Abstract
Aims
To assess the potential annual savings due to generic and therapeutic substitution of statin therapy for the general Dutch population, taking the patients medical history into account.
Methods
We conducted a population-based costing study using the PHARMO Record Linkage System (RLS). PHARMO RLS contains drug dispensing records from a representative sample of pharmacies located in more than 50 regions in the Netherlands. We selected all statin users in the database since 2003. The cost-savings of generic substitution of statin therapy for all simvastatin and pravastatin users, and of therapeutic substitution of statin therapy for other statin users were calculated. Substituting current users and new users of statins were considered separately. Therapeutic substitution was based on the medical history of the individual patient. Patients were only substituted if there was an appropriate substitute available. The appropriateness of substitution was based on drug–drug interactions between statins and possible comedication and the availability of an equipotent alternative.
Results
Substituting (generic and therapeutic) statin therapy for all current users would lead to potential annual savings of approximately €87 million. Substituting (generic and therapeutic) all starters on statin therapy would lead to potential annual savings of around €51 million. In the case of generic substitution only, the potential annual savings for all current simvastatin and pravastatin users would be €2.4 million and for the new users about €1.8 million.
Conclusions
From an economic point of view, society could gain a lot from substituting statin therapy, especially from therapeutic substitution.
Keywords: generic, savings, statins
Introduction
In the Netherlands, cost control in the healthcare sector is progressively becoming a more important factor on the political agenda. A much discussed method for cost containment is substitution of brand drugs with generics offered at lower prices. The discussion of the interchangeability of drugs within the same drug class [1] makes therapeutic substitution more controversial than generic substitution. Generic statins have been available on the Dutch market since 2003 (simvastatin) and 2004 (pravastatin). In spite of a general agreement to substitute brand drugs with generic drugs the conditions that determine eligibility for substitution are heavily discussed. Physicians might prefer not to substitute statin treatment of patients who already have used more than one different statin or patients who use comedication that might interact with the generic simvastatin. Only a few studies have established the savings due to substitution of cardiovascular drugs. To our best knowledge none of these has taken the individual patients history of switching and use of possibly interacting comedication into account. A US study considered therapeutic substitution of antihypertensive medication and found that substitution could lead to annual savings of approximately $1.2 billion [2]. A British study established the potential savings of the proprietary atorvastatin with generic simvastatin at approximately £2 billion over 5 years [3]. Dutch studies determined the potential annual savings of therapeutic substitution of statins in two databases to be approximately €53 million and €52 million [4]. In the year 2005, the pharmaceutical expenditure in the Netherlands on cardiovascular drugs was approximately €936 million, of which €309 million (33%) was spent on statin treatment [5]. The main objective of this study was to determine the potential savings due to both generic and therapeutic substitution of statins for the general Dutch population taking into account the individual patient's history of switching of statins and use of interacting comedication for both current users as well as new users.
Methods
Setting
The PHARMO Record Linkage System (RLS) links hospital admission records of patients to use and costs of prescription drugs. Cost information was obtained from the Z-index, which contains information regarding all products available at Dutch community pharmacies [6].
Participants
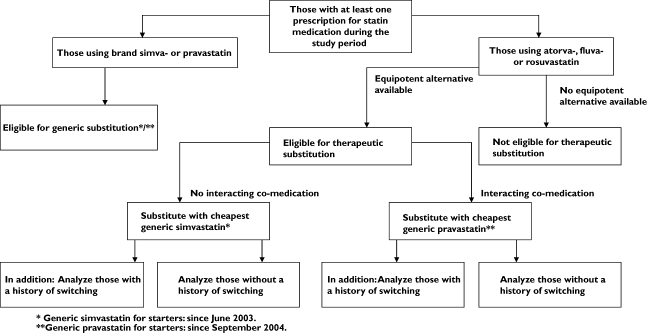
To determine which patients are eligible for substitution, all statin users between January 1, 2002 and December 31, 2005 in the database were identified. We distinguished between current (prevalent) users and new statin users (starters). Statin medication was substituted according to the scheme presented in Figure 1.
Figure 1.
Substitution scheme for statin users
Current users were defined as patients using statins on December 23, 2005 (the most recent date for which we had data). Drug costs were based on 2006 prices in Euros for the amount of statin medication dispensed during the study period. The calculated costs included national dispensing fees, €6.10 per dispensing record, and taxes (6%). To calculate annual savings due to generic substitution, subjects on brand simvastatin and pravastatin were hypothetically substituted with the cheapest generic simvastatin and generic pravastatin available. Other statin prescriptions (atorvastatin, rosuvastatin and fluvastatin) were hypothetically substituted with generic simvastatin or generic pravastatin (therapeutic substitution) based on the subject's comedication, possible history of previous statin use and equipotency of the statin with generic simvastatin and pravastatin (Figure 1). Possible interactions of statins with comedication were determined during the 3 months prior to the index date. Data on interacting comedication were derived from the literature [7]. Subjects not using interacting comedication were substituted with the cheapest simvastatin. Subjects using interacting comedication were substituted with the cheapest pravastatin, which is a more expensive but more appropriate substitute [7]. Furthermore, subjects eligible for therapeutic substitution were substituted according to a potency convertibility table [8–10] (Table 1). Subjects using statin medication for which there was no equipotent alternative available on the Dutch market were not considered eligible for substitution.
Table 1.
Potency convertibility and prices of the cheapest generics for statins per dose (in mg; adapted to availability on Dutch market) [6, 8–10].
| Potency | Rosuvastatin | Atorvastatin | Simvastatin | Pravastatin | Fluvastatin | Tc reduction | Price of cheapest generic simvastatin | Price of cheapest generic pravastatin |
|---|---|---|---|---|---|---|---|---|
| 1 (low) | 6–15% | |||||||
| 2 (low) | 5 | 10 | 20 | 15–17% | €6.40 | €10.07 | ||
| 3 (medium) | 10 | 20 | 40 | 22% | €6.40 | €16.78 | ||
| 4 (high) | 10 | 20 | 40 | 80 | 27% | €9.76 | €29.89 | |
| 5 (high) | 10 | 20 | 40 | 32% | €15.86 | |||
| 6 (high) | 20 | 40 | 80 | 37% | €31.72 | |||
| 7 (high) | 40 | 80 | 42% |
Starters were defined as subjects who had not received statins for at least 1 year before their first statin prescription. New users not starting with simvastatin or pravastatin and not using interacting comedication during the 3 months prior to their first statin prescription were substituted with simvastatin. As starters could not have been substituted before the availability of generic equivalents, starters without interacting comedication were considered eligible for substitution since June 1, 2003 (generic simvastatin available) and starters with interacting comedication since September 1, 2004 (generic pravastatin available). Subjects who had used different statins before they were identified as a starter (switchers) were analyzed separately because of the controversy of substitution in this group. Switchers identified as new users more than once, were included in the analysis once based on the first start with statin therapy. We estimated the prevalence and incidence of patients eligible for substitution projected to the general Dutch population using data from Statistics Netherlands [11]. The total annual savings were calculated by multiplying average potential savings per person according to their 10 year age and gender stratum by the total number of the individuals eligible for substitution within that stratum.
Results
We estimated that a total amount of approximately €311 million was spent in the year 2005 on statin treatment for the general Dutch population. Of the current simvastatin and pravastatin users (total n = 45.757) 5% and 8%, respectively, were eligible for generic substitution, while of the other current statin users (n = 30.903 excluding ezitimibe) more than 87% were eligible for therapeutic substitution. The percentages of patients eligible for substitution for new statin users (total n = 42.202) were comparable (Table 2). The observation that the percentage of patients eligible for therapeutic substitution was much higher than the percentage eligible for generic substitution was consistent with the relatively low potential savings following generic substitution (Table 3). The total potential annual savings due to generic substitution were approximately €2.4 million for current users and €1.8 million for starters. When therapeutic substitution was added the potential savings were €71 million for current users and €48 million for starters. When substitution was applied without consideration of a history of switching potential annual savings were €87 million for current users and €51 million for starters (Table 3).
Table 2.
Eligibility for substitution of current and new statin users
| ATC-code | Total number of current statin users | % of current statin users eligible for substitution | Total number of new statin users | % of new statin users eligible for substitution |
|---|---|---|---|---|
| Simvastatin | 33 101 | 5 | 17 693 | 1 |
| Pravastatin | 12 656 | 8 | 2 653 | 4 |
| Fluvastatin | 1 781 | 98 | 533 | 97 |
| Atorvastatin | 24 055 | 90 | 14 699 | 94 |
| Rosuvastatin | 5 067 | 87 | 6 531 | 96 |
| Ezitimibe/simvastatin | 23 | 0 | 93 | 0 |
| Total | 76 683 | 40 | 42 202 | 50 |
Table 3.
Potential savings of those eligible for substitution per substitution strategy extrapolated for the total Dutch population (n = 16 305 526)
| Substitution strategy | P* | Average savings per current statin user | Annual savings ** for the current statin users through substitution | I° | Average savings per new statin user | Annual savings ** for the new statin users through substitution |
|---|---|---|---|---|---|---|
| Eligible for generic substitution | ||||||
| Branded simvastatin | 0.26 | €121.49 | €1.9 | 0.04 | €65.91 | €0.3 |
| Branded pravastatin | 0.16 | €69.81 | €0.4 | 0.14 | €31.48 | €1.5 |
| Cumulative subtotal | €2.4 | €1.8 | ||||
| Eligible for therapeutic substitution | ||||||
| No history of switching | 18.8 | €141.26 | €68.4 | 14.2 | €182.18 | €46.1 |
| Cumulative subtotal† | €70.8 | €47.9 | ||||
| With a history of switching | 5.6 | €163.47 | €16.4 | 1.7 | €131.84 | €2.7 |
| Cumulative total‡ | €87.2 | €50.6 | ||||
P is the proportion, per 1000 persons, of the population on December 23, 2005 using either branded pravastatin or simvastatin, for generic substitution, or rosuvastatin, atorvastatin or fluvastatin for therapeutic substitution
Millions euros; I° is the proportion of the population per 1000 personyears since the availability of generic statins using either branded pravastatin or simvastatin, for generic substitution, or rosuvastatin, atorvastatin or fluvastatin for therapeutic substitution
Generic substitution and therapeutic substitution for those eligible for substitution without a history of switching of statins
Generic substitution and therapeutic substitution for those eligible for substitution.
Discussion
This study estimated the potential savings due to generic and therapeutic substitution of statins for the general Dutch population. The main findings were that therapeutic substitution could save society about €71 million annually (22.8% of the amount spent on statins in 2005) for all the current statin users and nearly €48 million annually for starters on atorvastatin, rosuvastatin and fluvastatin. For generic substitution of simvastatin and pravastatin potential savings were much lower (a total of €4.2 million annually).
The strength of this study is that it considered the patients' individual medication history to decide appropriateness of substitution and that it was population-based. A possible limitation of this study is that we did not include added costs due to therapeutic substitution of current users, e.g. laboratory costs for additional cholesterol measurements [4]. Yet, these costs are not ongoing and are relatively small compared with the costs of statins, and thus do not have a great impact on the economic benefits due to therapeutic substitution. Another possible limitation might be that the effectiveness of statin therapy might be affected by decreased adherence after switching or the inability to maintain lipid control after switching. A previous study has shown that generic substitution of antihypertensive drugs does not affect adherence or discontinuation rates in patients [12]. We have no reason to believe this would be very different for other cardiovascular drugs such as statins or for therapeutic substitution. A New Zealand study has shown deterioration of lipid control following therapeutic substitution. However, the patients were switched to insufficient doses of a less potent drug [13]. We believe our findings to be representative for the entire Dutch population. Our estimation of the €311 million spent in the year 2005 only differs by 0.6% of the €309 million as estimated by the Drug Information System of the Health Care Insurance Board [5]. Two Dutch studies estimated annual savings of €52 million and €53 million for therapeutic substitution and a third study of €100 million due to the generic substitution of simvastatin [14]. However these studies did not take the individual medication history into account. This study provides information regarding the extent to which (new) patients were already prescribed generic products which explained the very low potential savings due to generic substitution of the remaining new branded simvastatin and pravastatin users. Furthermore, this study provides policy makers with information regarding the economic consequences of generic and therapeutic substitution for the Netherlands. We believe there is enough support for implementation of both generic and therapeutic substitution. In the Netherlands drug expenditure will increase by 11% without interference from the government or other stakeholders in the field [15]. The market share of branded drugs, based on number of prescriptions, was 35% in 2005. The Dutch drug expenditure is lower than in other European countries. The NHS spends £11 billion a year on treatment of which £8 billion a year is on branded drugs and £3 billion on generics [16]. One of the five largest healthcare insurers in the Netherlands was involved in a lawsuit with four pharmaceutical companies following the introduction of a ‘rational prescribing program’ providing general practitioners (GPs) with incentives to prescribe more generic and less branded drugs. The highest civil judge in the Netherlands ruled that the ‘rational prescribing program’ was admissible [17]. The National Association for General Practitioners (LHV), the Dutch Patients Consumers Federation (NPCF) and The Dutch Society for General Practitioners (NHG) support the idea of using cheaper drugs with the same effect, generics should be prescribed whenever possible and branded drugs whenever necessary [17, 18]. The LHV supports third-party-payer incentives provided the GP invests the benefits in the provided healthcare, e.g. investing in new technology [17]. In England and Germany GPs already use convertibility programs for therapeutic substitution. The NHG is investigating the possibility of applying these programs in the Netherlands [18].
In conclusion, considerable savings can be obtained by therapeutic substitution of statins, but this is not the case for generic substitution since most patients already use generic statins when available.
References
- 1.Furberg CD, Herrington DM, Psaty BM. Are drugs within a class interchangeable? Lancet. 1999;354:1202–4. doi: 10.1016/S0140-6736(99)03190-6. [DOI] [PubMed] [Google Scholar]
- 2.Fischer MA, Avorn J. Economic implications of evidence-based prescribing for hypertension: can better care cost less? JAMA. 2004;291:1850–6. doi: 10.1001/jama.291.15.1850. [DOI] [PubMed] [Google Scholar]
- 3.Moon JC, Bogle RG. Switching statins. BMJ. 2006;332:1344–5. doi: 10.1136/bmj.332.7554.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterreus JJH. Diemen: College voor zorgverzekeringen; 2006. Lowering the reimbursement limit and therapeutic substitution (Verlaging GVS-limiet en therapeutische substitutie) [Google Scholar]
- 5.Hartvaatstelsel: GIP College voor zorgverzekeringen; 2006. Cardiovascular (Totale kosten 2001–2005 per ATC-hoofdgroep C) Total costs 2001–2005 per ATC-maingroup C. [Google Scholar]
- 6.G-standard descriptions (G-standaard beschrijvingen) Z-index. 2006 [Google Scholar]
- 7.Gier HD, editor. Commissie Medicatiebewaking, Pharmacovigilance Commentaries. [Commentaren Medicatiebewaking] Health Base: Houten; 2005. p. 956. [Google Scholar]
- 8.PHARMO-instituut. Utrecht: PHARMO; 2003. Efficiency first (Doelmatigheid Voorop) p. 23. [Google Scholar]
- 9.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–13. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 10.Illingworth DR, Tobert JA. A review of clinical trials comparingHMG-CoA reductase inhibitors. Clin Ther. 1994;16:366–85. discussion 365. [PubMed] [Google Scholar]
- 11.Statistics per theme. Statistics Netherlands; [Google Scholar]
- 12.Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Generic substitution of antihypertensive drugs: does it affect adherence? Ann Pharmacother. 2006;40:15–20. doi: 10.1345/aph.1G163. [DOI] [PubMed] [Google Scholar]
- 13.Thomas M, Mann J. Increased thrombotic vascular events after change of statin. Lancet. 1998;352:1830–1. doi: 10.1016/S0140-6736(05)79893-7. [DOI] [PubMed] [Google Scholar]
- 14.van der Linde H. MOL undermines prescription freedom: Influence of industry bound medical opinion leaders is underestimated (MOL ondergraaft prescriptievrijheid: Invloed industriegebonden medische opinieleiders wordt onderschat) Med Contact. 2006;61:1042–5. [Google Scholar]
- 15.SFK. Den Haag: Stichting Farmaceutische Kengetallen; 2007. p. 34. Data and facts 2006 (Data en Feiten 2006) [Google Scholar]
- 16.Office of Fair Trading. The pharmaceutical price regulation scheme. Office of Fair Trading; 2007. p. 114. [Google Scholar]
- 17.BOGIN. Highest civil court in the Netherlands decides bonus arrangements are admissible (Hoge raad acht bonusregelingen Menzis toelaatbaar) Bogin Bericht. 2006:4. [Google Scholar]
- 18.BOGIN. Dutch GP society: the front door is unnecessarily open for expensive drugs (NHG: voordeur onnodig wagenwijd open voor dure geneesmiddelen) Bogin Bericht. 2006:4. [Google Scholar]