Abstract
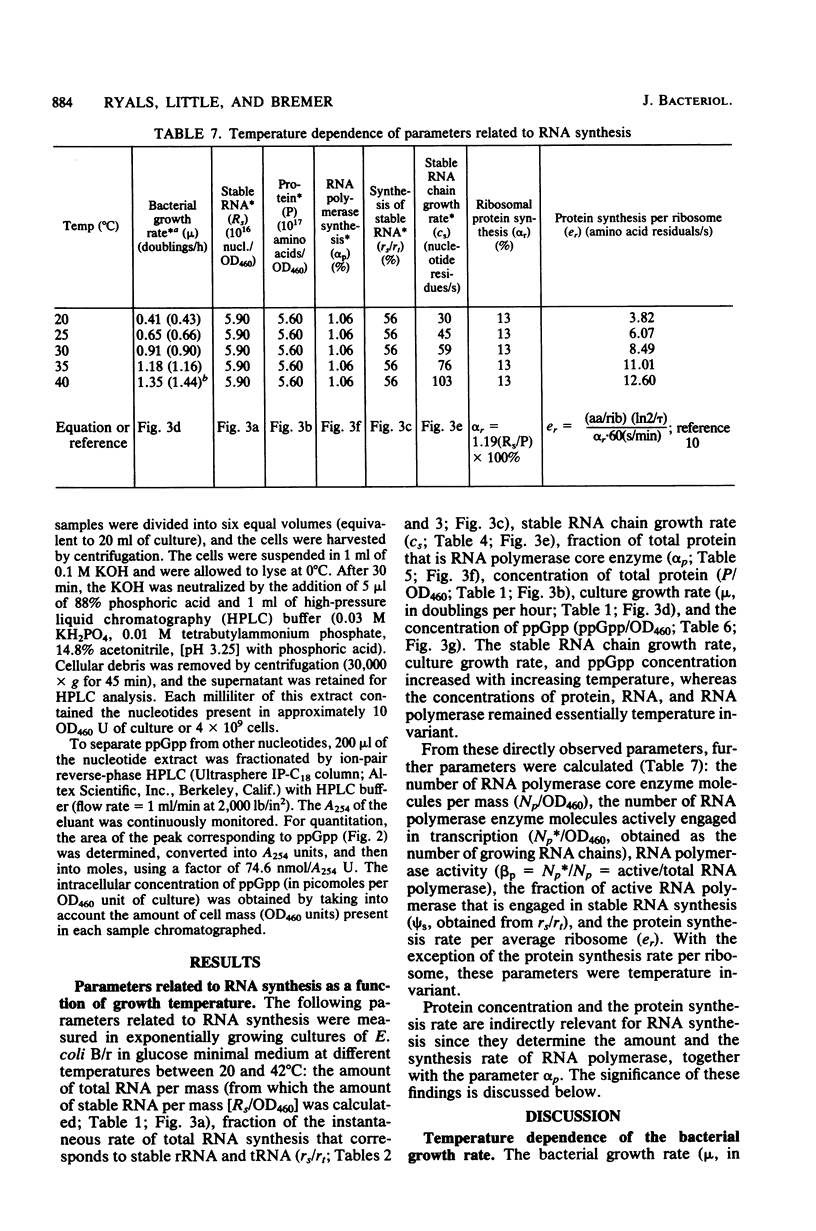
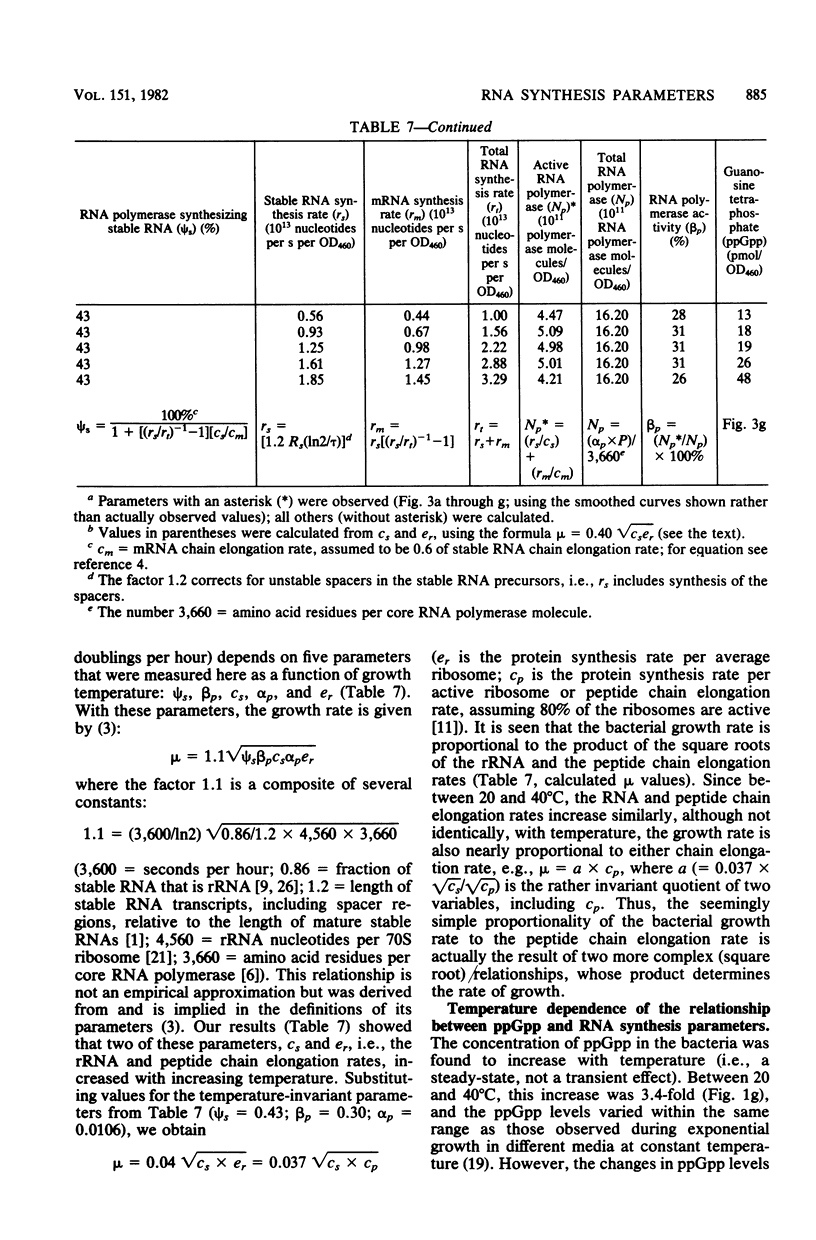
For Escherichia coli B/r growing in glucose minimal medium, the following parameters of RNA synthesis remained invariant between 20 and 40 degrees C: RNA polymerase concentration (RNA polymerase/mass), rRNA and tRNA concentration (RNA/mass), RNA polymerase activity (fraction of total RNA polymerase actively engaged in RNA chain elongation), and stable RNA synthesis relative to total RNA synthesis. The following parameters increased 3.4-fold over the same temperature range: rRNA chain elongation rate, guanosine tetraphosphate (ppGpp) concentration, and culture growth rate. Above 40 degrees C, the changes became more complex, and the growth rate began to decrease. The observation that most RNA synthesis parameters are temperature invariant despite the increase of ppGpp suggests that the mechanism of RNA synthesis control by ppGpp, assumed to involve an interaction of RNA polymerase wtih ppGpp, is itself temperature dependent such that, with increasing temperature, higher concentrations of ppGpp are required to affect the RNA polymerase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bremer H., Berry L., Dennis P. P. Regulation of ribonucleic acid synthesis in Escherichia coli B-r: an analysis of a shift-up. II. Fraction of RNA polymerase engaged in the synthesis of stable RNA at different steady-state growth rates. J Mol Biol. 1973 Mar 25;75(1):161–179. doi: 10.1016/0022-2836(73)90536-6. [DOI] [PubMed] [Google Scholar]
- Bremer H. Parameters affecting the rate of synthesis of ribosomes and RNA polymerase in bacteria. J Theor Biol. 1975 Sep;53(1):115–124. doi: 10.1016/0022-5193(75)90106-x. [DOI] [PubMed] [Google Scholar]
- Brunschede H., Dove T. L., Bremer H. Establishment of exponential growth after a nutritional shift-up in Escherichia coli B/r: accumulation of deoxyribonucleic acid, ribonucleic acid, and protein. J Bacteriol. 1977 Feb;129(2):1020–1033. doi: 10.1128/jb.129.2.1020-1033.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. Separation and characterization of the subunits of ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6168–6176. [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B-r. J Bacteriol. 1974 Jul;119(1):270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P. Regulation of ribosomal and transfer ribonucleic acid synthesis in Escherichia coli B-r. J Biol Chem. 1972 May 10;247(9):2842–2845. [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Gallant J., Palmer L., Pao C. C. Anomalous synthesis of ppGpp in growing cells. Cell. 1977 May;11(1):181–185. doi: 10.1016/0092-8674(77)90329-4. [DOI] [PubMed] [Google Scholar]
- Hayward R. S., Fyfe S. Over-synthesis and instability of sigma protein in a merodiploid strain of Escherichia coli. Mol Gen Genet. 1978 Feb 7;159(1):89–99. doi: 10.1007/BF00401752. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Schleif R. In vivo experiments on the mechanism of action of L-arabinose C gene activator and lactose repressor. J Mol Biol. 1973 Nov 5;80(3):433–444. doi: 10.1016/0022-2836(73)90414-2. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. II. control of RNA polymerase synthesis during nutritional shift up and down. Mol Gen Genet. 1975 Dec 23;142(1):67–84. [PubMed] [Google Scholar]
- Iwakura Y., Ito K., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. I. Control of RNA polymerase content at various growth rates. Mol Gen Genet. 1974;133(1):1–23. doi: 10.1007/BF00268673. [DOI] [PubMed] [Google Scholar]
- Kennel D. Titration of the gene sites on DNA by DNA-RNA hybridization. II. The Escherichia coli chromosome. J Mol Biol. 1968 May 28;34(1):85–103. doi: 10.1016/0022-2836(68)90236-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagosky P. A., Chang F. N. Correlation between RNA synthesis and basal level guanosine 5'-diphosphate 3'-diphosphate in relaxed mutants of Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11651–11656. [PubMed] [Google Scholar]
- Norris T. E., Koch A. L. Effect of growth rate on the relative rates of synthesis of messenger, ribosomal and transfer RNA in Escherichia coli. J Mol Biol. 1972 Mar 14;64(3):633–649. doi: 10.1016/0022-2836(72)90088-5. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Ryals J., Bremer H. relA-dependent RNA polymerase activity in Escherichia coli. J Bacteriol. 1982 Apr;150(1):168–179. doi: 10.1128/jb.150.1.168-179.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen V., Bremer H. Rate of ribosomal ribonucleic acid chain elongation in Escherichia coli B/r during chloramphenicol treatment. J Bacteriol. 1977 Jun;130(3):1109–1116. doi: 10.1128/jb.130.3.1109-1116.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd N. S., Churchward G., Bremer H. Synthesis and activity of ribonucleic acid polymerase in Escherichia coli. J Bacteriol. 1980 Mar;141(3):1098–1108. doi: 10.1128/jb.141.3.1098-1108.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Shen V. Stability of ribosomal and transfer ribonucleic acid in Escherichia coli B/r after treatment with ethylenedinitrilotetraacetic acid and rifampicin. J Bacteriol. 1975 May;122(2):425–432. doi: 10.1128/jb.122.2.425-432.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



