Abstract
What is already known about this subject
It is known that the dopamine receptor agonist pramipexole, used for the treatment of Parkinson's disease, often causes nausea that can be treated in patients by the co-administration of an antiemetic, for example domperidone.
In experimental studies of pramipexole it may be necessary to administer domperidone alongside pramipexole to alleviate nausea, and as such it is necessary to know how the co-administration of domperidone may alter the observed effects of pramipexole.
What this study adds
Results from our study indicate that the co-administration of pramipexole and domperidone may reduce the likelihood of observing an effect that is present when pramipexole is administered alone.
Although domperidone is mainly a peripherally acting drug, it appears that a high enough concentration of the drug crosses the blood–brain barrier to partially antagonize some of the autonomic actions of pramipexole.
Therefore, this report provides a cautionary note to the use of domperidone alongside pramipexole where the results of interest are those from pramipexole alone.
Aims
To investigate the effects of the D2-receptor agonist pramipexole with and without the co-administration of the peripherally acting D2-receptor antagonist domperidone on measures of alertness, autonomic and endocrine function.
Methods
Sixteen male volunteers participated in four weekly sessions of pramipexole 0.5 mg, domperidone 40 mg, their combination, and placebo administered according to a balanced, double-blind design. Alertness (visual analogue scales (VAS), critical flicker fusion frequency, pupillographic sleepiness test), autonomic (pupil diameter, light and darkness reflexes, blood pressure, heart rate, salivation, temperature) and endocrine (prolactin, thyroid-stimulating hormone (TSH), growth hormone (GH)) functions were assessed. Data were analyzed with anova with multiple comparisons.
Results
The pre-post treatment changes in VAS alertness were reduced by pramipexole with and without domperidone (mean difference from placebo (95% confidence interval), mm): pramipexole −15.75 (−23.38, −8.13), combination −11.84 (−20.77, −2.91). Treatment condition significantly affected pupil diameter measured in different ways (resting pupil diameter (F3,45 = 8.39, P < 0.001), initial diameter of the light reflex response (F3,42 = 3.78, P < 0.05), and light (F3,45 = 5.21, P < 0.005) and dark (F3,45 = 3.36, P < 0.05) diameters of the darkness reflex response). Pramipexole without domperidone consistently increased pupil diameter on all measures (P < 0.05), whereas with domperidone only the increase in resting and dark diameters reached significance. Pramipexole reduced light reflex amplitude and increased latency, whereas the combination affected latency only. Concentrations of prolactin and TSH were increased by domperidone. Pramipexole reduced prolactin and increased GH concentrations.
Conclusions
The attenuation of the central pupillary effects of pramipexole by domperidone indicates that domperidone had access to some central D2-receptors.
Keywords: alertness, darkness reflex, domperidone, light reflex, pramipexole, pupil
Introduction
Pramipexole, a nonergot dopamine D2/D3 receptor agonist, is prescribed for the treatment of the motor deficits associated with Parkinson's disease (PD), which include muscular rigidity, bradykinesia, tremor, postural instability, and hypokinesia [1–4]. However, the tolerability of pramipexole is reduced by a side-effect profile that includes sedation [5–10], dizziness [8–12], and nausea [8–11,13, 14]. Nausea is a common side-effect of dopamine receptor agonists in general (for example, apomorphine [15], piribedil and bromocriptine [16], and ropinirole [17]), and the co-administration of an antiemetic is often necessary to alleviate this symptom.
Domperidone is a frequently utilized antiemetic that acts as an antagonist at D2-dopamine receptors [18–20] and has been widely co-administered with a variety of dopamine receptor agonists (for example, apomorphine [21], bromocriptine [22], lisuride [23], pergolide [24], piribedil [25], and pramipexole [26]). The antiemetic action of domperidone results from the blockade of dopamine receptors in the chemoreceptor trigger zone, which is believed to lie outside the blood–brain barrier [18–20]. It is believed that domperidone acts almost exclusively in the periphery due to its inability to cross the blood–brain barrier [18–20].
In two previous experiments using single oral doses of pramipexole 0.5 mg in healthy volunteers we observed changes in central nervous system activity that included reduced alertness, increased pupil diameter, and reduced amplitude of the light reflex response following pramipexole administration [9, 10]. In both of these experiments nausea was reported as a side-effect. In the future, it may be necessary to make use of an antiemetic such as domperidone to alleviate this nausea in experimental research, as already practised in drug treatment regimes for patients with PD. Before routinely coadministering domperidone with pramipexole, however, it is important to verify the exclusively peripheral action of domperidone.
Therefore, the aim of this paper was to compare the effects of pramipexole administered with and without domperidone and domperidone administered alone on measures of alertness, autonomic (pupillary activity, cardiovascular functions, core temperature, and salivary output), and endocrine (blood concentrations of prolactin, thyroid stimulating hormone, and growth hormone) functions. The peripheral activity of domperidone suggests that on measures of central nervous system activity (alertness and some autonomic functions) the effect of pramipexole administration will be the same regardless of the presence or absence of domperidone co-administration. In contrast, domperidone may be expected to alter the effects of pramipexole on measures that reflect an interaction with D2-dopamine receptors outside the blood–brain barrier (hormone concentrations).
Methods
Subjects
Sixteen healthy male volunteers aged 18–27 years (mean ± SEM 20.75 ± 0.6 years), 167–189 cm (mean ± SEM 180.3 ± 1.9 cm) in height and weighing 52.4–86.8 kg (mean ± SEM 70.0 ± 2.3 kg) participated in the study. Subjects were all medication free for at least 3 months prior to the start of the study and completed a brief medical history and physical examination before inclusion in the study. Of the 16 volunteers, 14 were nonsmokers and two were occasional smokers (less than five cigarettes a day). All volunteers were requested to avoid drinking alcohol, coffee and other caffeine-containing beverages for at least 24 h before each experimental session and to avoid taking any medication for the duration of the study. Women were excluded from the study due to the slower renal clearance of pramipexole in this population [27]. The study protocol was approved by the University of Nottingham Medical School Ethics Committee, and all volunteers gave their written consent after reading a detailed information sheet.
Drugs
Matching capsules of pramipexole 0.5 mg, domperidone 20 mg, pramipexole 0.5 mg and domperidone 20 mg, and placebo were prepared for double-blind, oral administration. The time required to attain peak plasma concentration (tmax) following pramipexole administration is approximately 2 h [27], whilst the tmax following domperidone administration is approximately 1 h [28]. Therefore, a double-dummy procedure was used to ensure the assessment of drug effects at peak plasma concentrations: pramipexole was administered 2 h and domperidone 1 h prior to testing for drug effects. An additional dosage of domperidone was administered 2 h prior to testing in order to provide protection against nausea while the blood concentration of pramipexole was still rising. Thus the total dosage of domperidone administered was 40 mg split into two. Volunteers were required to ingest two capsules; the contents of the two capsules in the four experimental sessions are summarized in Table 1. The doses were chosen on the basis of the current literature available (pramipexole: Wright et al. [27], domperidone: Huang et al. [28]). Furthermore, single doses of pramipexole 0.5 mg have been used in two recent studies in our laboratory [9, 10].
Table 1.
Summary of treatments administered in experimental sessions
| Capsule I | Capsule II | |
|---|---|---|
| (i) | Placebo | Placebo |
| (ii) | Pramipexole 0.5 mg | Placebo |
| (iii) | Pramipexole 0.5 mg and domperidone 20 mg | Domperidone 20 mg |
| (iv) | Domperidone 20 mg | Domperidone 20 mg |
Design
Subjects participated in four sessions at weekly intervals, returning to the laboratory at the same time each week. Subjects were allocated to drug conditions according to a double-blind, balanced, cross-over design. The time course of the sessions was designed with regard to the pharmacokinetic profile of the two active drugs (see above).
Tests and apparatus
All tests used were conducted as in Samuels et al. [9, 10].
Measures of alertness
A computerized battery of 16 visual analogue scales (VAS, mm [29]), the critical flicker fusion frequency test (CFFF, Hz [30]), and the pupillographic sleepiness test (PST, total power of pupil diameter fluctuations, pupillary unrest index mm min−1 [31]) were used for the assessment of alertness level.
Pupillary functions
A binocular infra-red video pupillometer (Procyon Ltd, London, UK) was used to obtain resting pupil diameter measurements (static pupillometry) in darkness and at three luminance levels (6, 91 and 360 cd m−2). A binocular infra-red television pupillometer (TVC 1015b Applied Science Laboratories, Waltham, Mass., USA) was used to record pupillary reflexes (dynamic pupillometry): light reflex responses were evoked by four light flashes of incremental luminance levels (5.2, 41, 320 and 2050 cd m−2) and darkness reflex responses by switching off an illuminated screen of 1370 cd m−2 luminance.
Non-pupillary autonomic functions
Blood pressure and heart rate, measured in both standing and supine positions, temperature, and salivation were recorded conventionally.
Endocrine functions
A 10 ml blood sample was taken and analyzed for concentrations of the hormones prolactin and TSH by enzyme immunoassay and for GH by chemiluminescence immunoassay in the Clinical Chemistry Laboratory of Queen's Medical Centre, Nottingham.
Procedure
The pre- and post-treatment testing sessions consisted of standing and supine heart rate and blood pressure, temperature, salivation, CFFF, VAS, resting pupil diameter, light and darkness reflex responses, and PST measurement. After a 15 min acclimatization period of resting in the laboratory, subjects completed the pretreatment tests over a 45 min period. Two hours after ingestion of the first capsule the post-treatment tests were conducted over 45 min. A blood sample was collected following the post-treatment testing session.
Data analysis and statistics
Self-rated values of ‘alertness’, ‘calmness’ and ‘contentedness’ were derived from the VAS scores after weighting on these factors [29]. Pre-treatment/post-treatment differences for these ratings and for the CFFF and PST (power of pupillary fluctuations, pupillary unrest index [PUI]) values were calculated for further analysis. An increase in self-rated values of ‘alertness’ on the VAS and an increase in CFFF indicate an increase in alertness, whilst increases in PST values indicate a reduction in alertness (see Samuels et al. [9, 10]).
Pre-treatment/post-treatment differences were analyzed for all the autonomic measures (pupillary and nonpupillary) with the exception of the light dependent measures of resting pupil diameter and light reflex response. Pre-treatment/post-treatment differences were not calculated for these pupillary functions since measurements were taken at different luminance levels, and calculating the difference would have eliminated the effect of luminance on the measures studied. All pupil data were averaged across the left and right eyes. For the light reflex response the parameters studied were: initial diameter (diameter of the pupil before the light stimulus was presented, mm), latency (time from the onset of the light stimulus to the onset of the pupil response, s), and amplitude (change in pupil diameter from maximum to minimum diameter in response to presentation of the light stimulus, mm). For the darkness reflex, the parameters studied were: ‘light diameter’ (diameter of the pupil whilst the screen was illuminated, mm), ‘dark diameter’ (maximum diameter of the pupil when all illumination is removed, mm), latency (time from the offset of the light to the onset of the pupil response, s), amplitude (change in pupil diameter from minimum to maximum diameter as a response to the removal of the background luminance, mm), and initial velocity (calculated on the basis of the time required to obtain 25% of the maximum response following the onset of the response, mm s−1)
Prolactin, TSH, and GH effects were analyzed using post-treatment plasma concentration values.
All data were analyzed using repeated measures anova. The data were initially checked for skew, and subjected to a transformation where indicated. Pre-treatment values were analyzed using one- or two-way anova (treatment, or luminance × treatment) to find any session effects within the results. No significant pretreatment session effects were found, and so pretreatment/post-treatment differences were taken as the dependent variable where appropriate (see above). One-way anova with drug condition (four levels) as the within-subjects factor was used to compare the effects of drug condition on all measures except pupil diameter and light reflex responses, where two-way anova with drug condition (four levels) and luminance (four levels) as the within-subjects factors was used. Where there was a significant interaction, one-way anova were conducted for each luminance level separately. Where the interaction term was not significant, one-way anova were conducted on values averaged across luminance level. All significant main effects were further analyzed using Dunnett's corrected t-test (d.f. = 45, k = 4): active treatment conditions were compared with placebo (criterion of significance P < 0.05). In addition, where the overall anova was significant, Fisher's least significant difference (LSD) test was used to compare the combination administration of pramipexole and domperidone to the individual administration of the drugs ( d.f. = 45; criterion of significance P < 0.05).
Results
Alertness
The VAS, CFFF, and PST data are shown in Table 2. A significant effect of treatment condition was observed on the VAS measure of alertness (F3,45 = 7.35, P < 0.001), where pramipexole and the combination of pramipexole and domperidone reduced alertness compared with placebo. The mean (95% confidence interval (CI)) differences from the placebo condition were: pramipexole −15.75 (−23.38, −8.13) mm, combination −11.84 (−20.77, −2.91) mm. A significant effect of treatment condition on contentedness was also found (F3,45 = 6.24, P = 0.001), where pramipexole and the combination of pramipexole and domperidone reduced contentedness compared with placebo. Fisher's LSD showed that the combination of pramipexole and domperidone was not significantly different from pramipexole administered alone on either alertness or contentedness. There was no effect of treatment condition on calmness (square root transformation; F3,45 = 2.17, NS). Pramipexole and the combination of pramipexole and domperidone also appeared to reduce measures of CFFF and increase PUI and power measures of the PST, indicating reduced alertness level (see Table 2), but these effects failed to reach significance (CFFF: F3,45 = 1.18, NS; PUI: F3,45 = 2.45, P = 0.08; power: F3,45 = 1.83, NS).
Table 2.
Pretreatment/post-treatment differences in alertness (mean (95% confidence intervals))
| Placebo | Pramipexole | Domperidone | Combination | |
|---|---|---|---|---|
| VAS | ||||
| Alertness (mm) | −1.84 (−5.86, 2.19) | −17.59 (−24.45, −10.73)* | −1.73 (−10.26, 6.80) | −13.67 (−22.08, −5.27)* |
| Calmness (mm) | −1.97 (−10.66, 6.71) | −0.16 (−5.71, 5.40) | −2.90 (−10.87, 5.08) | −2.94 (−9.97, 4.09) |
| Contentedness (mm) | 0.07 (−2.74, 2.87) | −8.04 (−12.96, −3.12)* | −1.70 (−7.41, 4.01) | −7.71 (−13.40, −2.01)* |
| CFFF (Hz) | −0.39 (−1.11, 0.33) | −0.84 (−1.61, −0.08) | −0.25 (−1.13, 0.62) | −1.08 (−2.02, −0.15) |
| PST | ||||
| PUI (mm min−2) | 1.61 (−0.47, 3.69) | 3.05 (0.49, 5.62) | 1.17 (−0.21, 2.54) | 4.25 (1.08, 7.41) |
| Power (arbitrary units) | 663 (173, 1154) | 947 (256, 1637) | 499 (92, 905) | 1284 (383, 2186) |
| Pupil diameter (mm) | −0.20 (−0.48, 0.08) | −0.25 (−0.72, 0.22) | −0.33 (−0.61, −0.06) | −0.50 (−0.91, −0.08) |
significant effect compared with placebo (P < 0.05).
Pupillary functions
Resting pupil diameter at different luminance levels is shown in Figure 1. Pre-treatment data show a significanteffect of luminance (F3,45 = 446.0, P < 0.001), but no effect of session (F3,45 = 0.19, NS) and no significant interaction (F9,135 = 1.24, NS). Post-treatment data (log10 transformation) show a significant effect of treatment condition (F3,45 = 8.39, P < 0.001) and luminance level (F3,45 = 325.23, P < 0.001), and a significant interaction (F9,135 = 2.34, P < 0.05). One-way anova conducted at each luminance level show significant effects of treatment condition at 6 cd m−2 (F3,45 = 7.79, P < 0.001), 91 cd m−2 (F3,45 = 5.90, P < 0.005), and 360 cd m−2 (F3,45 = 7.10, P = 0.001), but no significant effect in the dark (F3,45 = 2.19, NS). Multiple comparisons indicate that the significant effect of treatment condition is due to an increase in pupil diameter following pramipexole administration at 6 cd m−2, and both pramipexole and the combination of pramipexole and domperidone administration at 91 cd m−2, and 360 cd m−2. The mean (95% CI) differences from the placebo condition were: 6 cd m−2 pramipexole −0.62 (0.12, −1.35) mm; 91 cd m−2 pramipexole −0.56 (0.15, −1.27) mm, combination −0.43 (0.22, −1.07) mm; 360 cd m−2 pramipexole −0.32 (0.16, −0.80) mm, combination −0.23 (0.26, −0.72) mm. In addition, at 6 cd m−2, Fisher's LSD found that the combination condition significantly increased pupil diameter in comparison with domperidone administered alone, but that this pupil dilatation was significantly less than the increase in pupil diameter following pramipexole administered alone. At 91 cd m−2 and 360 cd m−2, the combination condition was not significantly different from the pramipexole condition.
Figure 1.
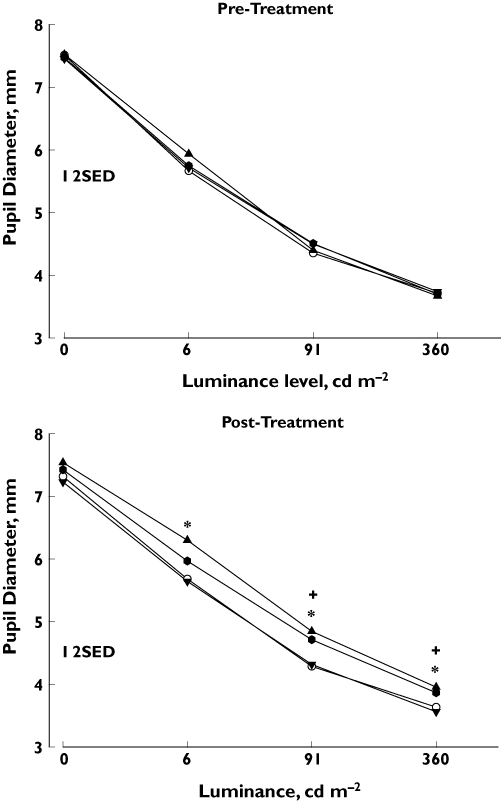
Resting pupil diameter: relationship between level of luminance and pupil diameter pretreatment (top) and post-treatment (bottom). The four treatment conditions are indicated by different symbols (see below). Ordinate: absolute pupil diameter (mm); abscissa: level of luminance (cd m−2). Each point corresponds to the mean obtained for the group (n = 16). Vertical bars represent two standard errors of the difference (2SED) obtained from the interaction term of the analysis of variance. Pramipexole (*) and the combination of pramipexole and domperidone (+) increased pupil diameter (P < 0.05; Dunnett's test: comparison with placebo condition). Placebo, (○); Pramipexole, (▴); Domperidone, (▾); Combination, ( )
)
Light reflex measures are shown in Figure 2. The data from one volunteer were excluded due to corruption of the data due to technical difficulties. For pretreatment measures of initial diameter, averaged across the eight measurements (i.e. two measurements at each luminance level), there was no effect of session (F3,42 = 0.19, NS). Post-treatment data show a significant effect of treatment condition (F3,42 = 3.78, P < 0.05), where pramipexole increased pupil diameter compared with placebo. The combination condition also increased pupil diameter, but this effect failed to reach statistical significance. For pretreatment measures of amplitude, there was a significant effect of luminance (F3,42 = 234.63, P < 0.001) but not of session (F3,42 = 2.05, NS) and there was no interaction (F9,126 = 1.00, NS). Post-treatment data (square root transformation) show a significant effect of treatment condition (F3,42 = 7.85, P < 0.001) and luminance level (F3,42 = 62.82, P < 0.001), but no interaction (F9,126 = 0.80, NS). Analysis of light reflex amplitude averaged across luminance levels revealed a significant effect of treatment condition (F3,42 = 7.85, P < 0.001), where pramipexole reduced light reflex amplitude. Fisher's LSD showed that the combination condition significantly reduced light reflex amplitude compared to domperidone alone, but was significantly less effective at reducing light reflex amplitude compared with pramipexole alone. For pretreatment measures of latency, there was a significant effect of luminance (F3,42 = 106.84, P < 0.001) but not of session (F3,42 = 0.39, NS) and there was no interaction (F9,126 = 0.78, NS). Post-treatment data (reciprocal transformation) showed a significant effect of treatment condition (F3,42 = 9.54, P < 0.001) and luminance level (F3,42 = 90.48, P < 0.001), but no interaction (F9,126 = 0.42, NS). Analysis of latency averaged across luminance levels revealed a significant effect of treatment condition (F3,42 = 8.05, P < 0.001), where pramipexole and the combination of pramipexole and domperidone increased the latency of the light reflex response. Fisher's LSD showed a trend for the increase in latency following pramipexole to be greater than the increase in latency following the combination condition.
Figure 2.

Pupillary light reflex responses: pretreatment (left) and post-treatment (right) measures of initial diameter in the dark (top), latency (middle), and light reflex amplitude (bottom). Initial diameter: columns correspond to mean changes in the initial diameter for the group (n = 15). Treatment condition is indicated at the bottom of the graphs: Pl = placebo, (□); Prami = pramipexole, ( ); Dom = domperidone, (
); Dom = domperidone, ( ); Combi = combination, (
); Combi = combination, ( ). Vertical bars represent standard errors of the mean (SEM). Pramipexole increased initial pupil diameter. *P < 0.05 (Dunnett's test: comparison with placebo condition). Latency: relationship between light stimulus intensity and the latency of the response. The four treatment conditions are indicated by different symbols (see below). Ordinate: time to respond to the light stimulus (s); abscissa: level of luminance (cd m−2). Each point corresponds to the mean obtained for the group (n = 15). Vertical bars represent two standard errors of the difference (2SED) obtained from the interaction term of the analysis of variance. Pramipexole and the combination of pramipexole and domperidone increased the latency of the light reflex (*P < 0.05; Dunnett's test: comparison with placebo condition). Amplitude: relationship between light stimulus intensity and the amplitude of the response. The four treatment conditions are indicated by different symbols (see below). Ordinate: maximal change in pupil diameter in response to the light stimulus (mm); abscissa: level of luminance (cd m−2). Each point corresponds to the mean obtained for the group (n = 15). Vertical bars represent two standard errors of the difference (2SED) obtained from the interaction term of the analysis of variance. Pramipexole (*) reduced light reflex amplitude (P < 0.05; Dunnett's test: comparison with placebo condition). Placebo, (○); Pramipexole, (▴); Domperidone, (▾); Combination (
). Vertical bars represent standard errors of the mean (SEM). Pramipexole increased initial pupil diameter. *P < 0.05 (Dunnett's test: comparison with placebo condition). Latency: relationship between light stimulus intensity and the latency of the response. The four treatment conditions are indicated by different symbols (see below). Ordinate: time to respond to the light stimulus (s); abscissa: level of luminance (cd m−2). Each point corresponds to the mean obtained for the group (n = 15). Vertical bars represent two standard errors of the difference (2SED) obtained from the interaction term of the analysis of variance. Pramipexole and the combination of pramipexole and domperidone increased the latency of the light reflex (*P < 0.05; Dunnett's test: comparison with placebo condition). Amplitude: relationship between light stimulus intensity and the amplitude of the response. The four treatment conditions are indicated by different symbols (see below). Ordinate: maximal change in pupil diameter in response to the light stimulus (mm); abscissa: level of luminance (cd m−2). Each point corresponds to the mean obtained for the group (n = 15). Vertical bars represent two standard errors of the difference (2SED) obtained from the interaction term of the analysis of variance. Pramipexole (*) reduced light reflex amplitude (P < 0.05; Dunnett's test: comparison with placebo condition). Placebo, (○); Pramipexole, (▴); Domperidone, (▾); Combination ( )
)
Darkness reflex measures are shown in Figure 3. A significant effect of treatment condition is shown on ‘light diameter’ (F3,45 = 5.21, P < 0.005) and ‘dark diameter’ (square root transformation; F3,45 = 3.36, P < 0.05). Multiple comparisons show that pramipexole increased ‘light diameter’ and tended to increase ‘dark diameter’, whilst the combination of pramipexole and domperidone increased only ‘dark diameter’. Fisher's LSD showed a trend for the pramipexole-induced increase in ‘light diameter’ to be greater than the increase following the combination condition. There were no significant effects of treatment condition on latency (log10 transformation; F3,45 = 0.32, NS), amplitude (square root transformation; F3,45 = 1.65, NS), or initial velocity (log10 transformation; F3,45 = 0.44, NS).
Figure 3.

Pupillary darkness reflex responses: pre/post treatment differences in measures of light diameter (top), dark diameter (middle), and amplitude (bottom). Columns correspond to mean changes for the group (n = 16). Treatment condition is indicated at the bottom of the graph: Pl = placebo, (□); Prami = pramipexole, ( ); Dom = domperidone, (
); Dom = domperidone, ( ); Combi = combination, (
); Combi = combination, ( ). Vertical bars represent standard errors of the mean (SEM). Ordinate (light diameter): pre/post treatment change in the diameter of the pupil under illumination (mm); ordinate (dark diameter): pre/post treatment change in the diameter of the pupil in the dark (mm); ordinate (amplitude): pre/post treatment change in the maximal difference in pupil diameter in response to removal of the light stimulus (mm). Pramipexole increased light diameter and the combination of pramipexole and domperidone increased dark diameter (*P < 0.05; Dunnett's test: comparison with placebo condition)
). Vertical bars represent standard errors of the mean (SEM). Ordinate (light diameter): pre/post treatment change in the diameter of the pupil under illumination (mm); ordinate (dark diameter): pre/post treatment change in the diameter of the pupil in the dark (mm); ordinate (amplitude): pre/post treatment change in the maximal difference in pupil diameter in response to removal of the light stimulus (mm). Pramipexole increased light diameter and the combination of pramipexole and domperidone increased dark diameter (*P < 0.05; Dunnett's test: comparison with placebo condition)
Non-pupillary autonomic functions
The data for nonpupillary autonomic functions are shown in Table 3. There was a significant effect of treatment condition on systolic blood pressure in the supine position (F3,45 = 2.85, P < 0.05), where domperidone tended to reduce blood pressure, and in the standing position (F3,45 = 4.19, P < 0.05), where pramipexole tended to reduce blood pressure. There was also a significant effect of treatment condition on orthostatic change (F3,45 = 3.05, P < 0.05), where domperidone increased the change in systolic blood pressure between the supine and standing positions. Fisher's LSD showed a significant difference between the effects of the combination treatment and pramipexole on systolic blood pressure in the supine and standing positions. There was a significant effect of treatment condition on diastolic blood pressure in the standing position (F3,45 = 4.74, P < 0.01), where pramipexole tended to reduce blood pressure, but there was no effect of treatment condition in the supine position (F3,45 = 1.48, NS) and no effect of treatment condition on orthostatic change (F3,45 = 2.33, NS). Fisher's LSD showed a significant difference between the effects of the combination treatment and pramipexole alone on diastolic blood pressure. Heart rate in the standing position showed an effect of treatment condition (F3,45 = 3.16, P < 0.05), where multiple comparisons showed a trend for domperidone to reduce heart rate. There was no effect of treatment condition on heart rate in the supine position (F3,45 = 0.95, NS), but there was a significant effect of treatment condition on orthostatic change in heart rate (F3,45 = 3.24, P < 0.05), where domperidone reduced the change in heart rate between the lying and standing positions. There was no effect of treatment condition on salivation (F3,45 = 1.29, NS) or temperature (F3,45 = 1.35, NS).
Table 3.
Pre-treatment/post-treatment differences in autonomic functions (mean (95% confidence interval))
| Placebo | Pramipexole | Domperidone | Combination | ||
|---|---|---|---|---|---|
| Heart rate (beats min−1) | Supine | −12.00 (−16.22, −7.78) | −12.31 (−17.14, −7.48) | −10.69 (−15.33, −6.05) | −8.88 (−13.66, −4.09) |
| Standing | −8.81 (−16.27, −1.35) | −7.19 (−13.31, −1.06) | −16.69 (−22.47, −10.91)T | −5.88 (−14.57, 2.82) | |
| Orthostatic change | 3.19 (-3.74, 10.11) | 5.13 (0.77, 9.48) | −6 (−11.09, −0.91)* | 3 (−3.55, 9.55) | |
| Systolic BP (mm Hg) | Supine | −1.88 (−8.94, 5.19) | −7.88 (−13.38, −2.37) | −9.88 (−15.85, −3.90)T | 0.31 (−5.80, 6.43) |
| Standing | −0.19 (−8.32, 7.94) | −9.13 (−16.93, −1.32)T | 4.25 (−1.61, 10.11) | 4.94 (−0.75, 10.63) | |
| Orthostatic change | 1.69 (−7.70, 11.08) | −1.25 (−8.54, 6.04) | 14.13 (5.11, 23.14)* | 4.63 (−1.49, 10.74) | |
| Diastolic BP (mm Hg) | Supine | 1.75 (−0.85, 4.35) | −0.88 (−4.20, 2.45) | 1.69 (−1.33, 4.70) | 2.88 (−0.06, 5.81) |
| Standing | 2.25 (−1.30, 5.80) | −2.94 (−9.01, 3.13)T | 4.25 (0.56, 7.94) | 6.94 (3.31, 10.57) | |
| Orthostatic change | 0.5 (−2.56, 3.56) | −2.06 (−7.74, 3.62) | 2.56 (−1.54, 6.67) | 4.06 (1.93, 6.20) | |
| Salivation (g) | −0.05 (−0.46, 0.37) | 0.20 (−0.23, 0.64) | −0.01 (−0.18, 0.15) | 0.34 (0.07, 0.60) | |
| Temperature (°C) | 0.04 (−0.10, 0.17) | −0.15 (−0.33, 0.03) | −0.07 (−0.25, 0.11) | 0.03 (−0.17, 0.23) |
significant effect compared with placebo (P < 0.05) T trend for significance compared with placebo.
Endocrine functions
Plasma concentrations of prolactin, TSH, and GH are shown in Figure 4. Data from three subjects were excluded from the analysis due to missing samples. A significant effect of treatment is shown on prolactin concentrations (log10 transformation; F3,36 = 209.8, P < 0.001), where domperidone and the combination of pramipexole and domperidone increased prolactin secretion and pramipexole reduced prolactin secretion. Fisher's LSD showed that the combination treatment had a significantly different effect on prolactin concentrations to pramipexole but not to domperidone. A significant effect of treatment is shown on TSH concentrations (log10 transformation; F3,36 = 11.02, P < 0.001), where domperidone and the combination of pramipexole and domperidone increased TSH secretion. Fisher's LSD showed that the combination treatment had a significantly different effect on TSH concentrations to pramipexole but not to domperidone. A significant effect of treatment was also shown on GH concentrations (square root transformation; F3,36 = 17.55, P < 0.001), where pramipexole and the combination of pramipexole and domperidone increased GH concentrations. Fisher's LSD showed that the effect of the combination treatment was not significantly different from the effect of pramipexole on GH concentration.
Figure 4.

Post-treatment plasma concentrations of prolactin (left), thyroid-stimulating hormone (TSH; centre), and growth hormone (GH; right). Columns correspond to mean plasma concentrations for the group (three samples missing: n = 13). Treatment condition is indicated at the bottom of the graphs: Pl = placebo, (□); Prami = pramipexole, ( ); Dom = domperidone, (
); Dom = domperidone, ( ); Combi = combination of pramipexole and domperidone, (
); Combi = combination of pramipexole and domperidone, ( ). The ordinate axis represents the plasma hormone concentration (mIU l−1). Vertical bars represent standard errors of the mean (SEM). Domperidone increased plasma concentrations of prolactin and TSH whilst pramipexole increased plasma concentrations of GH and reduced concentrations of prolactin. *P < 0.05 (Dunnett's test: comparison with placebo condition)
). The ordinate axis represents the plasma hormone concentration (mIU l−1). Vertical bars represent standard errors of the mean (SEM). Domperidone increased plasma concentrations of prolactin and TSH whilst pramipexole increased plasma concentrations of GH and reduced concentrations of prolactin. *P < 0.05 (Dunnett's test: comparison with placebo condition)
Discussion
In agreement with previous reports from our laboratory [9, 10], a single dose of pramipexole 0.5 mg produced sedation in a group of 16 healthy male volunteers as evidenced by a significant reduction in subjectively rated alertness on the VAS (see Table 2). Pramipexole also reduced the subjective rating of contentedness: this is consistent with reports that an increase in sedation is usually perceived as an unpleasant experience [32], and in the case of pramipexole the nausea associated with the drug is likely to have enhanced the unpleasantness of the drug experience. It has been proposed that pramipexole has a biphasic dose–response curve in relation to the modulation of alertness level [33, 34]: low doses are sedative while high doses are alerting. This effect is attributed to an action of pramipexole at presynaptic dopamine receptors at low doses and postsynaptic dopamine receptors at high doses. Inhibitory presynaptic D2 dopamine receptors have been identified on the cell bodies of neurones in the ventral tegmental area (VTA) of the midbrain [35], which sends a tonic excitatory projection to the noradrenergic locus coeruleus (LC) in the pons, a major wakefulness-promoting nucleus [36, 37]. The activation of the presynaptic dopamine receptors in the VTA by pramipexole may ‘switch off’ this mesocoerulear pathway, leading to a reduction in LC activity and consequently in alertness level [9, 10, 33, 38].
Administration of domperidone 40 mg had no effect on alertness level (see Table 2). The dosage administered was within the range commonly used (50 mg: Quinn et al. [22]; 40 mg: Lesser & Bateman [39]; 10–40 mg: Huang et al. [28]; 60 mg Jansen et al. [24]) and this lack of effect on alertness is consistent with the peripheral mode of action proposed for the drug [18–20] (see Introduction). In addition, the pramipexole-induced sedation was not antagonized by the concomitant administration of domperidone. On measures of alertness therefore the co-administration of domperidone with pramipexole did not appear to alter the sedative effects observed following the administration of pramipexole alone.
Pupil diameter was increased by pramipexole on a number of measures of pupillary function (resting pupil diameter at different levels of luminance, initial diameter of the light reflex response, ‘light diameter’ and ‘dark diameter’ of the darkness reflex response; see Figures 1, 2 and 3). Mydriasis following the administration of pramipexole has been found previously [9] and is in contrast to the usual observation of miosis following the administration of a sedative drug (see pp 786–788 in Loewenfeld [40]). We have suggested that the administration of pramipexole may attenuate the influence of a tonic excitatory dopaminergic output from the VTA to the Edinger-Westphal nucleus (EWN), a parasympathetic nucleus involved in pupil constriction, resulting in mydriasis [9, 10]. The presence of this putative meso-pupillomotor pathway is supported by evidence of a projection from the VTA to the periaqueductal grey matter (PAG) [41], and evidence of cells within the ventral PAG expressing D2 receptor mRNA [42], where the occulomotor complex (incorporating the EWN) is situated. Furthermore, alterations in the light reflex response following pramipexole administration (increased latency and reduced amplitude) reflect a parasympatholytic action of the drug that is consistent with removal of the effect of an excitatory input to the EWN.
Although domperidone on its own had no effect on any of the measures of pupillary function (resting pupil diameter, light reflex and darkness reflex responses; see Figures 1, 2 and 3), when co-administered with pramipexole it attenuated some of the pupillary effects of pramipexole. Thus the mydriatic effect of pramipexole at 6 cd m−2 and the pramipexole-induced increase in the ‘light diameter’ of the darkness reflex response were reduced by domperidone, together with a reduction in the effects of pramipexole on light reflex amplitude and latency. These observations suggest that domperidone can gain access to some central dopamine receptors, since the pupillary effects of pramipexole are likely to be centrally mediated. It should be noted, however, that a contribution to the pupillary effect of pramipexole by a peripheral mechanism cannot be entirely discounted since dopamine and/or dopaminergic mechanisms have been identified in ciliary ganglia [43] and the retina [44].
Pramipexole had relatively minor effects on measures of nonpupillary autonomic functions (see Table 3). Both systolic and diastolic BP tended to be reduced in the standing position following drug administration, consistent with reports of a reduction in BP in response to dopamine receptor agonists [45–48]. Indeed, it has been reported that dopamine receptor agonists may cause orthostatic hypotension and subjective dizziness [8–12, 14, 46]. This propensity of these drugs is consistent with a reduction in sympathetic activity which may be caused by central [47, 49, 50] and/or peripheral [51–53] mechanisms. Although the location of the central dopamine receptors mediating the sympatholytic effect of dopamine receptor agonists has not been established, it is likely that the VTA is involved since there is evidence that activation of the VTA results in an increase in BP in experimental animals [54]. Therefore, the hypotensive, sedative, and mydriatic effects of pramipexole may all be related to the ‘switching off’ of VTA activity via stimulation of inhibitory D2 autoreceptors on VTA neurones. Heart rate and core temperature were not affected by pramipexole in the present study, although small increases in heart rate [9] and decreases in core temperature [10] have been reported previously. In agreement with previous reports, pramipexole did not alter the rate of salivation [9, 10]. Domperidone had only minor effects on cardiovascular function (see Table 3), in agreement with previous reports [55, 56]. Domperidone, like pramipexole, was without any effect on core temperature and salivation.
In addition to alertness and autonomic measures, plasma concentrations of prolactin, TSH, and GH were analyzed, since the secretion of these hormones is modulated by the hypothalamic dopaminergic system. The secretion of prolactin and TSH is inhibited by the tuberoinfundibular dopaminergic neurones which exert an inhibitory influence on the secretion of these hormones via the activation of D2 dopamine receptors situated on the lactotropes and thyrotropes of the pituitary gland [57]. It is generally accepted that these postsynaptic inhibitory D2 dopamine receptors lie outside of the blood–brain barrier [57] and thus they were expected to be sensitive to both pramipexole and domperidone. Indeed, we have found that pramipexole and domperidone had opposite effects on the secretion of these hormones, pramipexole reducing and domperidone increasing prolactin concentrations and domperidone increasing TSH concentrations. The inhibitory effect of pramipexole on prolactin secretion has been reported before by ourselves [9, 10] and others [58], and although the TSH concentration was not affected by pramipexole in the present study, there are some reports describing an inhibitory effect of pramipexole [9, 58]. The opposite effects of pramipexole and domperidone are consistent with enhancement of the inhibitory effect of dopamine on prolactin and TSH secretion by pramipexole and attenuation of this inhibition by domperidone. The pattern observed by comparing a D2 dopamine receptor agonist and antagonist on the concentration of these hormones is identical to that observed by us in a previous study in which pramipexole and amisulpride were compared [10].
The secretion of GH is also modulated by the hypothalamic dopaminergic system. However, this modulation is indirect via somatostatin, which exerts an inhibitory influence on the secretion of GH. As the D2 dopamine receptors involved are not situated on the somatotropes of the pituitary gland but are on the somatostatin secreting neurones within the hypothalamus, they may lie inside rather than outside of the blood–brain barrier. Indeed, pramipexole had a profound facilitatory influence on the secretion of GH, presumably by disinhibiting the somatotropes from the influence of somatostatinergic neurones, whilst domperidone was without any effect. The predominant effect of pramipexole was also highlighted by the increased GH concentration recorded after the combination treatment. This is in contrast with the effect of the combination treatment on prolactin and TSH secretion where the effect of domperidone superseded any effect of pramipexole.
Overall, the results of this study demonstrate reliable sedative effects of pramipexole that are maintained in the presence of domperidone. However, some of the effects of pramipexole on the pupil (pupil diameter, light and darkness reflexes) and nonpupillary autonomic functions (BP, HR) were reduced or eliminated when domperidone was co-administered. This indicates that a small concentration of domperidone may enter the central nervous system when it is co-administered with a dopamine receptor agonist to prevent nausea. Our results have practical implications for experimental studies of dopamine receptor agonists when domperidone may be used to counteract nausea caused by the dopamine receptor agonist assuming that the effect of domperidone will be restricted to the periphery (for example Navan et al. [26] and Roesch-Ely et al. [59]). However, it is not possible to make a direct comparison with the clinical situation where both dopamine receptor agonists and domperidone are administered chronically using dosage regimens different from that employed in the present study.
Acknowledgments
ERS is an Institute of Neuroscience, University of Nottingham, Scholar.
References
- 1.Parkinson Study Group. Safety and efficacy of pramipexole in early Parkinson disease: a randomized dose-ranging study. JAMA. 1997;278:125–30. doi: 10.1001/jama.1997.03550020057038. [DOI] [PubMed] [Google Scholar]
- 2.Parkinson Study Group. Pramipexole vs. levodopa as initial treatment for Parkinson disease: a randomized controlled trial. JAMA. 2000;284:1931–8. doi: 10.1001/jama.284.15.1931. [DOI] [PubMed] [Google Scholar]
- 3.Piercey MF. Pharmacology of pramipexole, a dopamine D3-preferring agonist useful in treating Parkinson's disease. Clin Neuropharmacol. 1998;21:141–51. [PubMed] [Google Scholar]
- 4.Reichmann H, Brecht MH, Köster J, Kraus PH, Lemke MR. Pramipexole in routine clinical practice: a prospective observational trial in Parkinson's disease. CNS Drugs. 2003;17:965–73. doi: 10.2165/00023210-200317130-00003. [DOI] [PubMed] [Google Scholar]
- 5.Dooley M, Markham A. Pramipexole. A review of its use in the management of early and advanced Parkinson's disease. Drugs Aging. 1998;12:495–514. doi: 10.2165/00002512-199812060-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hauser RA, Gauger L, McDowell Anderson W, Zesiewicz TA. Pramipexole-induced somnolence and episodes of daytime sleep. Mov Disord. 2000;15:658–63. doi: 10.1002/1531-8257(200007)15:4<658::aid-mds1009>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.O'Suilleabhain PE, Dewey RB. Contributions of dopaminergic drugs and disease severity to daytime sleepiness in Parkinson's disease. Arch Neurol. 2002;59:986–9. doi: 10.1001/archneur.59.6.986. [DOI] [PubMed] [Google Scholar]
- 8.Etminan M, Gill S, Samii A. Comparison of the risk of adverse effects with pramipexole and ropinirole in patients with Parkinson's disease: a meta-analysis. Drug Saf. 2003;26:439–44. doi: 10.2165/00002018-200326060-00005. [DOI] [PubMed] [Google Scholar]
- 9.Samuels ER, Hou RH, Langley RW, Szabadi E, Bradshaw CM. Comparison of pramipexole and modafinil on arousal, autonomic, and endocrine functions in healthy volunteers. J Psychopharmacol. 2006;20:756–70. doi: 10.1177/0269881106060770. [DOI] [PubMed] [Google Scholar]
- 10.Samuels ER, Hou RH, Langley RW, Szabadi E, Bradshaw CM. Comparison of amisulpride and pramipexole on alertness, autonomic and endocrine functions in healthy volunteers. Psychopharmacology (Berl) 2006;187:498–510. doi: 10.1007/s00213-006-0443-y. [DOI] [PubMed] [Google Scholar]
- 11.Wermuth L The Danish Pramipexole Study Group. A double-blind, placebo-controlled, randomized, multi-center study of pramipexole in advanced Parkinson's disease. Eur J Neurol. 1998;5:235–42. doi: 10.1046/j.1468-1331.1998.530235.x. [DOI] [PubMed] [Google Scholar]
- 12.Weiner WJ, Factor SA, Jankovic J, Hauser RA, Tetrud JW, Waters CH, Shulman LM, Glassman PM, Beck B, Paume D, Doyle C. The long-term safety and efficacy of pramipexole in advanced Parkinson's disease. Parkinsonism Relat Disord. 2001;7:115–20. doi: 10.1016/s1353-8020(00)00031-6. [DOI] [PubMed] [Google Scholar]
- 13.Pogarell O, Gasser T, van Hilten JJ, Spieker S, Pollentier S, Meier D, Oertel WH. Pramipexole in patients with Parkinson's disease and marked drug resistant tremor: a randomised, double blind, placebo controlled multicentre study. J Neurol Neurosurg Psychiatry. 2002;72:713–20. doi: 10.1136/jnnp.72.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silber MH, Girish M, Izurieta R. Pramipexole in the management of restless legs syndrome: an extended study. Sleep. 2003;26:819–21. doi: 10.1093/sleep/26.7.819. [DOI] [PubMed] [Google Scholar]
- 15.Obering CD, Chen JJ, Swope DM. Update on apomorphine for the rapid treatment of hypomobility (‘off’) episodes in Parkinson's disease. Pharmacotherapy. 2006;26:840–52. doi: 10.1592/phco.26.6.840. [DOI] [PubMed] [Google Scholar]
- 16.Tan EK, Ratnagopal P, Han SY, Wong MC. Piribedil and bromocriptine in Parkinson's disease: a single-blind crossover study. Acta Neurol Scand. 2003;107:202–6. doi: 10.1034/j.1600-0404.2003.02104.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuzel MD. Ropinirole. a dopamine agonist for the treatment of Parkinson's disease. Am J Health-Syst Pharm. 1999;56:217–24. doi: 10.1093/ajhp/56.3.217. [DOI] [PubMed] [Google Scholar]
- 18.Champion MC, Hartnett M, Yen M. Domperidone, a new dopamine antagonist. CMAJ. 1986;135:457–61. [PMC free article] [PubMed] [Google Scholar]
- 19.Barone JA. Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother. 1999;33:429–40. doi: 10.1345/aph.18003. [DOI] [PubMed] [Google Scholar]
- 20.Dollery C. Therapeutic Drugs. 2. Edinburgh: Churchill Livingstone; 1999. D196-D199. [Google Scholar]
- 21.Kompoliti K, Wang QE, Goetz CG, Leurgans S, Raman R. Effects of central dopaminergic stimulation by apomorphine on speech in Parkinson's disease. Neurology. 2000;54:458–62. doi: 10.1212/wnl.54.2.458. [DOI] [PubMed] [Google Scholar]
- 22.Quinn N, Illas A, Lhermitte F, Agid Y. Bromocriptine and domperidone in the treatment of Parkinson disease. Neurology. 1981;31:662–7. doi: 10.1212/wnl.31.6.662. [DOI] [PubMed] [Google Scholar]
- 23.Capria A, Attanasio A, Quatrana M, Cannata D, Fioravanti M, Stocchi F, Ruggieri S. Cardiovascular effects of lisuride continuous intravenous infusion in fluctuating Parkinson's disease. Clin Neuropharmacol. 1989;12:331–8. doi: 10.1097/00002826-198908000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Jansen PA, Herings RM, Samson MM, De Vreede PL, Schuurmans-Daemen LM, Hovestadt A, Verhaar HJ, Van Laar T. Quick titration of pergolide in cotreatment with domperidone is safe and effective. Clin Neuropharmacol. 2001;24:177–80. doi: 10.1097/00002826-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Jenner P. Parkinson's disease. pathological mechanisms and actions of piribedil. J Neurol. 1992;239(Suppl 1):S2–S8. doi: 10.1007/BF00819559. [DOI] [PubMed] [Google Scholar]
- 26.Navan P, Findley LJ, Jeffs JAR, Pearce RKB, Bain PG. Double-blind, single-dose, cross-over study of the effects of pramipexole, pergolide, and placebo on rest tremor and UPDRS part III in Parkinson's disease. Mov Disord. 2003;18:176–80. doi: 10.1002/mds.10320. [DOI] [PubMed] [Google Scholar]
- 27.Wright CE, Lasher Sisson T, Ichhpurani AK, Peters GR. Steady-state pharmacokinetic properties of pramipexole in healthy volunteers. J Clin Pharmacol. 1997;37:520–5. doi: 10.1002/j.1552-4604.1997.tb04330.x. [DOI] [PubMed] [Google Scholar]
- 28.Huang YC, Colaizzi JL, Bierman RH, Woestenborghs R, Heykants JJ. Pharmacokinetics and dose proportionality of domperidone in healthy volunteers. J Clin Pharmacol. 1986;26:628–32. doi: 10.1002/j.1552-4604.1986.tb02962.x. [DOI] [PubMed] [Google Scholar]
- 29.Bond AJ, Lader MH. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–8. [Google Scholar]
- 30.Smith JM, Misiak H. Critical flicker frequency (CFF) and psychotropic drugs in normal human subjects- a review. Psychopharmacology (Berl) 1976;47:175–82. doi: 10.1007/BF00735818. [DOI] [PubMed] [Google Scholar]
- 31.Lüdtke H, Wilhelm B, Adler M, Schaeffel F, Wilhelm H. Mathematical procedures in data recording and processing of pupillary fatigue waves. Vision Res. 1998;38:2889–96. doi: 10.1016/s0042-6989(98)00081-9. [DOI] [PubMed] [Google Scholar]
- 32.Samuels ER, Hou RH, Langley RW, Szabadi E, Bradshaw CM. Modulation of the acoustic startle response by the level of arousal: comparison of clonidine and modafinil in healthy volunteers. Neuropsychopharmacol. 2007. Epub ahead of print. [DOI] [PubMed]
- 33.Rye DB, Jankovic J. Emerging views of dopamine in modulating sleep/wake state from an unlikely source: PD. Neurol. 2002;58:341–6. doi: 10.1212/wnl.58.3.341. [DOI] [PubMed] [Google Scholar]
- 34.Keating GL, Rye DB. Where you least expect it: dopamine in the pons and modulation of sleep and REM-sleep. Sleep. 2003;26:788–9. [PubMed] [Google Scholar]
- 35.Bagetta G, De Sarro G, Priolo E, Nistico G. Ventral tegmental area: site through which dopamine D2-recepor agonists evoke behavioural and electrocortical sleep in rats. Br J Pharmacol. 1988;95:860–6. doi: 10.1111/j.1476-5381.1988.tb11715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ornstein K, Milon H, McRae-Degueurce A, Alvarez C, Berger B, Würzner HP. Biochemical and radioautographic evidence for dopaminergic afferents of the locus coeruleus originating in the ventral tegmental area. J Neural Transm. 1987;70:183–91. doi: 10.1007/BF01253597. [DOI] [PubMed] [Google Scholar]
- 37.Maeda T, Kitahama K, Geffard M. Dopaminergic innervation of rat locus coeruleus: a light and electron microscope immunohistochemical study. Microsc Res Tech. 1994;29:211–8. doi: 10.1002/jemt.1070290306. [DOI] [PubMed] [Google Scholar]
- 38.Rye DB. Parkinson's disease and RLS: the dopaminergic bridge. Sleep Med. 2004;5:317–28. doi: 10.1016/j.sleep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 39.Lesser J, Bateman DN. Domperidone. Br Med J. 1985;290:241. doi: 10.1136/bmj.290.6463.241-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loewenfeld IE. Detroit, Michigan: Wayne State University Press; 1993. The pupil: anatomy, physiology, and clinical applications. [Google Scholar]
- 41.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 42.Mansour A, Watson SJ., Jr . Dopamine receptor expression in the central nervous system. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 207–19. [Google Scholar]
- 43.Landis SC, Jackson PC, Fredieu JR, Thibault J. Catecholaminergic properties of cholinergic neurons and synapses in adult rat ciliary ganglion. J Neurosci. 1987;7:3574–87. doi: 10.1523/JNEUROSCI.07-11-03574.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dearry A, Falardeau P, Shores C, Caron MG. D2 dopamine receptors in the human retina: cloning of cDNA and localization of mRNA. Cell Mol Neurobiol. 1991;11:437–53. doi: 10.1007/BF00734808. [DOI] [PubMed] [Google Scholar]
- 45.Luchsinger A, Velasco M, Urbina A, Morillo J, Romero E, Alvarez R, Hernandez Pieretti O. Comparative effects of dopaminergic agonists on cardiovascular, renal, and renin-angiotensin systems in hypertensive patients. J Clin Pharmacol. 1992;32:55–60. doi: 10.1002/j.1552-4604.1992.tb03788.x. [DOI] [PubMed] [Google Scholar]
- 46.Kujawa K, Leurgans S, Raman R, Blasucci L, Goetz CG. Acute orthostatic hypotension when starting dopamine agonists in Parkinson's disease. Arch Neurol. 2000;57:1461–3. doi: 10.1001/archneur.57.10.1461. [DOI] [PubMed] [Google Scholar]
- 47.Franchi F, Lazzeri C, Barletta Gianni L, Mannelli M. Centrally mediated effects of bromocriptine on cardiac sympathovagal balance. Hypertension. 2001;38:123–9. doi: 10.1161/01.hyp.38.1.123. [DOI] [PubMed] [Google Scholar]
- 48.Korchounov A, Kessler KR, Schipper HI. Differential effects of various treatment combinations on cardiovascular dysfunction in patients with Parkinson's disease. Acta Neurol Scand. 2004;109:45–51. doi: 10.1034/j.1600-0404.2003.00172.x. [DOI] [PubMed] [Google Scholar]
- 49.Jadhav AL, Willett RN, Sapru HN, Lokhandwala MF. Involvement of central dopamine receptors in the hypotensive action of pergolide. Naunyn-Schmiedeberg's Arch Pharmacol. 1983;324:281–6. doi: 10.1007/BF00502624. [DOI] [PubMed] [Google Scholar]
- 50.Ziegler MG, Kennedy B, Holland OB, Murphy D, Lake CR. The effects of dopamine agonists on human cardiovascular and sympathetic nervous systems. Int J Clin Pharmacol Ther Toxicol. 1985;23:175–9. [PubMed] [Google Scholar]
- 51.Szabo B, Crass D, Starke K. Effect of the dopamine D2 receptor agonist quinpirole on renal sympathetic nerve activity and renal norepinephrine spillover in anesthetized rabbits. J Pharmacol Exp Ther. 1992;263:806–15. [PubMed] [Google Scholar]
- 52.Toscano CF, Lahlou S. Blood pressure effects of intravenous apomorphine in conscious deoxycorticosterone-acetate salt-hypertensive rats. J Cardiovasc Pharmacol. 2003;42:772–81. doi: 10.1097/00005344-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Willems JL, Buylaert WA, Lefebvre RA, Bogaert MG. Neuronal dopamine receptors on autonomic ganglia and sympathetic nerves and dopamine receptors in the gastrointestinal system. Pharmacol Rev. 1985;37:165–216. [PubMed] [Google Scholar]
- 54.Cornish JL, van den Buuse M. Pressor responses to electrical and chemical stimulation of the rat brain A10 dopaminergic system. Neurosci Lett. 1994;176:142–6. doi: 10.1016/0304-3940(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 55.Worth DP, Harvey JN, Brown J, Worral A, Lee MR. Domperidone treatment in man inhibits the fall in plasma renin activity induced by intravenous gamma-L-glutamyl-L-dopa. Br J Clin Pharmacol. 1986;21:497–502. doi: 10.1111/j.1365-2125.1986.tb02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacDonald TM. Metoclopramide, domperidone and dopamine in man: actions and interactions. Eur J Clin Pharmacol. 1991;40:225–30. doi: 10.1007/BF00315200. [DOI] [PubMed] [Google Scholar]
- 57.Reichlin S. Neuroendocrinology. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams Textbook of Endocrinology. Philadelphia: W.B. Saunders Company; 1998. pp. 165–248. [Google Scholar]
- 58.Schilling JC, Adamus WS, Palluk R. Neuroendocrine and side effect profile of pramipexole, a new dopamine receptor agonist, in humans. Clin Pharmacol Ther. 1992;51:541–8. doi: 10.1038/clpt.1992.60. [DOI] [PubMed] [Google Scholar]
- 59.Roesch-Ely D, Scheffel H, Weiland S, Schwaninger M, Hundemer H-P, Kolter T, Weisbrod M. Differential dopaminergic modulation of executive control in healthy subjects. Psychopharmacol (Berl) 2005;178:420–30. doi: 10.1007/s00213-004-2027-z. [DOI] [PubMed] [Google Scholar]


