Abstract
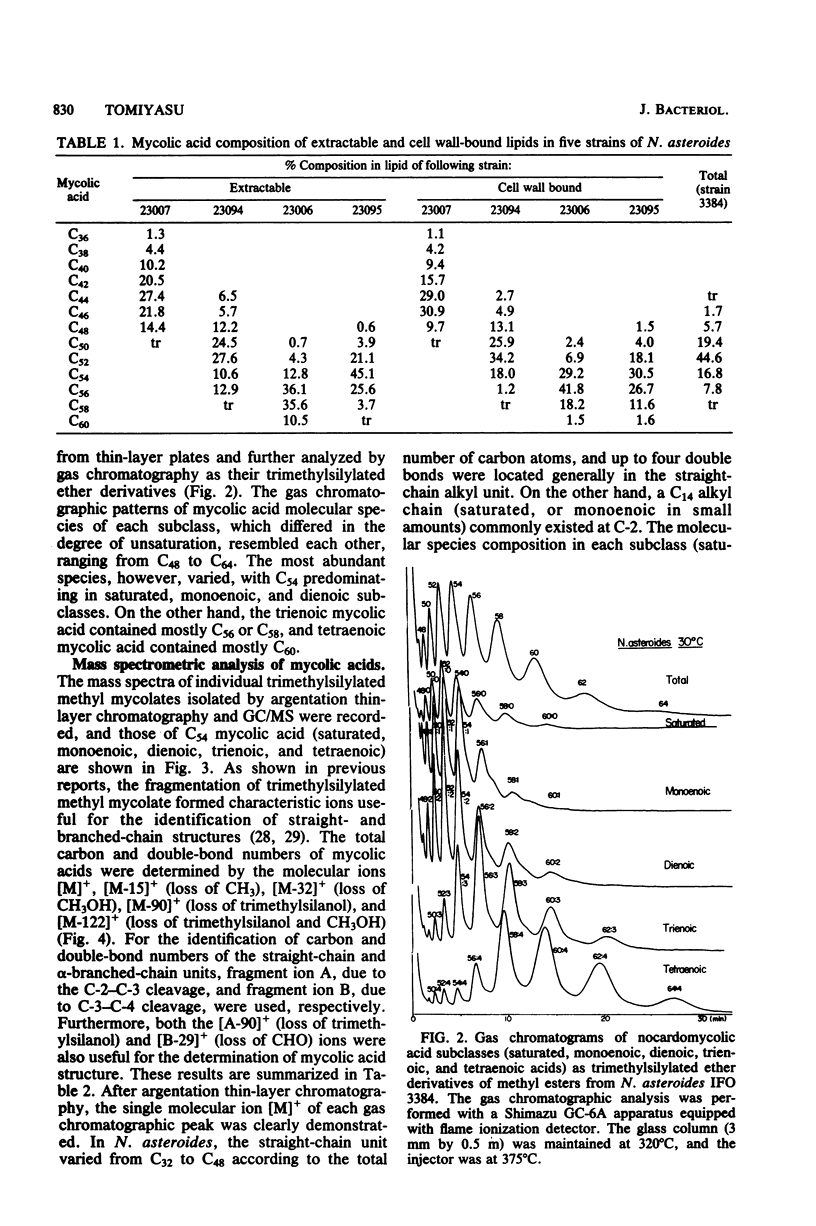
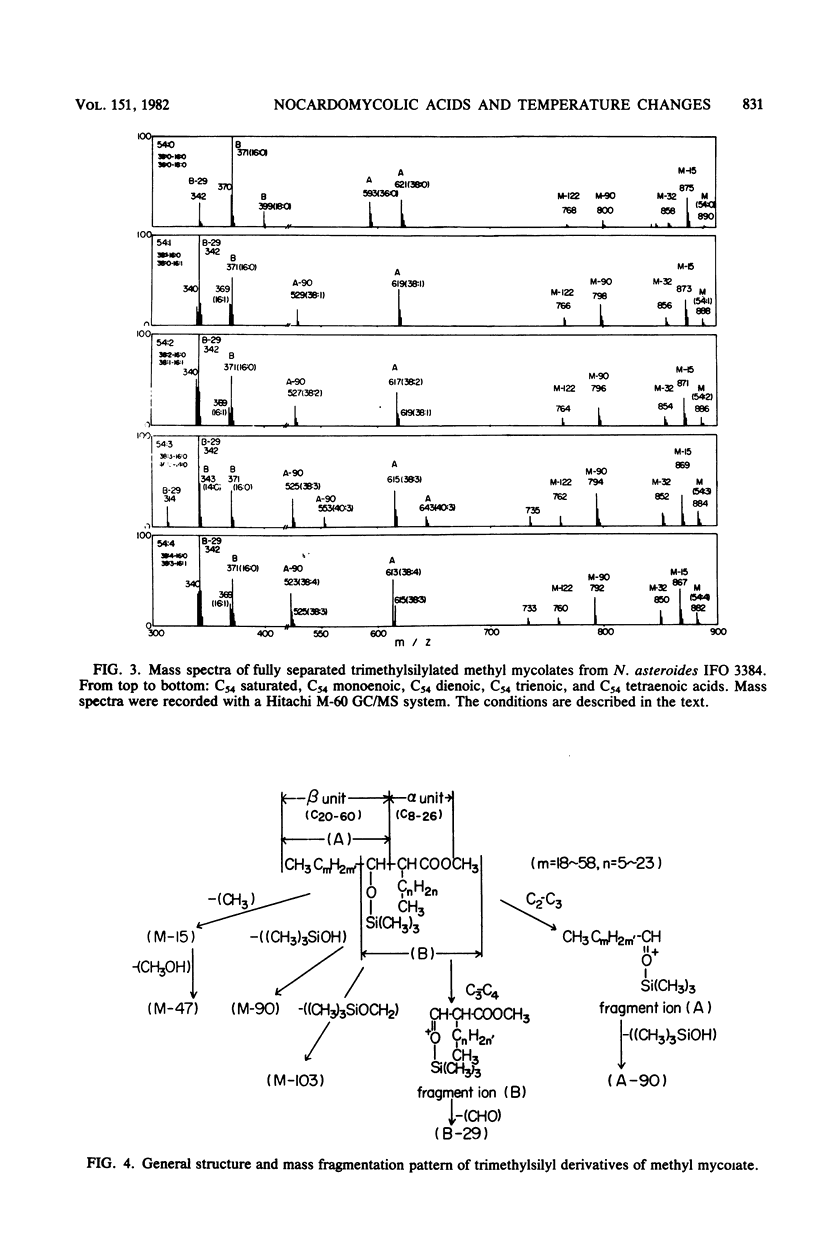
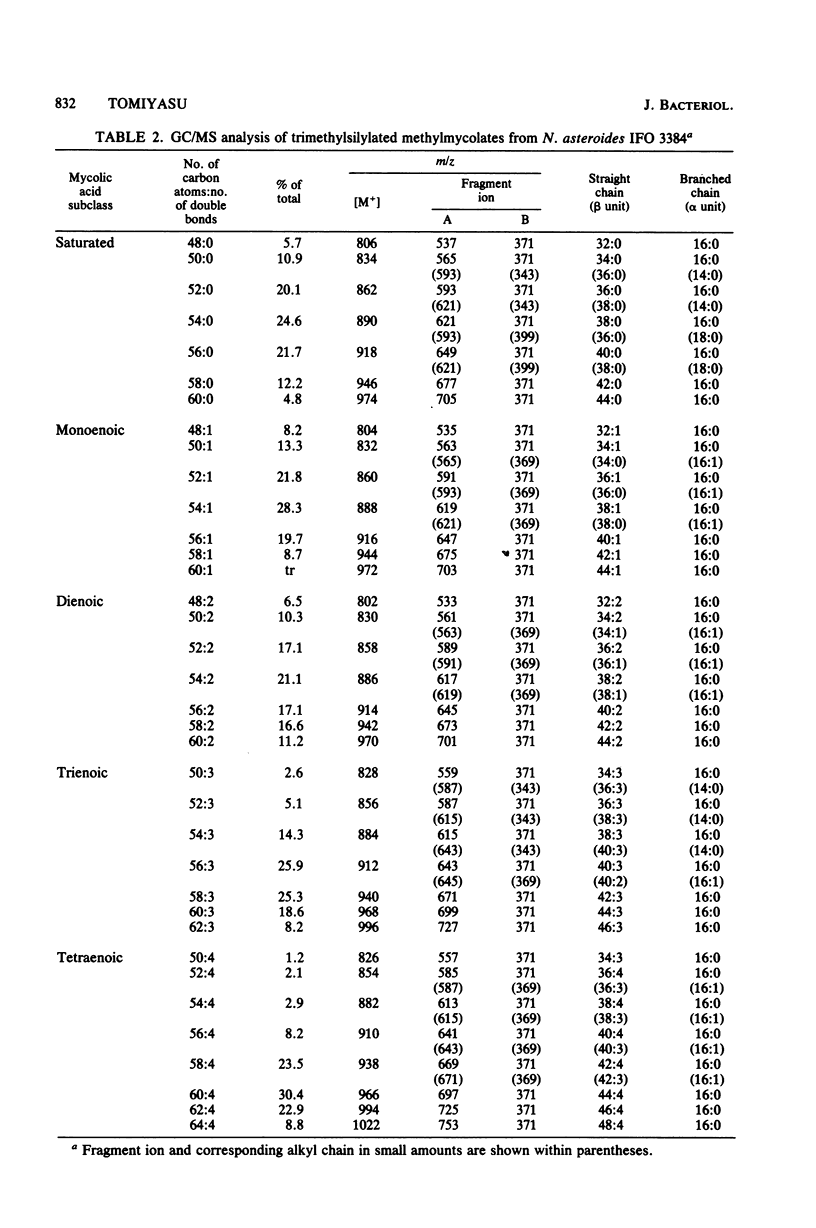
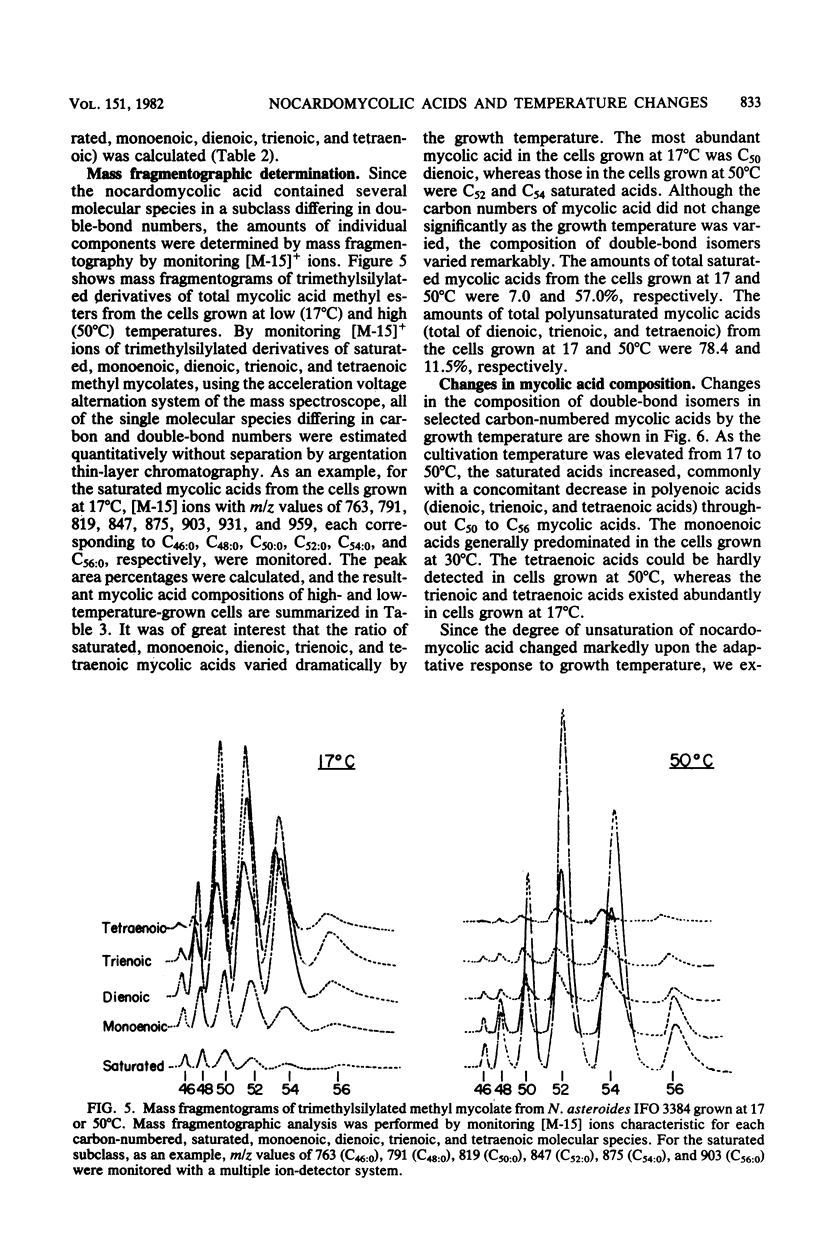
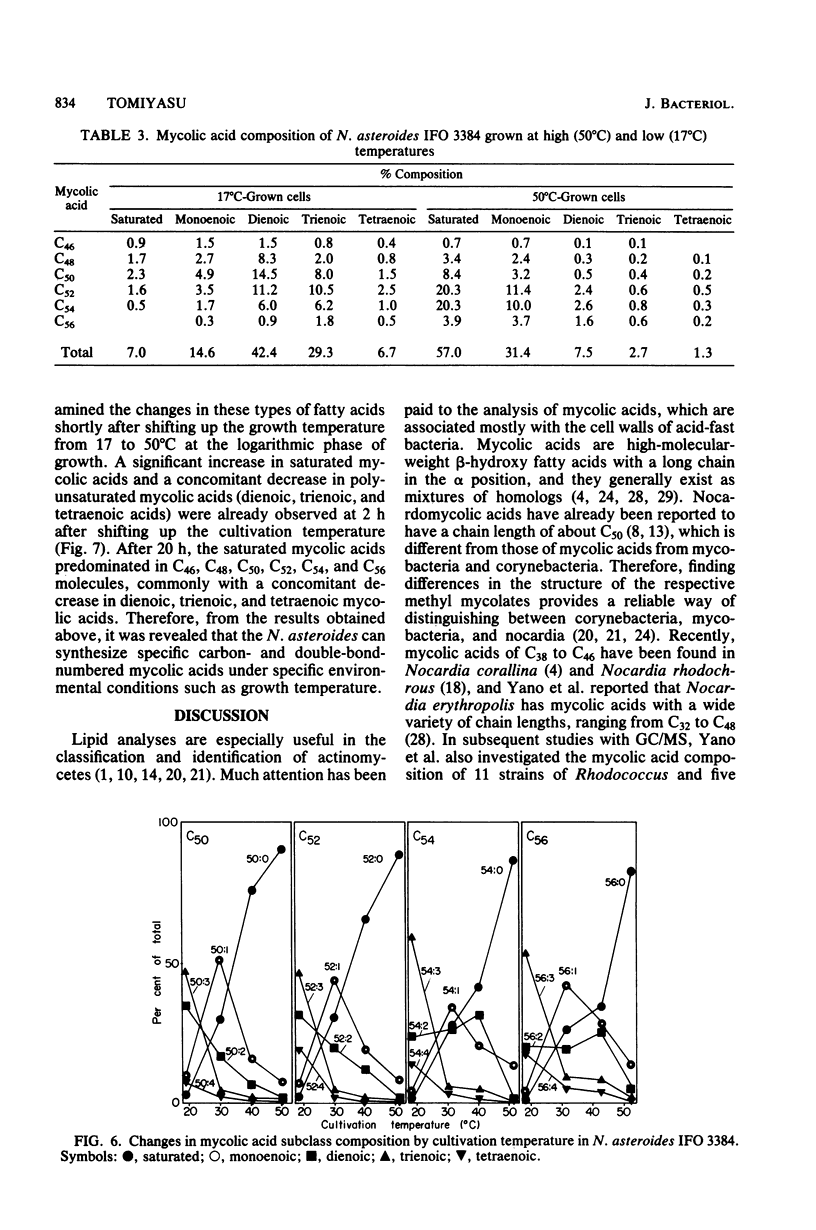
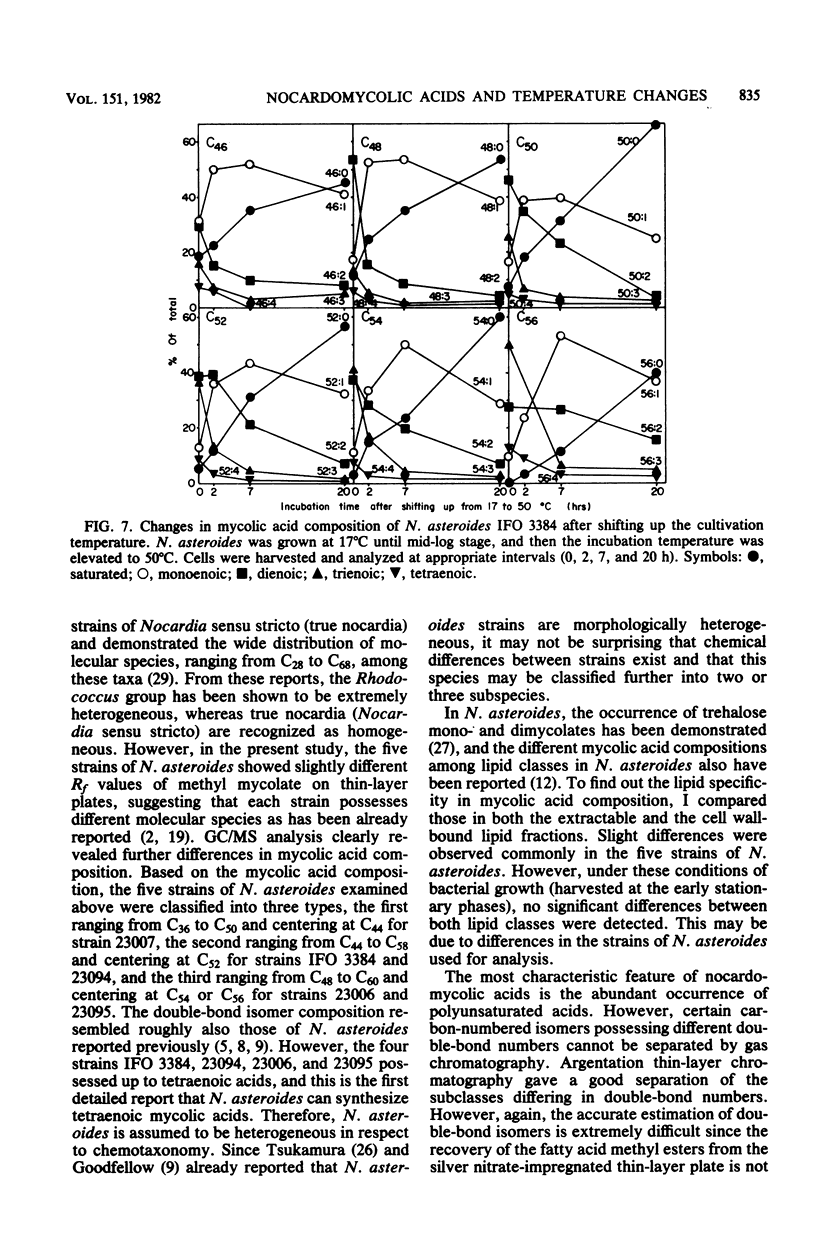
The nocardomycolic acid compositions of extractable and the cell wall-bound lipids from five strains of Nocardia asteroides (A-23007, A-23094, B-23006, B-23095, and IFO 3384) were compared by using gas chromatography-mass spectrometry. The molecular species composition of mycolic acid differed significantly among the strains of N. asteroides. The A-23007 strain possessed the shortest species, centering at C44(46), and the A-23094 and IFO-3384 strains followed, each centering at C52. The B-23006 and B-23095 strains possessed the longest species, centering at C56 or C54, thus indicating that N. asteroides strains accommodate a heterogeneous group in respect to carbon numbers of mycolic acids. The doublebond isomers of mycolic acids from the representative strain IFO 3384 were fully separated and analyzed by argentation thin-layer chromatography, followed by gas chromatography-mass spectrometry. The reference strain (IFO 3384) possessed up to four double bonds on the straight chain of mycolic acids ranging from C46 to C60. All of the species possessed a C14 alkyl branch at C-2. The more highly unsaturated subclasses consisted of the longer-chain mycolic acids. Marked changes in mycolic acid composition were induced by altering the growth temperature of strain IFO 3384. The cells grown at the higher temperature (50°C) contained more saturated mycolic acids, whereas those grown at the lower temperature (17°C) had more polyunsaturated (up to tetraenoic) mycolic acids, although a significant difference in carbon chain length was not detected. These changes in the degree of unsaturation of mycolic acids occurred shortly after shifting the growth temperature from 17 to 50°C at logarithmic stages of the bacterial growth, thus indicating that N. asteroides can adapt to changes in the environmental temperature by altering the structure of mycolic acids of the cell walls.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alashamaony L., Goodfellow M., Minnikin D. E. Free mycolic acids as criteria in the classification of Nocardia and the 'rhodochrous' complex. J Gen Microbiol. 1976 Jan;92(1):188–199. doi: 10.1099/00221287-92-1-188. [DOI] [PubMed] [Google Scholar]
- Alshamaony L., Goodfellow M., Minnikin D. E., Mordarska H. Free mycolic acids as criteria in the classification of Gordona and the 'rhodochrous' complex. J Gen Microbiol. 1976 Jan;92(1):183–187. doi: 10.1099/00221287-92-1-183. [DOI] [PubMed] [Google Scholar]
- Batt R. D., Hodges R., Robertson J. G. Gas chromatography and mass spectrometry of the trimethylsilyl ether methyl ester derivatives of long chain hydroxy acids from Nocardia corallina. Biochim Biophys Acta. 1971 Sep 1;239(3):368–373. doi: 10.1016/0005-2760(71)90028-2. [DOI] [PubMed] [Google Scholar]
- Bordet C., Michel G. Structure et biogenèse des lipides a haut poids moléculaire de Nocardia asteroides. Bull Soc Chim Biol (Paris) 1969 Jul 25;51(3):527–548. [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Etemadi A. H. Corrélations structurales et biogénétiques des acides mycoliques en rapport avec la phylogenèse de quelques genres d'Actinomycétales. Bull Soc Chim Biol (Paris) 1967;49(6):695–706. [PubMed] [Google Scholar]
- Goodfellow M., Minnikin D. E., Patel P. V., Mordarska H. Free nocardomycolic acids in the classification of nocardias and strains of the 'rhodochrous' complex. J Gen Microbiol. 1973 Jan;74(1):185–188. doi: 10.1099/00221287-74-1-185. [DOI] [PubMed] [Google Scholar]
- Goodfellow M. Numerical taxonomy of some nocardioform bacteria. J Gen Microbiol. 1971 Nov;69(1):33–80. doi: 10.1099/00221287-69-1-33. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Mycobacterial lipids: selected topics. Bacteriol Rev. 1972 Mar;36(1):33–64. doi: 10.1128/br.36.1.33-64.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANEELLE M. A., ASSELINEAU J., CASTELNUOVO G. ETUDES SUR LES MYCOBACT'ERIES ET LES NOCARDIAE. IV. COMPOSITION DES LIPIDES DE MYCOBACTERIUM RHODOCROUS, M. PELLEGRINO SP., ET DE QUELQUES SOUCHES DE NOCARDIAE. Ann Inst Pasteur (Paris) 1965 Jan;108:69–82. [PubMed] [Google Scholar]
- Lechevalier M. P., Horan A. C., Lechevalier H. Lipid composition in the classification of nocardiae and mycobacteria. J Bacteriol. 1971 Jan;105(1):313–318. doi: 10.1128/jb.105.1.313-318.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer E. Cord factor and related trehalose esters. Chem Phys Lipids. 1976 Mar;16(2):91–106. doi: 10.1016/0009-3084(76)90001-3. [DOI] [PubMed] [Google Scholar]
- Minnikin D. E., Alshamaony L., Goodfellow M. Differentiation of Mycobacterium, Nocardia, and related taxa by thin-layer chromatographic analysis of whole-organism methanolysates. J Gen Microbiol. 1975 May;88(1):200–204. doi: 10.1099/00221287-88-1-200. [DOI] [PubMed] [Google Scholar]
- Minnikin D. E., Patel P., Goodfellow M. Mycolic acids of representative strains of Nocardia and the 'rhodochrous' complex. FEBS Lett. 1974 Mar 1;39(3):322–324. doi: 10.1016/0014-5793(74)80140-7. [DOI] [PubMed] [Google Scholar]
- Toriyama S., Yano I., Masui M., Kusunose E., Kusunose M., Akimori N. Regulation of cell wall mycolic acid biosynthesis in acid-fast bacteria. I. Temperature-induced changes in mycolic acid molecular species and related compounds in Mycobacterium phlei. J Biochem. 1980 Jul;88(1):211–221. [PubMed] [Google Scholar]
- Toriyama S., Yano I., Masui M., Kusunose M., Kusunose E. Separation of C50--60 and C70--80 mycolic acid molecular species and their changes by growth temperatures in Mycobacterium phlei. FEBS Lett. 1978 Nov 1;95(1):111–115. doi: 10.1016/0014-5793(78)80063-5. [DOI] [PubMed] [Google Scholar]
- Yano I., Kageyama K., Ohno Y., Masui M., Kusunose E., Kusunose M., Akimori N. Separation and analysis of molecular species of mycolic acids in Nocardia and related taxa by gas chromatography mass spectrometry. Biomed Mass Spectrom. 1978 Jan;5(1):14–24. doi: 10.1002/bms.1200050104. [DOI] [PubMed] [Google Scholar]
- Yano I., Saito K., Furukawa Y., Kusunose M. Structural analysis of molecular species of nocardomycolic acids from Nocardia erythropolis by the combined system of gas chromatography and mass spectrometry. FEBS Lett. 1972 Mar 15;21(2):215–219. doi: 10.1016/0014-5793(72)80140-6. [DOI] [PubMed] [Google Scholar]