Abstract
An effective vaccine for genital herpes has been difficult to achieve because of the limited efficacy of subunit vaccines and the safety concerns about live viruses. As an alternative approach, mutant herpes simplex virus strains that are replication-defective can induce protective immunity. To increase the level of safety and to prove that replication was not needed for immunization, we constructed a mutant herpes simplex virus 2 strain containing two deletion mutations, each of which eliminated viral replication. The double-mutant virus induces protective immunity that can reduce acute viral shedding and latent infection in a mouse genital model, but importantly, the double-mutant virus shows a phenotypic defect in latent infection. This herpes vaccine strain, which is immunogenic but has defects in both productive and latent infection, provides a paradigm for the design of vaccines and vaccine vectors for other sexually transmitted diseases, such as AIDS.
Until recently, it was believed that, to be effective, viral vaccines must consist of a live, replication-competent virus or a large dose of inactivated virus (1). Replication of live virus was believed to be essential to provide sufficient immunogen to induce a strong immune response. However, several nonreplicating vaccines, including replication-incompetent viruses and even free DNA, have been shown to induce an immune response. For example, replication-defective mutant viruses of adenovirus (2), poxviruses (3–5), and herpes simplex virus (HSV; refs. 6–8) have been used as vaccines and vaccine vectors. An effective vaccine against genital herpes is highly desirable because of the morbidity and mortality caused by disseminated herpes infections in neonates and immunocompromised individuals (9). However, HSV subunit vaccines and live, attenuated vaccines have limitations because of low efficacy and safety concerns, respectively. Subunit vaccines have failed to provide any therapeutic protection against recurrent genital herpes in at least one clinical trial (10), presumably because of poor induction of cellular immunity needed to protect against HSV infection. In contrast, attenuated viruses have been difficult to engineer such that both safety and immunogenicity are optimized.** Immunization against HSV is also complicated by the need to protect against latent infection of neurons and by the potential of the vaccine virus to establish latent infection.
As alternative vaccine approaches, HSV replication-defective mutant strains with mutations in essential viral genes that fail to form progeny virions (6, 7) and single-cycle mutant viruses with mutations in structural protein genes that form uninfectious, progeny virions (8) have been used to immunize mice against virulent HSV challenge. We have shown that an HSV-2 mutant strain with a mutation in the UL29 gene encoding infected cell protein 8 (ICP8) could express many viral proteins without replicating its DNA in normal cells and could immunize mice and guinea pigs against genital challenge with virulent HSV-2 (11, 12). An HSV-2 strain with a mutation in the glycoprotein H gene also protected against genital challenge with HSV-2 (13). However, because all of these strains contained a single mutation, limited in vivo replication of the mutant virus was possible. We felt that it was advantageous to introduce at least two mutations into the HSV-2 genome to generate a candidate HSV-2 vaccine. Two or more mutations would lead to a safer vaccine candidate because of a reduced potential for generation of replication-competent virus due to recombination with the endogenous gene in the propagating cell line or recombination with wild HSV in the host. In addition, two mutations would eliminate the potential for viral replication in vivo; thus, if this virus were efficacious for inducing protective immunity, this result would argue that replication was truly not essential for vaccine efficacy.
MATERIALS AND METHODS
Cells and Viruses.
The wild-type (wt) HSV-2 strain 186 syn+-1 (14) was propagated and titrated on Vero Cells (American Type Culture Collection). We have isolated and characterized the dl5, dl29, and dl5-29 mutant HSV-2 strains (X.J.D. and D.M.K., unpublished work). Briefly, the dl5 mutant virus contains a deletion removing the UL5 gene and part of the nonessential UL4 ORF from nucleotides 12,244 to 15,143 (15) and was propagated on L5 cells (16), which were generously provided by Sandy Weller (University of Connecticut School of Medicine, Farmington, CT). The dl29 virus contains a deletion in the UL29 gene from nucleotides 58,784 to 62,527 (15) and was propagated on S-2 cells (17). The dl5-29 mutant virus was isolated by crossing the two single mutant viruses in V5-29 cells, which were derived from Vero cells by cotransformation of the HSV-1 ICP8 coding sequences and the HSV-2 UL5 coding sequences with a neomycin-resistance gene. The dl5-29 double-mutant virus was identified as a recombinant virus that grew only on the V5-29 cell line. The presence of deletions in the UL5 and UL29 genes was confirmed by Southern blot hybridization and DNA sequencing analysis.
The HSV-2 186ΔKpn mutant virus was isolated by introduction of a deletion of the KpnI fragment from the thymidine kinase (TK) gene and selection in the presence of acyclovir (C.A.J., T. J. Taylor, and D.M.K., unpublished work).
Mouse Studies.
All procedures were approved by the Institutional Animal Use Committee of Harvard Medical School. Viruses were inoculated intranasally as described (12). Subcutaneous immunization with viruses was performed as described (7). Intravaginal challenge with virulent HSV-2 strain G virus was performed as described (12).
Quantitative PCR.
Quantitative PCR was performed as described by Kramer and Coen (18), by using two primers from the portion of the HSV-2 TK gene not deleted in 186ΔKpn: 2TK-1 (TGG ATT ACG ATC AGT CGC C) and 2TK-2 (ACA CCA CAC GAC AAC AAT GC). Each set of samples was assayed with a series of HSV-2 DNA standards comprised of trigeminal ganglionic DNA and known amounts of HSV-2 virion DNA. The amplification products were resolved on 8% polyacrylamide gels, transferred to Genescreen Plus (New England Nuclear), and hybridized with end-labeled oligonucleotide 2TK-3 (CCA TCG CCG AGA TAC GCG AC). The bound probe was detected by PhosphorImage (Bio-Rad) analysis and quantified by using the multianalyst (Bio-Rad) software.
RESULTS
Biological Properties of an HSV-2 Double-Deletion Mutant.
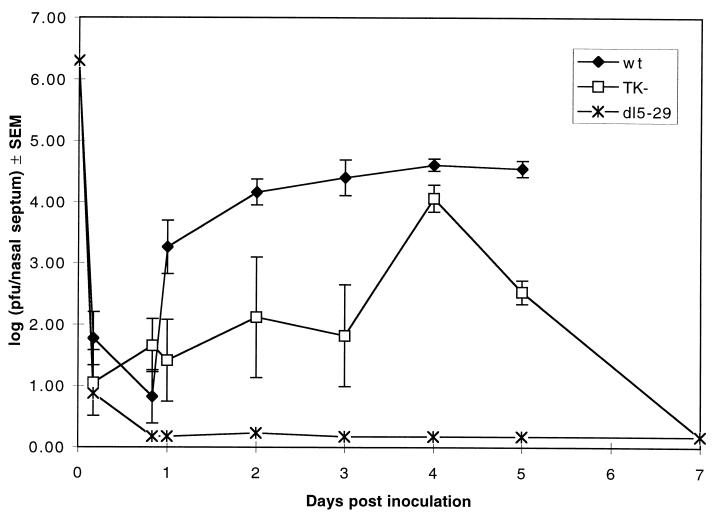
We have isolated an HSV-2 recombinant virus, dl5-29, which contains deletion mutations in both the UL29 (ICP8) gene and the UL5 gene (X.J.D. and D.M.K., unpublished work): the products of these genes are essential for HSV DNA synthesis (19, 20). This mutant virus grows only on V5-29 cells containing the ICP8 and UL5 genes and does not grow in normal Vero cells (X.J.D. and D.M.K., unpublished work). We wished to test the biological and immunization properties of dl5-29 in a murine model of genital herpes infection (12); therefore, we first examined the growth of dl5-29 in mouse cells in culture. Although wt HSV-2 showed a 62-fold increase in titer between 2 h and 18 h after infection, the UL5 single-deletion mutant dl5, the UL29 single-deletion mutant dl29, and the dl5-29 double mutant all showed decreases in viral titers during the same period (Table 1). Thus, the UL5 and UL29 mutations individually blocked viral growth in mouse cells, and dl5-29 contained a double block in viral growth. Similarly, when wt HSV-2, dl5-29, and the HSV-2 186 TK-negative (TK−) virus 186ΔKpn (C.A.J., J. T. Taylor, and D.M.K., unpublished work) were inoculated intranasally in BALB/c mice, the wt and TK− viruses showed increases in viral titer in nasal septum tissue, whereas all detectable infectivity of dl5-29 disappeared from the nasal septum tissue by 20 h after inoculation (Fig. 1). Thus, dl5-29 was defective for replication both in cultured mouse cells and in vivo.
Table 1.
Single-cycle growth of recombinant mutant viruses in mouse cells
| Virus | Yield on mouse 3T3 cells, pfu
|
|
|---|---|---|
| At 2 h | At 18 h | |
| wt | 1.1 × 104 | 6.8 × 105 |
| dl5 | 4.5 × 103 | 1.1 × 103 |
| dl29 | 5.2 × 103 | 4.2 × 102 |
| dl5-29 | 9.3 × 103 | 1.4 × 103 |
BALB/c 3T3 cells were infected with the indicated virus (multiplicity of infection = 5), and total intracellular and extracellular virus was harvested at the times indicated. Virus titers were then determined by plaque assay on V5-29 cells that complement growth of UL5 and UL29 mutant viruses. pfu, plaque-forming unit.
Figure 1.
Lack of dl5-29 mutant virus growth in mouse tissues. BALB/c mice (Taconic Farms; aged 6 weeks; n = 3 per time point per virus) were inoculated intranasally with 1 × 106 pfu of HSV-2 strain 186 wt virus (wt), HSV-2 strain 186ΔKpn TK− virus (TK−), or HSV-2 strain 186 dl5-29 virus (dl5-29). At the times shown, the nasal septa were removed and homogenized in sterile, low endotoxin PBS, and an aliquot of each homogenate was assayed for infectious virus by standard plaque assay on V5-29 cells, which complement the growth of dl5-29 mutant virus. Shown are the geometric mean values (log10) of the titers ± SEM.
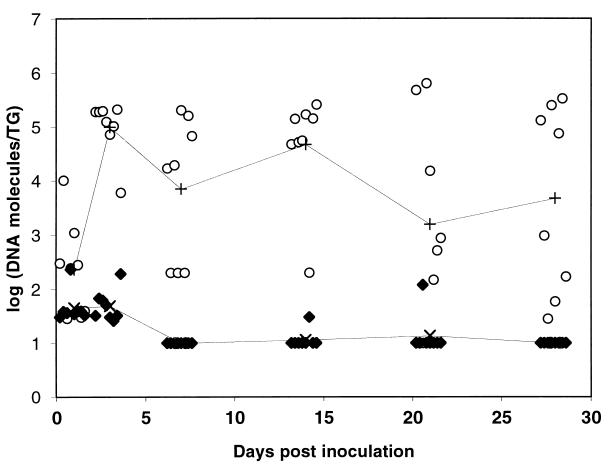
We investigated the ability of the dl5-29 and 186ΔKpn viruses to enter sensory ganglia and establish latency by quantifying viral DNA in sensory ganglia after intramuscular, intradermal, or intranasal inoculation of the viruses. At various times after inoculation, the innervating sensory ganglia were excised, and quantitative PCR was used to determine the levels of HSV-2 DNA in the tissue. The HSV-2 TK− mutant virus, 186ΔKpn, was used because it is attenuated—and thus not lethal like wt HSV-2—allowing establishment of latent infection in mice (C.A.J., J. T. Taylor, and D.M.K., unpublished work). No dl5-29 DNA was detected in the relevant ganglia after intradermal or intramuscular inoculation (not shown). A low level of dl5-29 viral DNA accumulated in the trigeminal ganglia of some mice at days 1–3 after intranasal inoculation, but after 3 days these levels decreased and became undetectable in nearly all ganglia (Fig. 2 and an additional experiment, not shown). The levels of dl5-29 DNA were significantly lower on days 7, 14, 21, and 28 as compared with the day 3 level, as determined by using the Mann–Whitney rank sum test (P < 0.01). In contrast, the 186ΔKpn viral DNA accumulated in trigeminal ganglia and persisted in that tissue (Fig. 2). The levels of 186ΔKpn DNA on days 7–28 were not statistically different from the day 3 level. Thus, dl5-29 was inefficient at reaching the ganglia, likely because of its inability to replicate, but even when it did reach the ganglia, dl5-29 was unable to persist stably in ganglionic tissue.
Figure 2.
Levels of HSV-2 DNA in trigeminal ganglia (TG) at various times after intranasal inoculation. Mice were inoculated with 1 × 106 pfu of the HSV-2 replication-defective double mutant dl5-29 (♦) or the replication-competent TK− mutant 186ΔKpn (○; n = 8 trigeminal ganglia per time point per virus) or given saline as a negative control (n = 4 trigeminal ganglia per time point; not shown). On days 1, 3, 7, 14, 21, or 28 after inoculation, the trigeminal ganglia were harvested and assayed for viral DNA by quantitative PCR. The lower limit of detection was 10 molecules per trigeminal ganglia. No viral DNA was detected in the mock-infected animals. Individual values of each trigeminal ganglia are shown along with the geometric mean for each series.
Immunization Against Genital Herpes.
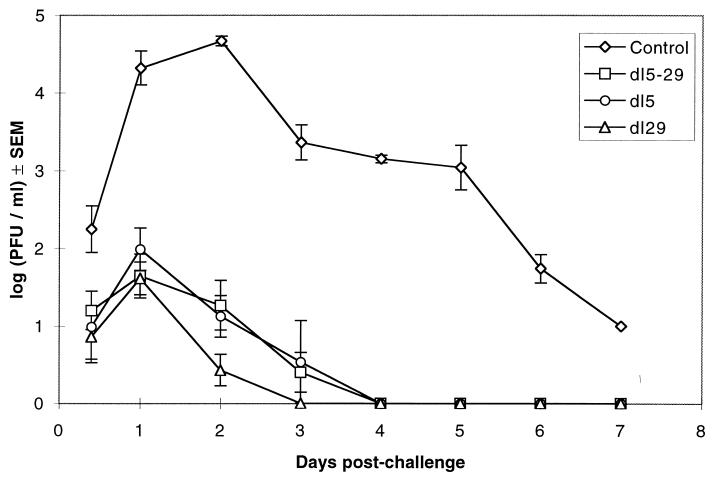
The abilities of the double dl5-29 and single dl5 and dl29 mutant viruses to induce protective immunity in a mouse genital-herpes model (12) then were compared. We chose this system, because it requires live virus for immunization (X.J.D., L. A. Morrison, and D.M.K., unpublished work) as compared with the guinea pig system in which inactivated virus can induce protective immunity (11). We immunized mice with dl5 virus, dl29 virus, dl5-29 virus, or uninfected cell-lysate as a control and then infected the mice intravaginally with virulent HSV-2 strain G virus (5 × 105 pfu, a dose 50 times the LD50). The control mice immunized with uninfected cell extract all showed genital lesions and died by 10 days after infection (six of six animals; X.J.D. and D.M.K., unpublished results). In contrast, the mice immunized with the mutant viruses were protected from lesion formation and survived the challenge infection (six of six animals for each of the three mutants; X.J.D. and D.M.K., unpublished results). Similarly, the control mice showed significant virus production in their genital tracts over 7 days after challenge, whereas the mutant-immunized mice showed greatly reduced levels of virus shedding (Fig. 3). Most importantly, the double-mutant dl5-29 virus was as effective as the single-mutant viruses at inducing immunity that reduced challenge virus replication.
Figure 3.
Reduction of the shedding of the challenge virus from the genital tract in animals immunized with recombinant mutant HSV strains. Female 6-week-old BALB/c mice were placed randomly into four groups of six mice each. All animals were injected twice, 4 weeks apart, by the subcutaneous route in the rear flank with 2 × 106 pfu of dl5 (○), dl29 (▵), dl5-29 (□), or as a control, uninfected cell lysate (⋄). The mice were challenged 4 weeks after the boost injection by intravaginal challenge with 5 × 105 pfu of HSV-2 strain G (≈50 times LD50). Virus shed in the genital tract was collected by vaginal swabs daily for 1 week after the challenge and quantified by titration in Vero cell monolayer cultures. Shown are the geometric means of the virus titers ± SEM. The lower limit of detection was 1 pfu/ml.
Prior immunization with dl5-29 also reduced latent infection after challenge infection with HSV-2. Mice were immunized with dl5-29 virus or uninfected cell lysate as a control and then challenged with HSV-2 186ΔKpn virus by intranasal infection. After 30 days, the trigeminal ganglia of the mice were removed, and latent HSV-2 DNA was quantified by PCR. Prior immunization with dl5-29 reduced the latent viral DNA content of the challenge virus by >6,000-fold (Table 2). Thus, productive infection or latent infection by the vaccine virus is not needed for effective immunization in this system.
Table 2.
Protection against latent infection by immunization with dl5-29 virus
| Immunogen | Challenge* | No. viral DNA molecules per trigeminal ganglia† |
|---|---|---|
| Cell extract | 186ΔKpn | 8.8 × 104 ± 4.9 × 104 |
| dl5-29 | 186ΔKpn | 1.4 × 101 ± 4.6‡ |
Intranasal challenge with 2 × 106 pfu.
Quantitative PCR as in ref. 13. Data are shown as means ±SEM.
P < 0.01, Mann–Whitney rank sum test.
DISCUSSION
At least two separate, nonreverting mutations are expected in microbial strains being used as live vaccines (21); thus, the dual mutations in dl5-29 provide one of the essential safety features of a live viral vaccine. These results show that it is possible to introduce two mutations into the HSV genome, each of which renders the virus defective for viral DNA synthesis and growth, without compromising the ability of the virus to induce protective immunity against genital herpes in an animal model. The double-mutant virus is stable on passage in the complementing cells, because homologous recombination between the viral genome and the viral gene in the host cell is not possible because of a lack of homologous sequences between the viral genome and the host-cell genome (X.J.D. and D.M.K., unpublished work). The deletions in the viral genes also preclude reversion of the mutations. A mutant virus with a single mutation in the UL29 gene does not cause any disease in immunodeficient mice (X.J.D., L. A. Morrison, and D.M.K., unpublished work), indicating that a double-mutant virus would be safe even in immunocompromised individuals. Thus, the dl5-29 double-mutant virus should provide a safe, stable virus that can serve as a vaccine candidate for immunization against genital herpes.
The additional biological property of a defect in latent infection was unexpected. This mutant HSV strain is the first that is nearly completely defective for stable establishment or maintenance of latent infection. In some studies, a virus deleted for the latency-associated transcript coding sequences has shown a few-fold reduction in latency (22, 23). All prior tests of replication-defective mutant viruses have shown an ability of these viruses to undergo latent infection, although these are somewhat reduced compared with the wt virus (8, 24–26). However, these tests were all with HSV-1 mutant viruses inoculated at other sites in mice. Thus, further studies are needed to compare dl5-29 and other mutant viruses. Nevertheless, all previous herpes viruses considered as vaccine candidates are capable of latent infection (8, 26–28). The dl5-29 virus is greatly decreased in its ability to reach the sensory ganglion, but the phenotype of this virus is such that, 3 days after inoculation, its viral DNA becomes undetectable (<10 DNA molecules per ganglion) in nearly all (30 of 32) ganglia. Previous studies of other replication-defective mutant viruses have detected an average of 100 DNA molecules per ganglion 30 days after infection (24, 25). Because of the unique latency phenotype of dl5-29, further studies of the genetic basis of this property are needed. Although the UL5 and UL29 genes and part of the nonessential UL4 gene are deleted, we do not know whether the latency defect is associated with any of these mutations. Restoration of each of these genes will be required to determine whether the phenotype is associated with these mutations. Nevertheless, ICP8 (the UL29 gene product) has been reported to play a role in repression of viral gene expression (29), thus possibly aiding in neuronal survival, evasion of the host immune response, and establishment of latent infection. Similarly, the UL5 gene has been associated with a neuron-specific replication function of HSV (30) that may be relevant to establishment of latent infection.
The general perception in the viral vaccine field has been that the “immunogenicity of a vector is closely tied to the extent of replication that vector undergoes in vivo” (31). Consequently, some have concluded that a live virus will be needed to induce the necessary cellular immunity to protect against sexually transmitted viruses, in particular HIV and genital herpes. Safety concerns then arise because “pathogenicity is similarly correlated with the extent of in vivo replication” (31). Our results show that a genital herpes vaccine virus strain can be defective for viral replication and for latent infection but still induce protective immunity. This finding provides a paradigm for a viral sexually transmitted disease vaccine strain, i.e., a virus that cannot replicate and cannot persist in the host but can induce protective immunity, features which should be considered in the design of other sexually transmitted disease vaccines including one for HIV. This mutant virus also has the properties desired in a safe vaccine vector for the expression of other antigens.
Acknowledgments
We thank Bryan Roberts, Dale Spriggs, Lendon Payne, and the members of our laboratory for their helpful comments on this work and John Mekalanos, John Young, and Ron Desrosiers for their helpful comments on the manuscript. These studies were supported by Public Health Service Grant AI38131 from the National Institutes of Health and by a grant from Virus Research Institute (Avant Immunotherapeutics). C.A.J. was supported by the Infectious Diseases Society of America–Connaught Laboratories Fellowship for Pediatricians in Infectious Disease.
ABBREVIATIONS
- HSV
herpes simplex virus
- wt
wild type
- pfu
plaque-forming unit
Footnotes
Cadoz, M., Micoud, M., Seigneurin, J. M., Mallaret, M. R., Baccard, C., Morand, P., Chatel, P., Meignier, B., Whitly, R. & Roizman, B. (1992) 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy, Oct. 11–14, Anaheim, CA, abstr. 341.
References
- 1.Murphy B R, Chanock R M. In: Fields Virology. 3rd Ed. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott-; 1996. pp. 467–497. [Google Scholar]
- 2.Eliot M, Gilardi-Hebenstreit P, Toma B, Perricaudet M. J Gen Virol. 1990;71:2425–2431. doi: 10.1099/0022-1317-71-10-2425. [DOI] [PubMed] [Google Scholar]
- 3.Baxby D, Paoletti E. Vaccine. 1992;10:8–9. doi: 10.1016/0264-410x(92)90411-c. [DOI] [PubMed] [Google Scholar]
- 4.Sutter G, Moss B. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutter G, Wyatt L S, Foley P L, Bennink J R, Moss B. Vaccine. 1994;12:1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen L H, Knipe D M, Finberg R W. J Virol. 1992;66:7067–7072. doi: 10.1128/jvi.66.12.7067-7072.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison L A, Knipe D M. J Virol. 1994;68:689–696. doi: 10.1128/jvi.68.2.689-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell H E, McLean C S, Harley C, Efstathiou S, Inglis S, McLean A C. J Virol. 1994;68:927–932. doi: 10.1128/jvi.68.2.927-932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitley R J. In: Fields Virology. 3rd Ed. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott; 1996. pp. 2297–2342. [Google Scholar]
- 10.Straus S E, Wald A, Kost R G, McKenzie R, Langenberg A G, Hohman P, Lekstrom J, Cox E, Nakamura M, Sekulovich R, et al. J Infect Dis. 1997;176:1129–1134. doi: 10.1086/514103. [DOI] [PubMed] [Google Scholar]
- 11.DaCosta X J, Bourne N, Stanberry L R, Knipe D M. Virology. 1997;232:1–12. doi: 10.1006/viro.1997.8564. [DOI] [PubMed] [Google Scholar]
- 12.Morrison L A, DaCosta X J, Knipe D M. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- 13.Boursnell M E G, Entwisle C, Blakeley D, Roberts C, Duncan I A, Chisholm S E, Martin G M, Jennings R, Ni Challanain D, Sobek I, et al. J Infect Dis. 1997;175:16–25. doi: 10.1093/infdis/175.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spang A E, Godowski P J, Knipe D M. J Virol. 1983;45:332–342. doi: 10.1128/jvi.45.1.332-342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolan A, Jamieson F E, Cunningham C, Barnett B C, McGeoch D J. J Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu L A, Weller S K. J Virol. 1992;66:458–468. doi: 10.1128/jvi.66.1.458-468.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao M, Knipe D M. J Virol. 1989;63:5258–5267. doi: 10.1128/jvi.63.12.5258-5267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer M F, Coen D M. J Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Challberg M D. Proc Natl Acad Sci USA. 1986;83:9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C A, Nelson N J, McGeoch D J, Challberg M D. J Virol. 1988;62:435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtiss R R, Kelly S M, Tinge S A, Tacket C O, Levine M M, Srinivasan J, Koopman M. Dev Biol Stand. 1994;82:23–33. [PubMed] [Google Scholar]
- 22.Sawtell N M, Thompson R L. J Virol. 1992;66:2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson R L, Sawtell N M. J Virol. 1997;71:5432–5440. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz J P, Bodin E T, Coen D M. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedarati F, Margolis T P, Stevens J G. Virology. 1993;192:687–691. doi: 10.1006/viro.1993.1089. [DOI] [PubMed] [Google Scholar]
- 26.Speck P G, Efstathiou S, Minson A C. J Gen Virol. 1996;77:2563–2568. doi: 10.1099/0022-1317-77-10-2563. [DOI] [PubMed] [Google Scholar]
- 27.Meignier B, Longnecker R, Roizman B. J Infect Dis. 1988;158:602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- 28.Spector F C, Kern E R, Palmer J, Kaiwar R, Cha T A, Brown P, Spaete R R. J Infect Dis. 1998;177:1143–1154. doi: 10.1086/515278. [DOI] [PubMed] [Google Scholar]
- 29.Godowski P J, Knipe D M. J Virol. 1983;47:478–486. doi: 10.1128/jvi.47.3.478-486.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloom D C, Stevens J G. J Virol. 1994;68:3761–3772. doi: 10.1128/jvi.68.6.3761-3772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letvin N L. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]