Abstract
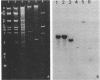
The multiple antibiotic resistance plasmid R100 renders Escherichia coli resistant to the bactericidal action of serum complement. We constructed a plasmid (pOW3) consisting of a 1,900-base-pair-long restriction fragment from R100 joined to a 2,900-base-pair-long fragment of pBR322 carrying ampicillin resistance. E. coli strains carrying pOW3 or R100 were up to 10,000-fold less sensitive to killing by serum complement than were plasmid-free bacteria or bacteria carrying pBR322. Nucleotide sequencing revealed that 875 of the 1,900 bases from R100 correspond exactly to part of the bacterial insertion sequence IS2. The remaining 1,075 bases contained only one sizeable open reading frame; it covered 729 base pairs (243 amino acids) and was preceded by nucleotide sequences characteristic of bacterial promoters and ribosome binding sites. The first 20 amino acids of the predicted protein showed features characteristic of a signal sequence. The remainder of the predicted protein showed an amino acid composition almost identical with that determined for the traT protein from the E. coli F factor. Southern blot analysis showed that the resistance gene from R100 does not hybridize to the serum resistance gene from ColV,I-K94 isolated by Binns et al.; we concluded that these genes are distinct.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Kennedy N., Skurray R. Cell--cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnello V. Complement deficiency states. Medicine (Baltimore) 1978 Jan;57(1):1–23. doi: 10.1097/00005792-197801000-00001. [DOI] [PubMed] [Google Scholar]
- Beutin L., Manning P. A., Achtman M., Willetts N. sfrA and sfrB products of Escherichia coli K-12 are transcriptional control factors. J Bacteriol. 1981 Feb;145(2):840–844. doi: 10.1128/jb.145.2.840-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns M. M., Davies D. L., Hardy K. G. Cloned fragments of the plasmid ColV,I-K94 specifying virulence and serum resistance. Nature. 1979 Jun 28;279(5716):778–781. doi: 10.1038/279778a0. [DOI] [PubMed] [Google Scholar]
- Binns M. M., Mayden J., Levine R. P. Further characterization of complement resistance conferred on Escherichia coli by the plasmid genes traT of R100 and iss of ColV,I-K94. Infect Immun. 1982 Feb;35(2):654–659. doi: 10.1128/iai.35.2.654-659.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstén B., Kaijser B. Interaction of human serum and neutrophils with Escherichia coli strains: differences between strains isolated from urine of patients with pyelonephritis or asymptomatic bacteriuria. Infect Immun. 1978 Nov;22(2):308–311. doi: 10.1128/iai.22.2.308-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Shipley P. L. Plasmid-mediated factors associated with virulence of bacteria to animals. Annu Rev Microbiol. 1980;34:465–496. doi: 10.1146/annurev.mi.34.100180.002341. [DOI] [PubMed] [Google Scholar]
- Ferrazza D., Levy S. B. Biochemical and immunological characterization of an R plasmid-encoded protein with properties resembling those of major cellular outer membrane proteins. J Bacteriol. 1980 Oct;144(1):149–158. doi: 10.1128/jb.144.1.149-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrazza D., Levy S. B. Biosynthesis of a plasmid-encoded outer membrane surface exclusion protein involves processing from a precursor polypeptide. J Biol Chem. 1980 Oct 10;255(19):8955–8958. [PubMed] [Google Scholar]
- Ghosal D., Sommer H., Saedler H. Nucleotide sequence of the transposable DNA-element IS2. Nucleic Acids Res. 1979 Mar;6(3):1111–1122. doi: 10.1093/nar/6.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F. Two periplasmic transport proteins which interact with a common membrane receptor show extensive homology: complete nucleotide sequences. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6038–6042. doi: 10.1073/pnas.78.10.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh J., Schleif R. High resolution electron microscopic studies of genetic regulation. J Mol Biol. 1976 Dec;108(2):471–490. doi: 10.1016/s0022-2836(76)80131-3. [DOI] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Lederberg J, Cavalli L L, Lederberg E M. Sex Compatibility in Escherichia Coli. Genetics. 1952 Nov;37(6):720–730. doi: 10.1093/genetics/37.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Beutin L., Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol. 1980 Apr;142(1):285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickel S., Ohtsubo E., Bauer W. Heteroduplex mapping of small plasmids derived from R-factor R12: in vivo recombination occurs at IS1 insertion sequences. Gene. 1977;2(3-4):193–210. doi: 10.1016/0378-1119(77)90017-8. [DOI] [PubMed] [Google Scholar]
- Miki T., Easton A. M., Rownd R. H. Mapping of the resistance genes of the R plasmid NR1. Mol Gen Genet. 1978 Jan 17;158(3):217–224. doi: 10.1007/BF00267192. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Ippen-Ihler K. Identification of a membrane protein associated with expression of the surface exclusion region of the F transfer operon. J Bacteriol. 1977 Mar;129(3):1613–1622. doi: 10.1128/jb.129.3.1613-1622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Levine R. P. Characterization of complement resistance in Escherichia coli conferred by the antibiotic resistance plasmid R100. J Immunol. 1980 Oct;125(4):1494–1498. [PubMed] [Google Scholar]
- Reynard A. M., Beck M. E., Cunningham R. K. Effects of antibiotic resistance plasmids on the bactericidal activity of normal rabbit serum. Infect Immun. 1978 Mar;19(3):861–866. doi: 10.1128/iai.19.3.861-866.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynard A. M., Beck M. E. Plasmid-mediated resistance to the bactericidal effects of normal rabbit serum. Infect Immun. 1976 Sep;14(3):848–850. doi: 10.1128/iai.14.3.848-850.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roantree R. J., Rantz L. A. A STUDY OF THE RELATIONSHIP OF THE NORMAL BACTERICIDAL ACTIVITY OF HUMAN SERUM TO BACTERIAL INFECTION. J Clin Invest. 1960 Jan;39(1):72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Smith H. W. A search for transmissible pathogenic characters in invasive strains of Escherichia coli: the discovery of a plasmid-controlled toxin and a plasmid-controlled lethal character closely associated, or identical, with colicine V. J Gen Microbiol. 1974 Jul;83(0):95–111. doi: 10.1099/00221287-83-1-95. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. Further observations on the association of the colicine V plasmid of Escherichia coli with pathogenicity and with survival in the alimentary tract. J Gen Microbiol. 1976 Feb;92(2):335–350. doi: 10.1099/00221287-92-2-335. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanak N., Cramer J. H., Rownd R. H. EcoRI restriction endonuclease map of the composite R plasmid NR1. J Bacteriol. 1976 Jul;127(1):619–636. doi: 10.1128/jb.127.1.619-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Hughes C. Plasmid carriage and the serum sensitivity of enterobacteria. Infect Immun. 1978 Oct;22(1):10–17. doi: 10.1128/iai.22.1.10-17.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Cabello F., Cohen S. N. Cloning and characterization of EcoRI and HindIII restriction endonuclease-generated fragments of antibiotic resistance plasmids R6-5 and R6. Mol Gen Genet. 1978 Jun 14;162(2):121–137. doi: 10.1007/BF00267869. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. A single UGA codon functions as a natural termination signal in the coliphage q beta coat protein cistron. J Mol Biol. 1973 Nov 15;80(4):837–855. doi: 10.1016/0022-2836(73)90213-1. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. I. Location of the lethal lesion. J Immunol. 1981 Sep;127(3):1146–1151. [PubMed] [Google Scholar]