Abstract
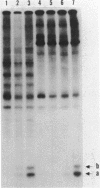
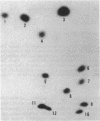
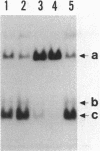
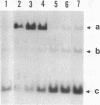
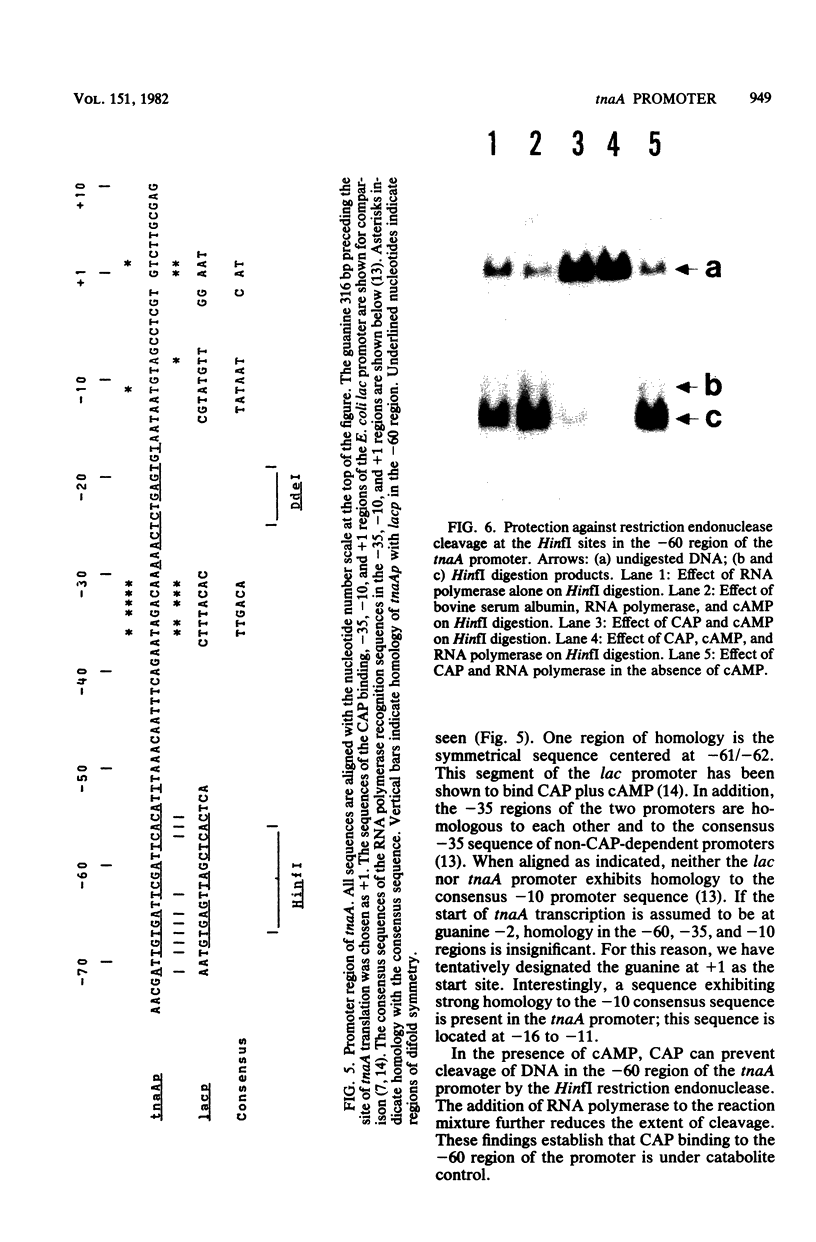
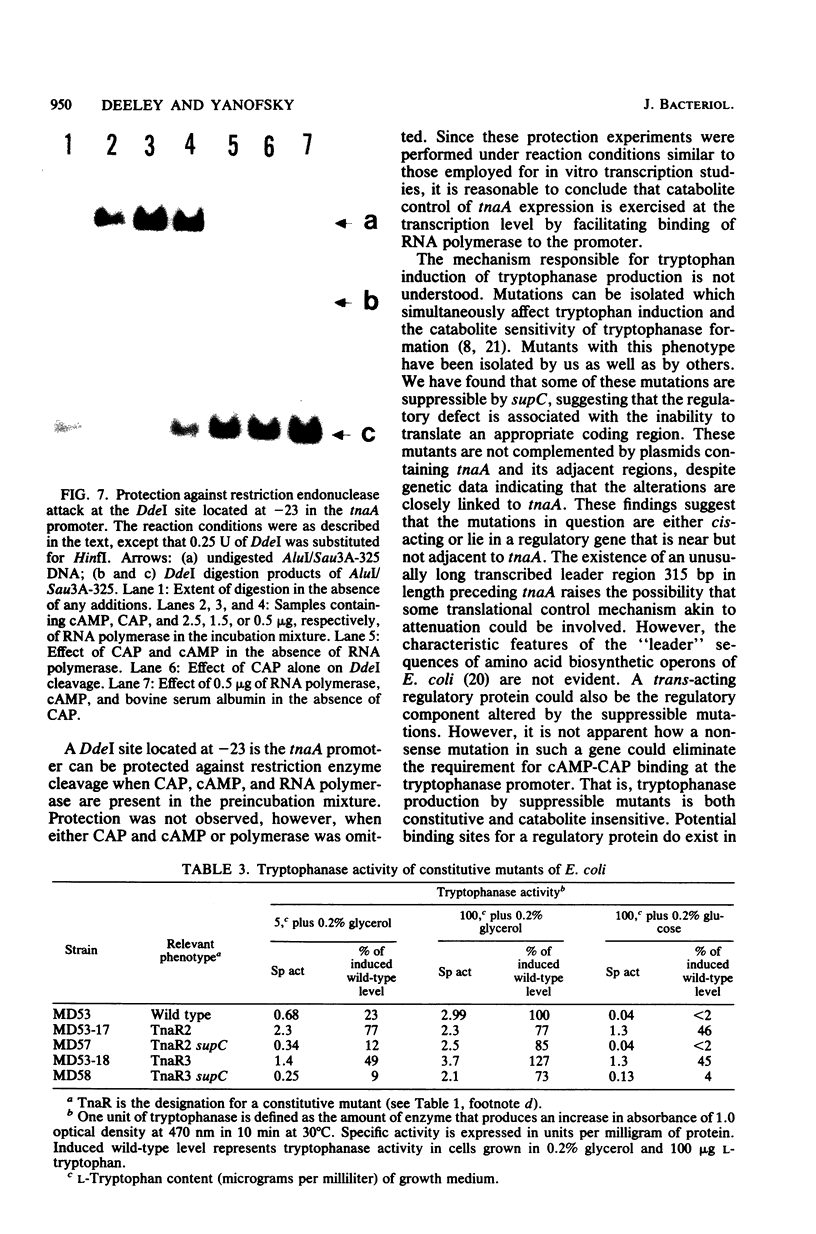
Restriction fragments containing the region preceding the tryptophanase structural gene, tnaA, were used as templates for in vitro transcription experiments. A transcription initiation site was detected that was dependent on the catabolite gene activator protein (CAP) plus cyclic AMP (cAMP). The mRNA produced in vitro was fingerprinted, and the nucleotide at which transcription was initiated was localized to the vicinity of two guanine residues 316 and 318 base pairs upstream of tnaA. A region exhibiting extensive difold symmetry and homology to the CAP binding site adjacent to the lactose operon promoter exists approximately 60 base pairs preceding the site of transcription initiation. Two HinfI restriction sites are located in this region. Restriction enzyme cleavage at these sites was prevented when DNA containing the promoter region was preincubated with CAP and cAMP. RNA polymerase was incapable of protecting these sites against this cleavage. CAP and cAMP addition did not protect against cleavage at a DdeI restriction site located in the -20 region of the promoter. RNA polymerase did protect against DdeI cleavage but only in the presence of CAP and cAMP. Thus, transcription initiation at the tryptophanase promoter involves cAMP-dependent, CAP-facilitated binding of RNA polymerase to the DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone T., Wilcox G. A rapid high-yield purification procedure for the cyclic adenosine 3',5'-monophosphate receptor protein from Escherichia coli. Biochim Biophys Acta. 1978 Jul 17;541(4):528–534. doi: 10.1016/0304-4165(78)90162-9. [DOI] [PubMed] [Google Scholar]
- Botsford J. L., DeMoss R. D. Catabolite repression of tryptophanase in Escherichia coli. J Bacteriol. 1971 Jan;105(1):303–312. doi: 10.1128/jb.105.1.303-312.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley M. C., Yanofsky C. Nucleotide sequence of the structural gene for tryptophanase of Escherichia coli K-12. J Bacteriol. 1981 Sep;147(3):787–796. doi: 10.1128/jb.147.3.787-796.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Majors J. Initiation of in vitro mRNA synthesis from the wild-type lac promoter. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4394–4398. doi: 10.1073/pnas.72.11.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. CATALYTIC PROPERTIES OF TRYPTOPHANASE, A MULTIFUNCTIONAL PYRIDOXAL PHOSPHATE ENZYME. Proc Natl Acad Sci U S A. 1964 Mar;51:382–389. doi: 10.1073/pnas.51.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Zurawski G., Yanofsky C. Attenuation in the Escherichia coli tryptophan operon: role of RNA secondary structure involving the tryptophan codon region. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5524–5528. doi: 10.1073/pnas.76.11.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Bishop R. J., Gelfand D. H. A plasmid cloning vehicle allowing regulated expression of eukaryotic DNA in bacteria. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3900–3904. doi: 10.1073/pnas.73.11.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker E., Brown K., Yanofsky C. Mitomycin C-induced expression of trpA of Salmonella typhimurium inserted into the plasmid ColE1. J Bacteriol. 1977 Jan;129(1):388–394. doi: 10.1128/jb.129.1.388-394.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Simpson R. B. Interaction of the cAMP receptor protein with the lac promoter. Nucleic Acids Res. 1980 Feb 25;8(4):759–766. [PMC free article] [PubMed] [Google Scholar]
- Snell E. E. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv Enzymol Relat Areas Mol Biol. 1975;42:287–333. doi: 10.1002/9780470122877.ch6. [DOI] [PubMed] [Google Scholar]
- Squires C., Lee F., Bertrand K., Squires C. L., Bronson M. J., Yanofsky C. Nucleotide sequence of the 5' end of tryptophan messenger RNA of Escherichia coli. J Mol Biol. 1976 May 15;103(2):351–381. doi: 10.1016/0022-2836(76)90317-x. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Ward D. F., Yudkin M. D. Mutations in Escherichia coli that relieve catabolite repression of tryptophanase synthesis. Tryptophanase promoter-like mutations. J Gen Microbiol. 1976 Jan;92(1):133–137. doi: 10.1099/00221287-92-1-133. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Snell E. E. Reversibility of the tryptophanase reaction: synthesis of tryptophan from indole, pyruvate, and ammonia. Proc Natl Acad Sci U S A. 1972 May;69(5):1086–1090. doi: 10.1073/pnas.69.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Yudkin M. D. Mutations in Escherichia coli that relieve catabolite repression of tryptophanase synthesis. Mutations distant from the tryptophanase gene. J Gen Microbiol. 1976 Jan;92(1):125–132. doi: 10.1099/00221287-92-1-125. [DOI] [PubMed] [Google Scholar]