 15.7 | 1.37 (1.19, 1.57) | 0.001 |
| Cmax, ng ml−1 | 423 ± 75.2 | 528 ± 106 | 1.24 (1.08, 1.42) | 0.01 |
| AUC24 h, ng h−1 ml−1 | 3789 ± 499 | 4633 ± 735 | 1.22 (1.10, 1.35) | 0.003 |
| Median (range) | ||||
| Tmax, h | 1.5 (0.5–2.5) | 1.0 (1.0–4.0) | – | 0.74 |
CI, Confidence interval; LS, least square; n, number of volunteers; SD, standard deviation.
n = 11 for C0 h and Cmin.
The pharmacokinetic characteristics of ritonavir were not significantly affected when coadministered with TDF and TMC114, with LS mean ratios for C0 h, Cmin, Cmax and AUC12 h being 0.95, 0.92, 0.87 and 0.94, respectively, compared with administration of TMC114/r alone. Based on predose plasma concentrations, steady-state conditions for TMC114, ritonavir and tenofovir were reached on the days when full pharmacokinetic profiles were taken. No significant sequence or period effects were observed (P > 0.1).
Safety and tolerability
Overall, TMC114/r was generally well tolerated when used alone or in combination with TDF. The most commonly reported AEs during the trial were gastrointestinal (diarrhoea, flatulence and loose stools) and headache. All reported AEs were classified as mild and no grade 3 or 4 abnormality of biochemistry parameters was recorded.
Discussion
We report here the steady-state pharmacokinetic characteristics of TMC114, ritonavir and tenofovir during coadministration in HIV– healthy volunteers. Coadministration of TMC114/r and TDF increased systemic exposure to tenofovir by 22% compared with administration of TDF alone; this is not considered clinically relevant. Administration of TDF had no significant influence on systemic exposure to TMC114. There were no changes in the urinary excretion of unchanged tenofovir or TMC114 during coadministration. Since pharmacokinetic sampling was performed over the dosing interval (12 h), the terminal half-life of TMC114 was not determined.
Pharmacokinetic drug interactions can be complex and unpredictable, and may involve processes such as drug absorption, distribution, metabolism and excretion. Of these processes, effects on metabolism are the most common factors that contribute to drug interactions. The most efficient and well-known enzymes responsible for drug metabolism are the CYP450 enzymes.
Drug interactions between TDF and other PIs have been reported, most notably the reduction in systemic concentrations of atazanavir achieved in the presence of TDF (both with and without ritonavir) [10]. In contrast, coadministration of TDF, saquinavir hard gel and ritonavir has no significant effect on the pharmacokinetic concentrations achieved for any of the three drugs [11, 12]. Systemic exposure to fosamprenavir is not altered by coadministration of TDF, when fosamprenavir is administered in the presence of ritonavir [13]. Similarly, no change is seen in lopinavir or ritonavir concentrations when lopinavir/ritonavir and TDF are coadministered. However, a 32% increase in tenofovir plasma AUC has been observed in the presence of lopinavir and ritonavir, compared with TDF alone [8, 14]. Importantly, the interaction between tenofovir and TMC114/r is of similar magnitude to that observed with tenofovir and lopinavir/ritonavir, for which no dose adjustments are advised.
To date, the mechanisms of tenofovir–PI interaction are unknown. However, since tenofovir is not metabolized by CYP450 enzymes, interactions are unlikely to be mediated by this pathway [15].
Increases in tenofovir concentrations could theoretically be associated with increased renal side-effects, but the magnitude of this potential problem is unclear. Tenofovir is an acyclic nucleoside phosphate, excreted by glomerular filtration and active tubular secretion in a similar manner to cidofovir and adefovir. Cidofovir and adefovir are substrates of renal transporter proteins such as human renal organic anion transporter 1 (hOAT1) [16, 17]. Ritonavir is a potent inhibitor of the drug-transporting ATPases P-glycoprotein and multidrug resistance protein 2 (Mrp-2) [18]. Inhibition of Mrp-2-mediated transport could theoretically increase tubular concentrations of tenofovir by reducing its efflux from the kidneys. This hypothesis, however, remains unproven. In the dose ranging studies of TMC114/r plus OBR, TDF was the most frequently used reverse transcriptase inhibitor. TMC114/r plus OBR was generally well tolerated and renal disorders related to tenofovir were infrequent [19–21].
Cilhar and coworkers investigated tenofovir transport by hOAT1, hOAT2 and hOAT3 to assess further the involvement of tenofovir in renal drug interactions [22]. The study showed that at clinically relevant plasma concentrations of tenofovir, this substrate would occupy only a limited fraction of the renal transport capacity and so would not exert an inhibitory effect. Therefore, the authors concluded that renal drug interactions due to the inhibition of active transport are unlikely to occur. Recent data from Ray and colleagues have confirmed that, at the molecular level, there is no evidence of a pharmacokinetic renal drug–drug interaction between lopinavir or ritonavir and tenofovir [23]. This is congruent with our findings, which showed no alteration in tenofovir or TMC114 renal excretion during coadministration.
This interaction study between TMC114/r and tenofovir has been conducted at a TMC114 dose lower than the recommended dosage and with an oral solution. Exposure (AUC) to TMC114 has been found to increase less than dose proportionally. In HIV-infected patients receiving TMC114/r doses of 400/100 mg bid or 600/100 mg bid regimens, a considerable overlap in exposure to TMC114 was obtained with these two dosing regimens. The 50% increase in dose between the 400/100 mg bid and 600/100 mg bid regimens resulted in only a 29% increase in median TMC114 exposure [24]. On this basis, the results of this study conducted with the TMC114/r 300/100 mg bid regimen is considered applicable to subjects receiving the recommended 600/100 mg bid dosing regimen. Since no clinically relevant interaction was observed, and no dose adjustment is recommended with the 300/100 mg bid regimens, it is reasonable to extrapolate this finding to the 600/100 mg bid regimen on account of the overlap in TMC114 exposures seen with these dosing regimens. In addition, in the TMC114/r clinical trials in HIV-infected patients, analysis of covariance was used to investigate the impact of the covariate of concomitant use of TDF on the pharmacokinetics of TMC114. This analysis showed that the concomitant use of TDF did not significantly influence the exposure to TMC114. The geometric mean TMC114 AUC24 h was 114 898 and 121 890 ng h−1 ml−1 for subjects without (n = 21) and with (n = 98) concomitant use of TDF, respectively, in the combined analysis of the dose ranging studies when using the recommended dose of TMC114/r 600/100 mg bid. This further supports the conclusion that the observed interaction between TMC114/r and tenofovir in this study is not clinically relevant and no dose adjustments are required when these drugs are coadministered.
Acknowledgments
Editorial support was provided by Joanne Williams of Gardiner-Caldwell Communications (GCC) Ltd (Macclesfield, UK). Editorial assistance was provided by Marta Boffito of Chelsea and Westminster Hospital (London, UK). Tibotec Pharmaceuticals Ltd, Dublin, Ireland, provided funding for this support and for the conduct of this clinical trial. Some of the data shown in this study have previously been presented as a poster at the 15th World AIDS Conference, 11–16 July 2004, Bangkok, Thailand.
References
- 1.De Meyer S, Azijn H, Surleraux D, Jochmans D, Tahri A, Pauwels R, Wigerinck P, de Bethune M-P. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother. 2005;49:2314–21. doi: 10.1128/AAC.49.6.2314-2321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prezista™. NDA no. 021976. Available at http://www.fda.gov/cder/foi/label/2006/021976lbl.pdf Last accessed 15 December 2006.
- 3.Hoetelmans R, Van der Sandt I, De Pauw M, Struble K, Peeters M, van der Geest R. TMC114, A Next Generation HIV Protease InhibitorPharmacokinetics and Safety Following Oral Administration of Multiple Doses with and without Low Doses of Ritonavir in Healthy Volunteers [549] 2003. Presented at 10th Conference on Retroviruses and Opportunistic Infections, Boston.
- 4.Katlama C, Carvalho MTM, Cooper D, De Backer K, Lefebvre E, Pedro R, Rombouts K, Stoehr A, Vangeneugden T, Woehrmann A. TMC114/r Outperforms Investigator-Selected PI (s) in 3-Class-Experienced Patients: Week 24 Primary Efficacy Analysis of POWER 1 (TMC114-C213) [WeOaLB0102] 2005. Presented at 3rd International AIDS Society Conference on HIV Pathogenesis and Treatment, Rio de Janeiro, Brazil.
- 5.Wilkin T, Haubrich R, Steinhart CR, Becker S, Conant M, Vangeneugden T, Spinosa-Guzman S, Godderis F, Stoffels P, Parys W. TMC114/r Superior to Standard of care in 3-Class-Experienced Patients: 24-wk Primary Analysis of the POWER 2 study (TMC114-C202) [2860] 2005. Presented at: 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington DC.
- 6.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JMAH, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. 903 Study Group. [DOI] [PubMed] [Google Scholar]
- 7.Squires K, Pozniak AL, Pierone G, Jr, Steinhart CR, Berger D, Bellos NC, Becker SL, Wulfsohn M, Miller MD, Toole JJ, Coakley DF, Cheng A. Tenofovir disoproxil fumarate in nucleoside-resistant HIV-1 infection: a randomized trial. Ann Intern Med. 2003;139:313–20. doi: 10.7326/0003-4819-139-5_part_1-200309020-00006. Study 907 Team. [DOI] [PubMed] [Google Scholar]
- 8.Viread™[Tenofovir Disoproxil Fumarate] Foster City, CA: Gilead Sciences; 2004. [Google Scholar]
- 9.Bouche MP, Michielsen L, Piot M, Timmerman P. Swift and Simultaneous Determination of Darunavir (TMC114) and Ritonavir in Human Plasma Using LC-MS/MS [Tup-042] 2006. Presented at: 17th International Mass Spectrometry Conference, Prague, Czech Republic.
- 10.Reyataz™[Atazanavir] Princeton, NJ: Bristol-Myers Squibb; 2004. [Google Scholar]
- 11.Boffito M, Back D, Stainsby-Tron M, Hill A, Di Perri G, Moyle G, Nelson M, Tomkins J, Gazzard B, Pozniak A. Pharmacokinetics of saquinavir hard gel/ritonavir (1000/100 mg twice daily) when administered with tenofovir diproxil fumarate in HIV-1-infected subjects. Br J Clin Pharmacol. 2005;59:38–42. doi: 10.1111/j.1365-2125.2004.02240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zong J, Chittick G, Blum MR, Hill D, Begley J, Adda N, Shah J, Kearney BP. Pharmacokinetic Assessment of Tenofovir Disoproxil Fumarate and Ritonavir-Boosted Saquinavir in Healthy Subjects [A-444] 2004. Presented at 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington DC.
- 13.Wilkins E. Pharmacokinetics of Telzir (fosamprenavir) J HIV Ther. 2004;9:87–91. [PubMed] [Google Scholar]
- 14.Kaletra®[Lopinavir/ritonavir] Chicago, IL: Abbott Laboratories; 2005. [Google Scholar]
- 15.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 16.Cundy KC. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin Pharmacokinet. 1999;36:127–43. doi: 10.2165/00003088-199936020-00004. [DOI] [PubMed] [Google Scholar]
- 17.Cihlar T, Ho ES, Lin DC, Mulato AS. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucl Acids. 2001;20:641–8. doi: 10.1081/NCN-100002341. [DOI] [PubMed] [Google Scholar]
- 18.Gutmann H, Fricker G, Drewe J, Toeroek M, Miller DS. Interactions of HIV protease inhibitors with ATP-dependent drug export proteins. Mol Pharmacol. 1999;56:383–9. doi: 10.1124/mol.56.2.383. [DOI] [PubMed] [Google Scholar]
- 19.Grinsztejn B, Arasteh K, Clotet B, Cunha CA, Goffard JC, Spinosa Guzman S, Hill A, Molina JM, Tanski C, Walgraeve H. TMC114/r Well Tolerated in 3-Class-Experienced Patients: Week 24 Primary Analysis of POWER 1 (TMC114-C213) [WePeLB6201] 2005. Presented at: 3rd International AIDS Society Conference on HIV Pathogenesis and Treatment, Rio de Janeiro, Brazil.
- 20.Berger D, Bellos N, Farthing C, Stryker R, Colson A, Pierone G, Lefebvre E, Cefalone M, Koester A, Van Baelen K, De Pauw M. TMC114/r in 3-Class-Experienced Patients 24-wk Primary Safety Analysis of the POWER 2 Study (C202) [H-1094] 2005. Presented at: 45th Interscience Conference on Antimicrobial Agents and Chemotherapy,Washington DC.
- 21.Molina J-M, Cohen C, Katlama C, Grinsztejn B, Timerman A, Pedro R, De Meyer S, de Bethune M-P, Vangeneugden T, Lefebvre E. POWER 3 Trial: 24-Week Efficacy and Safety Results of TMC114/r in Treatment-Experienced HIV Patients [P4] 2006. Presented at: 12th Annual Conference of the British HIV Association (BHIVA), Brighton, UK.
- 22.Cihlar T, Bleasby K, Pritchard J. Antiviral Acyclic Nucleotide Analogs (ANAs) Tenofovir and Adefovir are Substrates for Human Kidney Organic Anion, but not Cation Transporters: Implications for Potential Renal Drug Interactions [A-443] 2004. Presented at: 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington DC.
- 23.Ray AS, Cihlar T, Robinson KL. Mechanism of Active Tubular Secretion of Tenofovir and Potential for a Renal Drug–Drug Interaction with HIV Protease Inhibitors [A-39] 2006. Presented at: 7th International Workshop on Clinical Pharmacology of HIV Therapy, Lisbon, Portugal.
- 24.Sekar V, DeMeyer S, Vangeneugden T, Lefebvre E, De Pauw M, Van Baelen B, De Paepe E, de Bethune M-P, Hoetelmans R, Parys W. Pharmacokinetic/Pharmacodynamic (PK/PD) Analyses of TMC114 in the POWER 1 and POWER 2 Trials in Treatment-Experienced HIV-Infected Patients [J-121] 2006. Presented at: 13th Conference on Retroviruses and Opportunistic Infections, Denver.


