Abstract
Aging and age-related disorders such as Alzheimer’s disease (AD) are usually accompanied by oxidative stress as one of the main mechanisms contributing to neurodegeneration and cognitive decline. Aging canines develop cognitive dysfunction and neuropathology similar to those seen in humans, and the use of antioxidants results in reductions in oxidative damage and in improvement in cognitive function in this canine model of human aging. In the present study, the effect of a long-term treatment with an antioxidant fortified diet and a program of behavioral enrichment on oxidative damage was studied in aged canines. To identify the neurobiological mechanisms underlying these treatment effects, the parietal cortex from 23 beagle dogs (8.1-12.4 years) were treated for 2.8 years in one of four treatment groups: i.e., control food- control behavioral enrichment (CC); control food - behavioral enrichment (CE); antioxidant food-control behavioral enrichment (CA); and enriched environment - antioxidant fortified food (EA). We analyzed the levels of the oxidative stress biomarkers, i.e., protein carbonyls, 3-nitrotyroine (3NT), and the lipid peroxidation product, 4-hydroxynonenal (HNE), and observed a decrease in their levels on all treatments when compared to control, with the most significant effects found in the combined treatment, EA. Since EA treatment was most effective, we also carried out a comparative proteomics study to identify specific brain proteins that were differentially expressed and used a parallel redox proteomics approach to identify specific brain proteins that were less oxidized following EA. The specific protein carbonyl levels of glutamate dehydrogenase [NAD (P)], glyceraldehyde-3-phosphate dehydrogenase (GAPDH), α-enolase, neurofilament triplet L protein, glutathione S-transferase (GST) and fascin actin bundling protein were significantly reduced in brain of EA-treated dogs compared to control. We also observed significant increases in expression of Cu/Zn superoxide dismutase, fructose-bisphosphate aldolase C, creatine kinase, glutamate dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase. The increased expression of these proteins and in particular Cu/Zn SOD correlated with improved cognitive function. In addition, there was a significant increase in the enzymatic activities of glutathione-S-transferase (GST) and total superoxide dismutase (SOD), and significant increase in the protein levels of heme oxygenase (HO-1) in EA treated dogs compared to control. These findings suggest that the combined treatment reduces the levels of oxidative damage and improves the antioxidant reserve systems in the aging canine brain, and may contribute to improvements in learning and memory. These observations provide insights into a possible neurobiological mechanism underlying the effects of the combined treatment. These results support the combination treatments as a possible therapeutic approach that could be translated to the aging human population who are at risk for age-related neurodegenerative disorders, including Alzheimer’s disease.
Keywords: Oxidative stress, Canine, Cognition, Antioxidants, Aging, Behavioral enrichment, β-Amyloid, Redox proteomics, Memory, Cognition, Proteomics
1. Introduction
Aged dogs naturally develop cognitive deficits and accumulate brain pathology that is similar to aging humans providing a useful model for studying the neurobiological mechanisms underlying age- related cognitive dysfunction [52, 53]. Aged canines show reduced cerebral volume, cortical atrophy and ventricular widening by in vivo magnetic resonance imaging [103, 110, 111]. The aging canine also shows impairments in visuospatial working memory and executive function [36, 102, 108]. Aged beagle brain accumulates amyloid-β-peptide (Aβ) that is of the same sequence as humans [63, 96] and is correlated with decline in cognitive function with age [43, 51]. Beagle dogs are accessible, easy to handle, capable of learning a broad repertoire of cognitive tasks, do not need food deprivation to be motivated and absorb dietary nutrients in similar ways as humans, hence making them a good model for dietary treatments [44]. The deposition of Aβ could play a significant role in molecular pathways involving free radical generation and oxidative stress as previously shown in AD-related studies from our laboratory [18, 23].
The brain is particularly vulnerable to oxidative damage due to its relative lack of antioxidant capacity, high concentration of unsaturated fatty acids, and high consumption rate of oxygen [74]. Oxidative stress leads to damaged to DNA, proteins and lipids that may consequently lead to dysfunction in various proteins or enzymes involved in several neurodegenerative disorders [16, 71, 76].
The aging process is associated with a progressive accumulation of oxidative damage that could play a role in the development or accumulation of neuropathology typically observed in age-related neurodegenerative disorders like AD [17, 57, 73, 74]. When compared to age-matched controls, the AD brain shows a higher levels of protein and DNA oxidation, and lipid peroxidation leading to loss of function of key enzymes [56, 73, 99]. In various AD studies from our laboratory, we have shown that Aβ 1-42 plays a central role in the oxidative stress observed and that the key to this link is a key amino acid residue methionine 35 [18, 23]. Similar events may also occur in the canine model of aging as deposits of Aβ1-42 may account for increased oxidative damage, a decline in glutathione content and decreased glutamine synthetase (GS) activity reported previously [54].
The use of antioxidants and/or related compounds reduces the level of oxidative damage and delays or reduces age-related cognitive decline in both animal models and in humans [10, 64, 78]. Previous studies in aged canines show that oxidative damage may be critically involved in the maintenance of cognitive function and long-term treatment with antioxidants and a program of behavioral enrichment reduces cognitive decline [40, 54, 79, 80]. In the canine model of human aging, short term and long term treatment with a diet rich in a broad spectrum of antioxidants leads to rapid and sustained learning ability and improved spatial attention; these effects were further enhanced with the addition of behavioral enrichment [78, 40]. However, the neurobiological changes elicited by these two interventions alone or in combination have yet to be established.
In the present study, we hypothesized that a possible mechanism for the improvement of cognition in aged treated animals may be mediated through the protection of neuronal function as a consequence of reduced oxidative damage and improved antioxidant reserves and possibly an increase in the expression of key brain proteins associated with neuronal improvement. We report that the use of antioxidants composed of mitochondrial cofactors and cellular antioxidants and a program of behavioral enrichment in the present study could potentially protect proteins from oxidative damage and enhance mitochondrial function leading to the observed improved memory and cognitive function in this model.
Methods
2.1 Subjects
Twenty-four beagle dogs ranging in from 8.05-12.35 yrs at the start of the study (Mean = 10.69 yrs SE=0.25) were obtained from the colony at the Lovelace Respiratory Research Institute (Table 1). These study animals were bred and maintained in the same environment and all had documented dates of birth and comprehensive medical histories. At the time of euthanasia, 23 dogs had received the intervention and ranged in age from 10.72-15.01 yrs (Mean=13.31 yrs, SE=0.26) with one animal not completing the baseline phase of the study. All research was conducted in accordance with approved IACUC protocols.
Table 1.
Ages and treatment times of study animals
| Dog | Date | Animal | Treatment | Age at Start of Study (yrs) | Age at End of Study (yrs) | Duration of Intervention (yrs) | Cause of Death |
|---|---|---|---|---|---|---|---|
| 1494D | 10/15/01 | 1 | C/C | 12.1 | 15.0 | 2.8 | Study End |
| 1508U | 10/15/01 | 2 | C/C | 11.4 | 13.5 | 1.9 | *Congestive heart failure |
| 1510A | 10/15/01 | 3 | C/C | 11.3 | 14.2 | 2.7 | Study End |
| 1521S | 10/15/01 | 4 | C/C | 10.7 | 13.6 | 2.8 | Study End |
| 1543S | 10/15/01 | 5 | C/C | 10.1 | 13.0 | 2.8 | Study End |
| B2150 | 10/15/01 | 6 | C/C | 11.6 | 14.5 | 2.8 | Study End |
| Mean C/C | 11.2 | 14.0 | 2.6 | ||||
| 1492B | 10/15/01 | 7 | E/C | 12.1 | 12.5 | 0.3 | *Liver degeneration, pancreatitis |
| 1506B | 10/15/01 | 8 | E/C | 11.5 | 14.4 | 2.8 | Study End |
| 1518D | 10/15/01 | 9 | E/C | 10.8 | 13.7 | 2.8 | Study End |
| 1523U | 10/15/01 | 10 | E/C | 9.6 | 12.2 | 2.5 | Anorexia |
| 1529S | 10/15/01 | 11 | E/C | 10.4 | 13.3 | 2.8 | Study End |
| 1542S | 10/15/01 | 12 | E/C | 10.1 | 13.0 | 2.8 | Study End |
| Mean E/C | 10.7 | 13.2 | 2.3 | ||||
| 1491B | 10/15/01 | 13 | C/A | 12.1 | 15.0 | 2.7 | Study End |
| 1508A | 10/15/01 | 14 | C/A | 11.4 | 14.3 | 2.8 | Study End |
| 1509U | 10/15/01 | 15 | C/A | 11.3 | 13.8 | 2.4 | Abscess in left axilla |
| 1523B | 10/15/01 | 16 | C/A | 9.6 | 12.5 | 2.7 | Study End |
| 1532S | 10/15/01 | 17 | C/A | 10.4 | 13.3 | 2.8 | Study End |
| 1581S | 10/15/01 | 18 | C/A | 8.1 | 11.0 | 2.7 | Study End |
| Mean C/A | 10.5 | 13.3 | 2.7 | ||||
| 1502S | 10/15/01 | 19 | E/A | 11.9 | 14.8 | 2.8 | Study End |
| 1521B | 10/15/01 | 20 | E/A | 10.7 | 13.6 | 2.7 | Study End |
| 1541B | 10/15/01 | 21 | E/A | 10.1 | 13.0 | 2.7 | Study End |
| 1542T | 10/15/01 | 22 | E/A | 10.1 | 13.0 | 2.8 | Study End |
| 1581T | 10/15/01 | 23 | E/A | 8.1 | 11.0 | 2.7 | Study End |
| 1585A | 10/15/01 | 24 | E/A | 7.8 | 10.7 | 2.7 | Study End |
| Mean E/A | 10.5 | 13.3 | 2.7 | ||||
C/C - control enrichment/control diet
E/C - behavioral enrichment/control diet
C/A - control enrichment/antioxidant diet
E/A - behavioral enrichment/antioxidant diet
2.2 Group Assignments and Study Timeline
All study dogs underwent extensive baseline cognitive testing as described previously [78]. Animals were subsequently ranked based on cognitive test scores and placed into 4 groups. These four groups were randomly assigned as one of the treatment conditions as follows: C/C – control enrichment/control diet, E/C – behavioral enrichment /control diet, C/A – control enrichment/antioxidant diet, E/A – behavioral enrichment /antioxidant diet.
2.3 Behavioral Enrichment Treatment
The behavioral enrichment protocol consisted of social enrichment, by housing animals in pairs, environmental enrichment, by providing play toys, physical enrichment, by providing two 20-minute outdoor walks per week, and cognitive enrichment, through continuous cognitive testing. The cognitive enrichment consisted of a landmark discrimination task, an oddity discrimination task [78], and size concept learning [109]. In addition, all animals, regardless of treatment condition were evaluated annually on a test of visuospatial memory [36], object recognition memory [30] and either size discrimination and reversal learning [108], or black/white discrimination and reversal on consecutive years.
2.4 Diet Treatment
The two foods were formulated to meet the adult maintenance nutrient profile for the American Association of Feed Control Officials recommendations for adult dogs (AAFCO 1999). Control and test foods were identical in composition, other than inclusion of a broad-based antioxidant and mitochondrial co-factor supplementation to the test food. The control and enriched foods had the following differences on an as-fed basis, respectively: dl-alpha-tocopherol acetate (vitamin E, approximately 100 vs. 1000 ppm), L-carnitine (<20 ppm vs. approximately 250 ppm), dl-alpha-lipoic acid (<20 ppm vs. approximately 120 ppm), ascorbic acid or vitamin C as Stay-C (<30 ppm vs. approximately 80 ppm), and 1% inclusions of each of the following (1 to 1 exchange for corn): spinach flakes, tomato pomace, grape pomace, carrot granules and citrus pulp. The rationale for these inclusions were as follows: Vitamin E is lipid soluble and acts to protect cell membranes from oxidative damage; vitamin C is essential in maintaining oxidative protection for the soluble phase of cells as well as preventing vitamin E from propagating free radical production [20]; alpha-lipoic acid is a cofactor for the mitochondrial respiratory chain enzymes, pyruvate and alpha-ketoglutarate dehydrogenase, as well as an antioxidant capable of redox recycling other antioxidants and raising intracellular glutathione levels [85]; L-carnitine is a precursor to acetyl-L-carnitine and is involved in mitochondrial lipid metabolism and maintaining efficient function [29]. Fruits and vegetables are rich in flavonoids and carotenoids and other antioxidants [10, 65]. To define this further, added inclusions were measured for oxygen radical absorbing capacity (ORAC) as well as carotenoid and flavonoid profiles [31]. Fruit and vegetables selected for inclusion were based on ORAC content and general commercial availability. Results of this analysis revealed that ORAC content of the individual fruit and vegetable inclusions were higher than the corn for which they were substituted. In addition, inclusion of these ingredients, in combination with the vitamins, resulted in increased ORAC content of the finished product. The food was produced by an extrusion process and a production batch was fed for no more than 6 months before a new lot was manufactured.
2.5 Cognitive Testing
All animals were given annual tests of cognition to detect changes in response to the different treatments. Within 8 months of euthanasia, animals were given an black/white discrimination and reversal problem that is impaired in aged animals and is significantly improved in both antioxidant treated and/or behaviorally enriched animals [80]. Also within a year of the end of the study, spatial memory was tested using a nonmatching to position paradigm described previously to be sensitive to age in dogs [36]. All of the testing procedures were described in previous publications [36, 80].
2.6 Animal Euthanasia
Twenty minutes before induction of general anesthesia, animals were sedated by subcutaneous injection with 0.2 mg/kg acepromazine. General anesthesia was induced by inhalation with 5% isoflurane. While being maintained under anesthesia, dogs were exsanguinated by cardiac puncture and blood samples were collected to obtain plasma and serum for future studies. Within 15 minutes, the brain was removed from the skull and a cerebrospinal fluid sample was obtained from the lateral ventricles. The brain was sectioned midsagitally, with the entire left hemisphere being immediately placed in 4% paraformaldehyde for 48-72 hours at 4°C prior to long term storage in phosphate buffered saline with 0.05% sodium azide at 4°C. The remaining hemispheres were sectioned coronally and flash frozen at -80°C and the parietal cortex was dissected for use in the current studies. The dissection procedure was completed within 20 minutes. Thus, the post mortem interval for all animals was 35-45 minutes.
2.7 Measurement of protein carbonyls
Protein carbonyls are an index of protein oxidation and were determined as described previously [15]. Briefly, samples (5 μg of protein) were derivatized with 10 mM 2, 4-dinitrophenylhydrazine (DNPH) in the presence of 5 μL of 12% sodium dodecyl sulfate for 20 min at room temperature (23°C). The samples were then neutralized with 7.5 μL of the neutralization solution (2 M Tris in 30% glycerol). Derivatized protein samples were then blotted onto a nitrocellulose membrane with a slot-blot apparatus (250 ng per lane). The membrane was then washed with wash buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) and blocked by incubation in the presence of 5% bovine serum albumin, followed by incubation with rabbit polyclonal anti-DNPH antibody (1:100 dilution) as the primary antibody for 1 h. The membranes were washed with wash buffer and further incubated with alkaline phosphatase-conjugated goat anti-rabbit antibody as the secondary antibody for 1 h. Blots were developed using fast tablet (BCIP/NBT; Sigma-Aldrich) and quantified using Scion Image (PC version of Macintosh-compatible NIH Image) software. No non-specific background binding of the primary or secondary anti bodies was found.
2.8 Measurement of 3-nitrotyrosine (3-NT)
Nitration of proteins is another form of protein oxidation [34, 106]. The nitrotyrosine content was determined immunochemically as previously described [45]. Briefly, samples were incubated with Laemmli sample buffer in a 1:2 ratio (0.125 m Trizma base, pH 6.8, 4% sodium dodecyl sulfate, 20% glycerol) for 20 min. Protein (250 ng) was then blotted onto the nitrocellulose paper using the slot-blot apparatus and immunochemical methods as described above for protein carbonyls. The mouse anti-nitrotyrosine antibody (5: 1000 dilution) was used as the primary antibody and alkaline phosphatase-conjugated anti-mouse secondary antibody was used for detection. Blots were then scanned using scion imaging and densitometric analysis of bands in images of the blots was used to calculate levels of 3-NT. No non-specific binding of the primary or secondary antibodies was found.
2.9 Measurement of 4-hydroxynonenal (HNE)
HNE is a marker of lipid oxidation and the assay was performed as previously described [68]. Briefly, 10 μl of sample were incubated with 10 μl of Laemmli buffer containing 0.125 M Tris base pH 6.8, 4 % (v/v) SDS, and 20% (v/v) Glycerol. The resulting sample (250 ng) was loaded per well in the slot blot apparatus containing a nitrocellulose membrane under vacuum pressure. The membrane was blocked with 3% (w/v) bovine serum albumin (BSA) in phosphate buffered saline containing 0.01% (w/v) sodium azide and 0.2% (v/v) Tween 20 (PBST) for 1 h and incubated with a 1:5000 dilution of anti-4-hydroxynonenal (HNE) polyclonal antibody in PBST for 90 min. Following completion of the primary antibody incubation, the membranes were washed three times in PBST. An anti-rabbit IgG alkaline phosphatase secondary antibody was diluted 1:8000 in PBST and added to the membrane. The membrane was washed in PBST three times and developed using Sigmafast Tablets (BCIP/NBT substrate). Blots were dried, scanned with Adobe Photoshop, and quantified by Scion Image. A small background of the primary antibody binding to the membrane was found, but this was scattered from both control and subject blots.
2.10 Two-dimensional electrophoresis
Brain samples (200 μg) were incubated with 4 volumes of 2N HCl at room for electrophoresis or 20mM 2, 4 dinitrophenyl hydrazine (DNPH) for western blotting at room temperature for 20 min. Proteins were then precipitated by the addition of ice-cold 100% trichloroacetic acid (TCA) to obtain a final concentration of 15% TCA. Samples were then placed on ice for 10 min and precipitates centrifuged at 16,000 g for 3min. The resulting pellet was then washed three times with a 1:1(v/v) ethanol/ethyl acetate solution. The samples were then suspended in 200 μl of rehydration buffer composed of a 1:1 ratio (v/v) of the Zwittergent solubilization buffer (7M urea, 2M thiourea, 2% Chaps, 65 mM DTT, 1% Zwittergent 0.8% 3-10 ampholytes and bromophenol blue) and ASB-14 solubilization buffer (7M urea, 2M thiourea 5Mn TCEP, 1% (w/v) ASB-14, 1% (v/v) Triton X-100, 0.5% Chaps, 0.5% 3-10 ampholytes) for 1 h.
2.11 First dimension electrophoresis
For the first-dimension electrophoresis, 200 μL of sample solution was applied to a 110-mm pH 3–10 ReadyStrip™ IPG strips (Bio-Rad, Hercules CA). The strips were then actively rehydrated in the protean IEF cell (Bio-Rad) at 50 V for 18 h. The isoelectric focusing was performed in increasing voltages as follows; 300 V for 1 h, then linear gradient to 8000 V for 5 h and finally 20 000 V/h. Strips were then stored at −80 °C until the 2nd dimension electrophoresis was to be performed.
2.12 Second dimension electrophoresis
For the second dimension, the IPG® Strips, pH 3–10, were equilibrated for 10 min in 50 mM Tris–HCl (pH 6.8) containing 6 M urea, 1% (w/v) sodium dodecyl sulfate (SDS), 30% (v/v) glycerol, and 0.5% dithiothreitol, and then re-equilibrated for 15 min in the same buffer containing 4.5% iodacetamide instead of dithiothreitol. Linear gradient precast criterion Tris–HCl gels (8–16%) (Bio-Rad) were used to perform second dimension electrophoresis. Precision Protein™ Standards (Bio-Rad, CA) were run along with the sample at 200 V for 65 min.
2.13 SYPRO ruby staining
After the second dimension electrophoresis, the gels were incubated in fixing solution (7% acetic acid, 10% methanol) for 20 min and stained overnight at room temperature with 50ml SYPRO Ruby gel stain (Bio-Rad). The SYPRO ruby gel stain was then removed and gels stored in DI water.
2.14 Western Blotting
Brain samples (200 μg) incubated with 20mM DNPH were used for western blotting. The strips and gels were run as described above. After the second dimension, the proteins from the gels were transferred onto nitrocellulose papers (Bio-Rad) using the Transblot-Blot® SD semi-Dry Transfer Cell (Bio-Rad), at 15 V for 4 h. The 2,4-dinitrophenyl hydrazone (DNP) adduct of the carbonyls of the proteins was detected on the nitrocellulose paper using a primary rabbit antibody (Chemicon, CA) specific for DNP-protein adducts (1:100), and then a secondary goat anti-rabbit IgG (Sigma, MO) antibody was applied. The resulting stain was developed by application of Sigma-Fast (BCIP/NBT) tablets.
2.15 Image analysis
The nitrocellulose blots (oxyblots) were scanned and saved in TIFF format using Scan jet 3300C (Hewlett Packard, CA). SYPRO ruby-stained gel images were obtained using a STORM phosphoimager (Ex. 470 nm, Em. 618 nm, Molecular Dynamics, Sunnyvale, CA, USA) and also saved in TIFF format. PD-Quest (Bio-Rad) imaging software was then used to match and analyze visualized protein spots among differential 2D gels and 2D oxyblots, with one blot and one gel for each individual sample.
2.16 In-gel trypsin digestion
In those brain proteins less oxidized from EA dogs compared to CC dogs as judged by PDQuest analysis, protein spots were digested by trypsin using protocols previously described [112]. Briefly, spots of interest were excised using a clean blade and placed in Eppendorf tubes, which were then washed with 0.1 M ammonium bicarbonate (NH4HCO3) at room temperature for 15 min. Acetonitrile was then added to the gel pieces and incubated at room temperature for 15 min. This solvent mixture was then removed and gel pieces dried. The protein spots were then incubated with 20 μL of 20 mM DTT in 0.1 M NH4HCO3 at 56 °C for 45 min. The DTT solution was removed and replaced with 20 μL of 55 mM iodoacetamide in 0.1 M NH4HCO3. The solution was then incubated at room temperature for 30 min. The iodoacetamide was removed and replaced with 0.2 mL of 50 mM NH4HCO3 and incubated at room temperature for 15 min. Acetonitrile (200 μL) was added. After 15 min incubation, the solvent was removed, and the gel spots were dried in a flow hood for 30 min. The gel pieces were rehydrated with 20 ng/μL-modified trypsin (Promega, Madison, WI) in 50 mM NH4HCO3, with the minimal volume enough to cover the gel pieces. The gel pieces were incubated overnight at 37 °C in a shaking incubator.
2.17 Mass spectrometry
A MALDI-TOF mass spectrometer in the reflectron mode was used to generate peptide mass fingerprints. Peptides resulting from in-gel digestion with trypsin were analyzed on a 384 position, 600 μm AnchorChip™ Target (Bruker Daltonics, Bremen, Germany) and prepared according to AnchorChip recommendations (AnchorChip Technology, Rev. 2, Bruker Daltonics, Bremen, Germany). Briefly, 1 μL of digestate was mixed with 1 μL of alpha-cyano-4-hydroxycinnamic acid (0.3 mg/mL in ethanol: acetone, 2:1 ratio) directly on the target and allowed to dry at room temperature. The sample spot was washed with 1 μL of a 1% TFA solution for approximately 60 seconds. The TFA droplet was gently blown off the sample spot with compressed air. The resulting diffuse sample spot was recrystallized (refocused) using 1 μL of a solution of ethanol: acetone: 0.1 % TFA (6:3:1 ratio). Reported spectra are a summation of 100 laser shots. External calibration of the mass axis was used for acquisition and internal calibration using either trypsin autolysis ions or matrix clusters and was applied post acquisition for accurate mass determination.
2.18 Analysis of peptide sequences
Peptide mass fingerprinting was used to identify proteins from tryptic peptide fragments by utilizing the MASCOT search engine based on the entire NCBI and SwissProt protein databases. Database searches were conducted allowing for up to one missed trypsin cleavage and using the assumption that the peptides were monoisotopic, oxidized at methionine residues, and carbamidomethylated at cysteine residues. Mass tolerance of 150 ppm, 0.1 Da peptide tolerances and 0.2 Da fragmentation tolerances was the window of error allowed for matching the peptide mass values. Probability-based MOWSE scores were estimated by comparison of search results against estimated random match population and were reported as -10*log10 (p), where p is the probability that the identification of the protein is a random event. MOWSE scores greater than 63 were considered to be significant (P < 0.05). All protein identifications were in the expected size and isoelectric point (pI) range based on the position in the gel.
2.19 Immunoprecipitation
Immunoprecipitation of specific proteins was performed as previously described [104]. Brain samples (200 μg) from control or treated animals were incubated overnight with anti-GAPDH and anti-CuZnSOD antibody. This was followed by three washing steps with buffer B (50Mm Tris HCl (pH-8.0), 150 Mm NaCl, and 1% NP40). The proteins were resolved by SDS-PAGE followed by immunoblotting on a nitrocellulose membrane (BioRad). In addition, for the GAPDH, after immunoprecipitation, a set of the samples were on-blot derivatized by DNPH as previously described [39]. The proteins were then detected with alkaline phosphate labeled secondary antibody (Sigma).
2.20 Protein Interacteome
The functional protein interacteome was obtained by using Interaction Explorer ™ Software Pathway Assist software package (Stratagene, La Jolla, CA). Pathway Assist is software for functional interaction analysis. It allows for the identification and visualization of pathways, gene regulation networks and protein interaction maps. The proteins are first imported as the gene symbols as a set of data. This data set is then searched against ResNet, a database containing over 500,000 biological interactions built by applying the MedScan text-mining algorithms to all PubMed abstracts. These interactions are then visualized by building interaction networks with shortest-path algorithms. This process can graphically identify all known interaction among the proteins. The information of the function of these proteins and their relevance to diseases are then obtained by using the BIOBASE’s Proteome BioKnowledge Library form Incyte Corporation (Incyte, Wilmington, DE) [59].
2.21 Determination of glutathione-S-transferase (GST) activity
Glutathione-S-transferase activity was measured as previously described using 1-chloro-2, 4-dinitrobenzene (CDNB) as substrate [49]. Briefly, the standard assay mixture contained CDNB (1 mM), reduced glutathione (1 mM), and potassium phosphate buffer (100 mM; pH = 6.5) in a volume of 100 μL. The changes in absorbance were monitored at 340 nm. The thioether formed was determined by reading the absorbance at 340 nm, and quantification was performed by using a molar absorptivity of 9.6 M−1.
2.22 Determination of superoxide dismutase (SOD) activity
Superoxide dismutase (SOD) activity was measured as previously described [101]. Briefly, the reaction mixture of total volume 184 μL contained 160 μL of 50 mmol/l glycine buffers, pH 10.4, and 20.0 μL sample. The reaction was initiated by the addition of 4.0 μL of a 20 mg/ml solution of (–)-epinephrine. Due to its poor solubility, (–)-epinephrine (40 mg) was suspended in 2 ml water and was solubilized by adding 2–3 drops of 2N HCl. The auto-oxidation of (–)-epinephrine was monitored at 480nm and the millimolar absorptivity (4.02 mmol · l−1 · cm−1) was used for calculations.
2.23 Measurement of HO-1 Protein levels
Mixtures of loading buffer and brain samples (50 ng) were denatured and electrophoresed on a 10% SDS-polyacrylamide gel. Proteins were transferred to nitrocellulose at 90 mA/gel for 2 h. The blots were blocked for 1h in fresh wash buffer and incubated with HO-1 primary antibody for 2 h. The membrane was then washed for three times in PBS for 5 min and the incubated with a secondary alkaline phosphatase-conjugated antibody. Proteins were visualized by developing with Sigma fast tablets (BCIP/NBT substrate). Blots were dried, scanned with Adobe Photoshop, and quantified using Scion Image (PC version of Macintosh-compatible NIH Image) software.
2.24 Statistics
An analysis of variance was used to compare the 4 treatment groups on measures of oxidative damage (protein carbonyls, 3-NT, HNE). Post hoc comparisons were made using both the Bonferroni correction and using Dunnett’s t-test. For measures of antioxidant enzymes and HO-1 protein levels, independent t-tests were used. In all of these analyses, raw data were used but percent changes are presented in the plots. Pearson product moment correlations were used to test the linear association between oxidative damage, antioxidant enzymes and cognition. A linear stepwise multiple regression was used to determine which of the measures of oxidative damage best predicted cognition. SPSS for Windows was used and a p value of <.05 was used to establish statistical significance. Statistical analysis of specific protein carbonyl levels matched with anti-DNP-positive spots on 2D-oxyblots from brain samples from animals on an enriched environment and antioxidant fortified diet (EA) and age matched control of dogs that were on control food- control environment (CC) was carried out using Student’s t-tests. A value of p < 0.05 was considered statistically significant. Only proteins that are considered significantly different by Student’s t-test were subjected to in-gel trypsin digestion and subsequent proteomic analyses. This is the normal procedure for proteomics studies, as sophisticated statistical analysis used for microarray studies are not applicable for proteomics studies [75].
3. Results
3.1 Decrease in the levels of protein oxidation
As shown in Fig.1A and 1B, total protein oxidation measured by the accumulation of protein carbonyls (F (3, 22) =4.93 p=0.011) and 3-nitrotyrosine (3NT) (F (3, 22) =3.82 p=0.027), respectively, were reduced in all treatment conditions. Post hoc comparisons show that the extent of neuroprotection was greater for the combined treatment of the enriched environment and antioxidant-fortified food (EA) (p=0.013 and p=0.031 for protein carbonyls and 3NT, respectively). The levels of lipid peroxidation, detected as protein-bound HNE, (Fig.1C), showed a tendency towards reduction when the groups were compared, but was not significantly different than controls (F (3, 22) =1.34 p=0.29).
Figure 1.
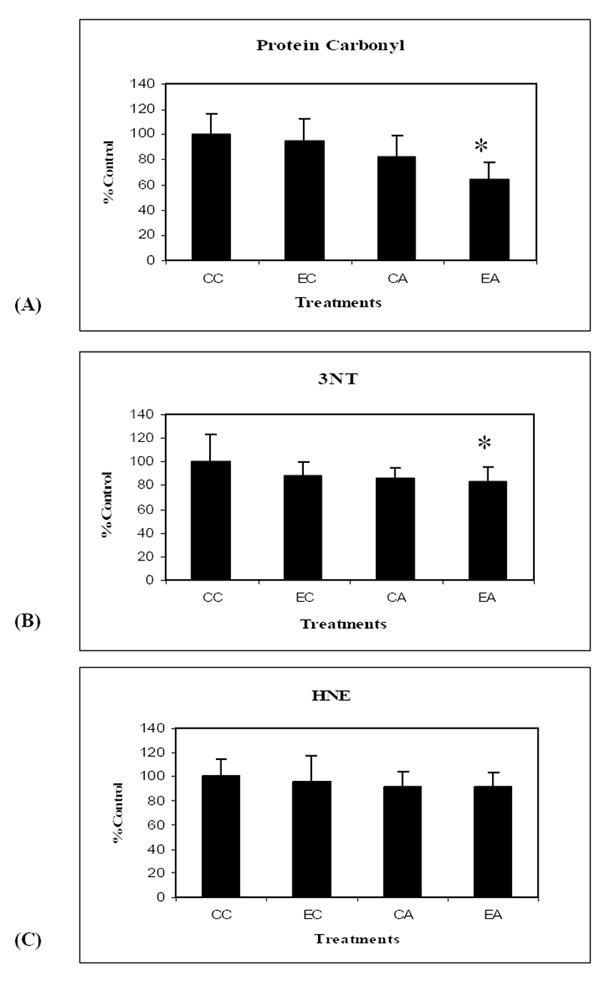
Changes in protein carbonyls (A), 3NT (B) and HNE (C) levels in canine brain homogenate samples following treatment. There was a decrease in the levels of protein carbonyls, 3NT and HNE measured from the various treatments, i.e. EC, CA and EA compared to the control group CC. Data are represented as % control ± SEM for animals in each treatment group. Measured values are normalized to the CC values (n=6) * p < 0.05 for canines on EA treatment.
3.2 Specific Protein Carbonyl Levels
We next estimated of the carbonyl levels of specific proteins by dividing the carbonyl level of a protein spot on the 2-D nitrocellulose membrane by the protein level of its corresponding protein spot on the 2-D gel. This ratio gives the carbonyl level per unit of protein. We used a parallel approach to quantify the specific protein carbonyl levels by the extent of DNP-bound proteins by immunoblotting (Fig. 2). For these comparisons, we focused on the CC and EA groups, which showed the largest differences in total protein oxidation in the first experiment. Six proteins were identified that were significantly less oxidized. As shown in (Table 2), these proteins were: glutamate dehydrogenase [NAD (P)], glyceraldehyde-3-phosphate dehydrogenase (GAPDH), α-enolase, neurofilament triplet L protein, glutathione S-transferase (GST), and fascin actin bundling protein. The summary of specific carbonyl levels of the six identified proteins is shown in Table 3. The probability of an incorrect identification was established to be minimal (Table 2). Nevertheless, to confirm the proteomics identification of the proteins were correct; GAPDH was used as a representative protein. We used immunoprecipitation of GAPDH with an anti-GAPDH antibody, oxidized it with DNPH, and used Western blot analysis (Fig. 4B) to show decreased level of oxidation in the combined treatment group EA compared to control CC. It should be noted that n=3, since this was a representative validation and confirmation of our results.
Figure 2.
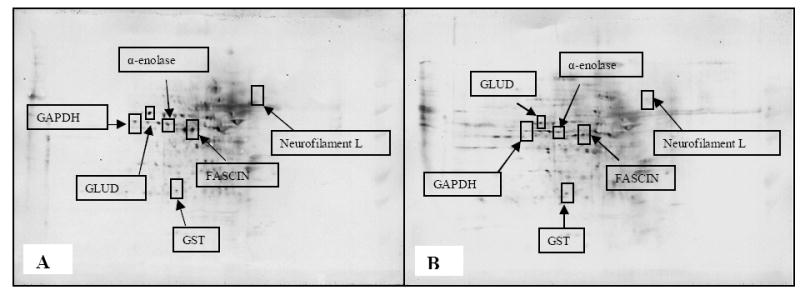
Combined treatment of aged dogs with an antioxidant enriched diet and behavioral enrichment leads to reduced protein oxidation. Carbonyl immunoblots showing proteins with less oxidation in the parietal cortex of canines given a combined treatment with an antioxidant fortified diet and an exposure to a behavioral enrichment programme EA) (B) as compared to control (CC) (A).
Table 2.
Mass spectrometric characteristics of oxidized canine brain proteins identified in this study
| Identified Protein | GI accession number | Number of peptide matches identified | % Coverage of matched peptides | pI, MrW | Mowse score | Probability of a random identification |
|---|---|---|---|---|---|---|
| Glutamate dehydrogenase [NAD(P)] | gi∣81884222 | 10 | 22 | 8.05,61640 | 123 | 5.0 ×10-13 |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | gi∣62296789 | 7 | 25 | 8.23,35935 | 69 | 1.3 ×10-7 |
| Alpha enolase | gi∣13637776 | 8 | 21 | 6.36,47322 | 94 | 4.0 ×10-10 |
| Neurofilament triplet L protein | gi∣1709260 | 13 | 29 | 4.63,61224 | 132 | 6.3 ×10-14 |
| Glutathione S-transferase P | gi∣73975748 | 5 | 30 | 6.30,23518 | 71 | 7.9 ×10-8 |
| Fascin- actin bundling protein | gi∣2498357 | 14 | 39 | 6.81,54992 | 137 | 2.0 ×10-14 |
Table 3.
Specific carbonyl levels of oxidized proteins
| Identified Protein | Specific protein carbonyl levels of canine on EA(% control ± SEM) (n=5) | P value |
|---|---|---|
| Glutamate dehydrogenase | 27 ± 5 | < 0.04 |
| GAPDH | 18 ± 8 | < 0.05 |
| Alpha enolase | 14 ± 3 | < 0.05 |
| Neurofilament triplet L protein | 16 ± 3 | < 0.04 |
| Glutathione S-transferase P | 20 ±6 | < 0.02 |
| Fascin actin bundling protein | 23 ± 7 | < 0.008 |
Figure 4.
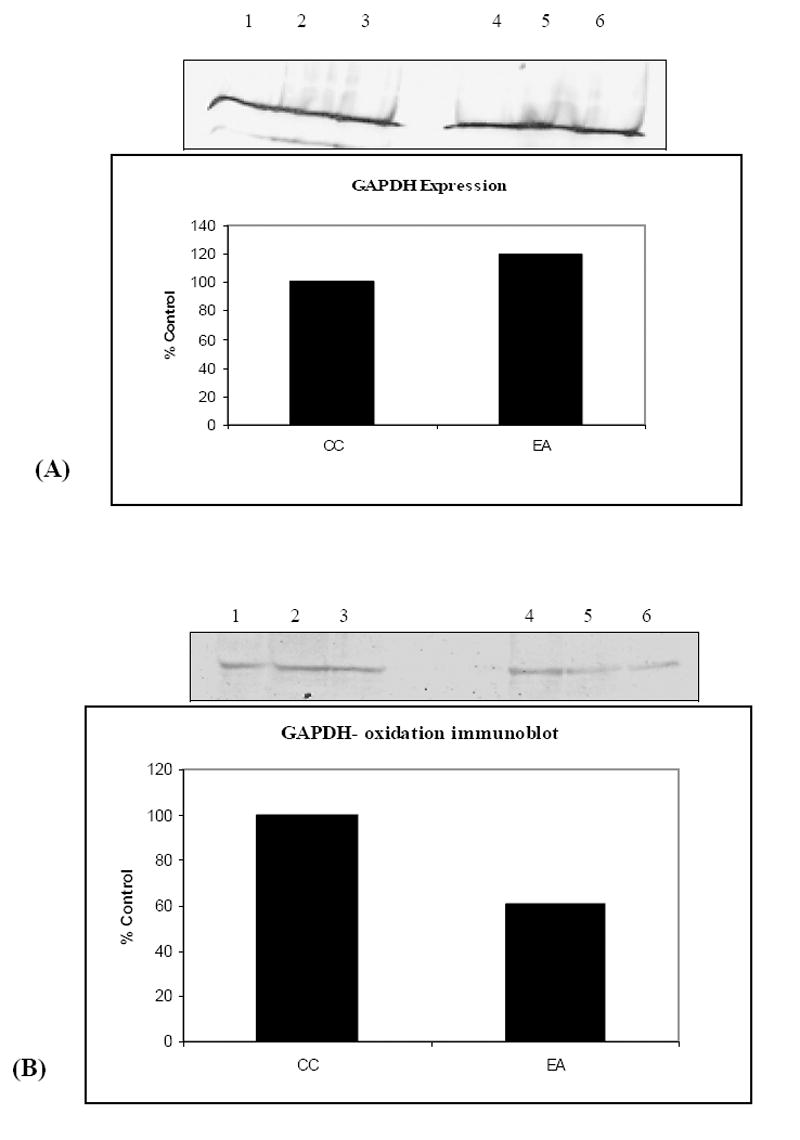
Validation of proteins identified by proteomics. (A) shows immunoblot probed with anti GADPH antibody. Lanes 1-3 represent CC, while lanes 4-6 represent EA, and a graphical quantification of the blot also is shown. (B) shows an immuno-oxyblot of GAPDH and a graphical representation, validating the identification of reduced oxidation of GAPDH (n=3).
3.3 Protein Expression Levels
Two-dimensional electrophoresis offers an excellent tool for the screening of abundant protein changes in various disease states [21, 22, 82, 88]. In the present study, we investigated the pattern of protein expression in the parietal cortex in the four different groups. The final comparison was made as follows 1) CC vs. CE; 2) CC vs. CA; and 3) CC vs. EA. Fig 3A, 3B and 3C shows SYPRO ruby stained 2D gels of the groups mentioned above with identified protein boxed and labeled. Compared to control (CC), all treatment groups showed a significant increase in the expression of specific proteins. Some proteins showed an increase in expression in all treatment groups while others were specific for a particular treatment. Proteins associated with energy metabolism and antioxidant reserve were identified by mass spectrometry and included: Cu/Zn superoxide dismutase, fructose-bisphosphate aldolase C, creatine kinase, glutamate dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase (Table 4). Table 5 provides the changes in protein levels expressed as % control ± S.E.M across the treatment conditions. As representative proteins to validate these proteomic identifications, we used immunoprecipitation of GAPDH and CuZn SOD with anti-GAPDH and anti- CuZn SOD antibodies as shown Fig 4A and Fig 5, respectively. As can be seen there was an increase in the expression of in both proteins confirming our previous identification by mass spectrometry.
Figure 3.
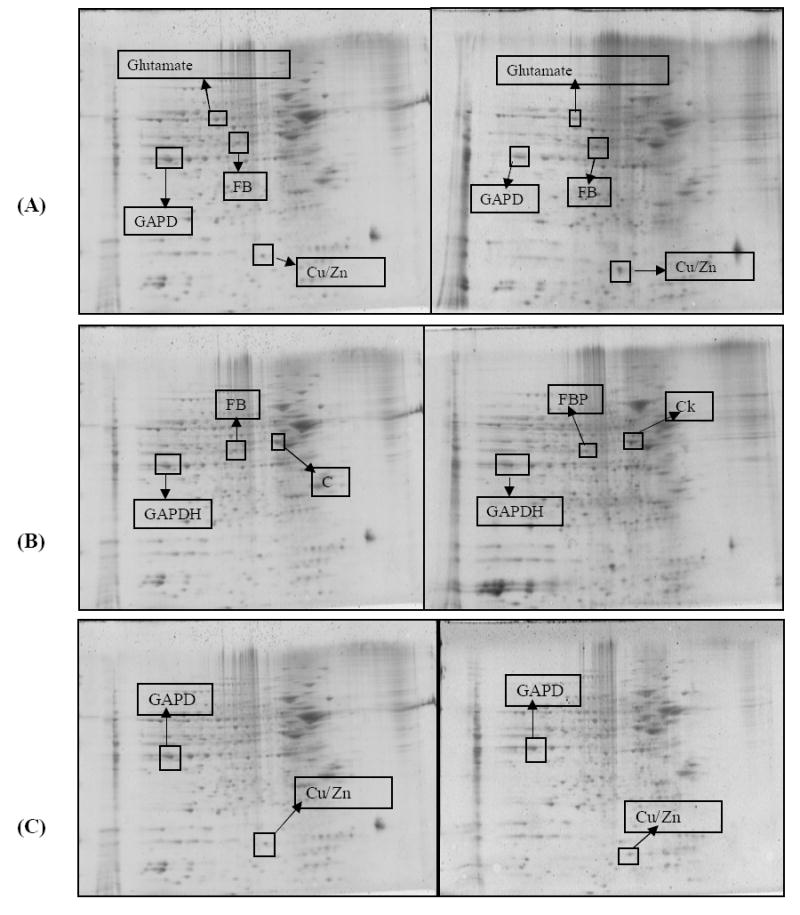
SYPRO Ruby-stained 2D-gels maps, (A) CC vs. CE, (B) CC vs. CA and (C) CC vs. EA of canine parietal cortex homogenates samples from the CC CE,CA and EA treated animals are presented. Proteins identified by mass spectrometry are presented as the boxed spots.
Table 4.
Proteomic characterization of differentially expressed canine brain proteins identified
| Identified Protein | GI accession number | Number of Peptide matches identified | % Coverage of matched peptides | pI, MrW | Mowse score | Probability of a random identification |
|---|---|---|---|---|---|---|
| Cu/Zn Superoxide dismutase | gi∣50978674 | 5 | 45 | 5.69,16074 | 69 | 1.3 ×10-7 |
| Fructose-Bisphosphate aldolase C | gi∣56748614 | 10 | 37 | 6.46,39665 | 108 | 1.6 ×10-11 |
| Creatine Kinase B Chain | gi∣320114 | 10 | 37 | 5.47,42960 | 115 | 3.2 ×10-12 |
| Glutamate dehydrogenase [NAD(P)] | gi∣2494097 | 10 | 20 | 7.66,61701 | 95 | 3.2 ×10-10 |
| glyceraldehyde-3-phosphate dehydrogenase (phosphorylating) | gi∣65987 | 8 | 23 | 6.90,35914 | 92 | 6.3 ×10-10 |
Table 5.
Canine brain proteins differentially expressed by different treatment paradigms
| Identified Protein | %Control ± S.E.M | CC vs. CE | CC vs. CA | CC vs. EA | P value |
|---|---|---|---|---|---|
| Cu/Zn SOD | 204 ± 44 | √ | √ | < 0.02 | |
| FBP | 139 ± 28 | √ | √ | < 0.03 | |
| CK | 171 ± 19 | √ | < 0.04 | ||
| GLUD | 152 ± 23 | √ | < 0.01 | ||
| GAPDH | 234 ± 42 | √ | √ | √ | < 0.05 |
Figure 5.
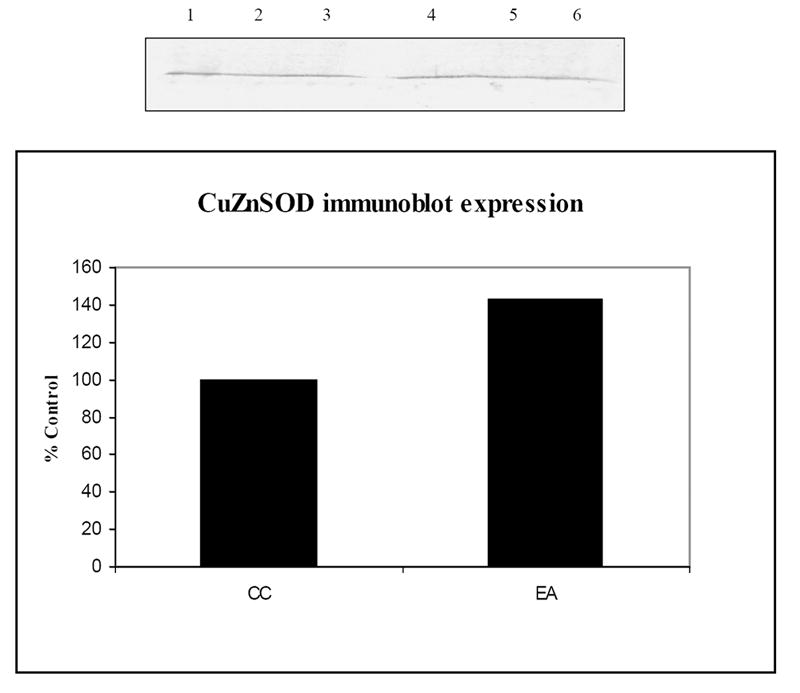
Validation of reduced oxidation of proteins (CuZnSOD) identified by proteomics. An immunoblot of the expression of Cu-Zn SOD, blots probed with anti CuZnSOD is shown. Lanes 1-3 represent CC, while lanes 4-6 represent EA. A graphical quantification of the blot also is shown. (n=3).
3.4 Enzyme activities
We hypothesized that in addition to reduced protein oxidation that antioxidant enzyme activity would be increased in response to treatment. We directly compared the CC animals with the EA animals for these experiments as they showed the largest difference in protein oxidation treatment effects.
3.4.1 Superoxide dismutase (SOD) and GST activity
We have previously shown that the oxidative modification of specific enzymes generally decreases their activity [82, 92]. Therefore, in the present study we hypothesized that since increased oxidation leads to loss of enzymatic activity, then protection from oxidative damage could restore or maintain the activity of enzymes with up-regulated expression levels. To test this hypothesis, we measured the activities of total SOD and GST. The activity of GST in aged canine brain isolated from dogs that had been treated long-term with antioxidants and a program of behavioral enrichment (EA) was found to be significantly (t (8) =3.3 p=0.011) increased by approximately 25% in aged EA animals compared to controls (Fig 6). The activity of a second antioxidant enzyme, superoxide dismutase (SOD) was also increased by approximately a 50% (Fig 6) in the aging canines after the combined treatment (EA) compared to controls (CC) (t (8) =2.29 p=0.05). This result is consistent with the hypothesis that oxidative modification of an enzyme leads to a loss or decrease in function and the reversal of this oxidative damage can restore the function of an enzyme [89].
Figure 6.
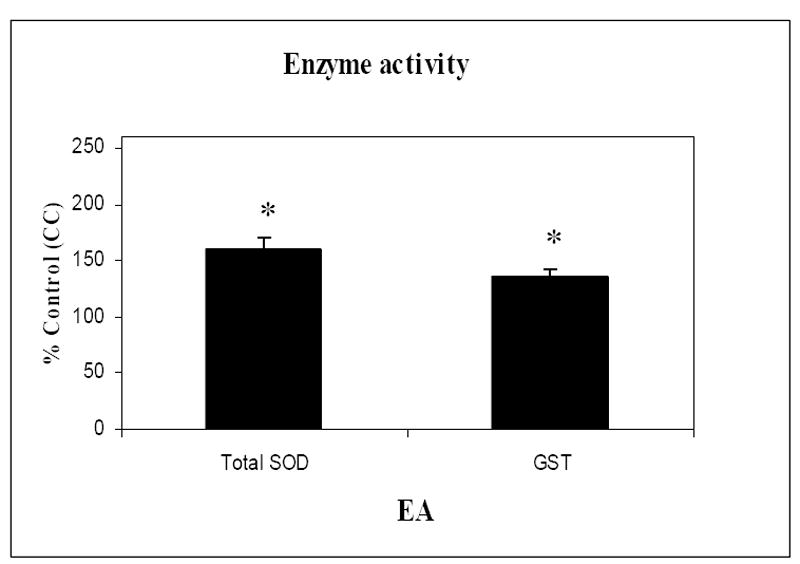
SOD and GST activity are significant increased in response to treatment in aged dogs. Dogs provided with the combination of an antioxidant-fortified diet and behavioral enrichment show significantly increased GST and total SOD enzyme activity relative to controls. Activities of GST and SOD are expressed as units per milligram of protein and data are presented as % control ± SEM for animals in each treatment group, (n=5) * p <0.05.
3.5 Induction of HO-1
Previous studies from our laboratory and others have shown induction of HO-1 at both gene and protein levels as a protective response to oxidative challenge [26, 105]. In the current study we observed a significant increase in expression of HO-1 following a program of behavioral enrichment and an antioxidant fortified diet in the parietal cortex of the aging canine (t (10)=5.17 p<.0005). Fig 7A shows the results of a Western immunoblot analysis of brain homogenates for HO-1 protein levels. Lanes 1-6 represent brains from the canines that underwent the combined treatment (EA), while lanes 7-12 represent age-matched control animals (CC). Fig 7 B presents the quantification of these blots. Thus, lower levels of oxidative stress may be linked in part to protection provided by increased protein levels of HO-1 in response to the fortified diet and behavioral enrichment program.
Figure 7.
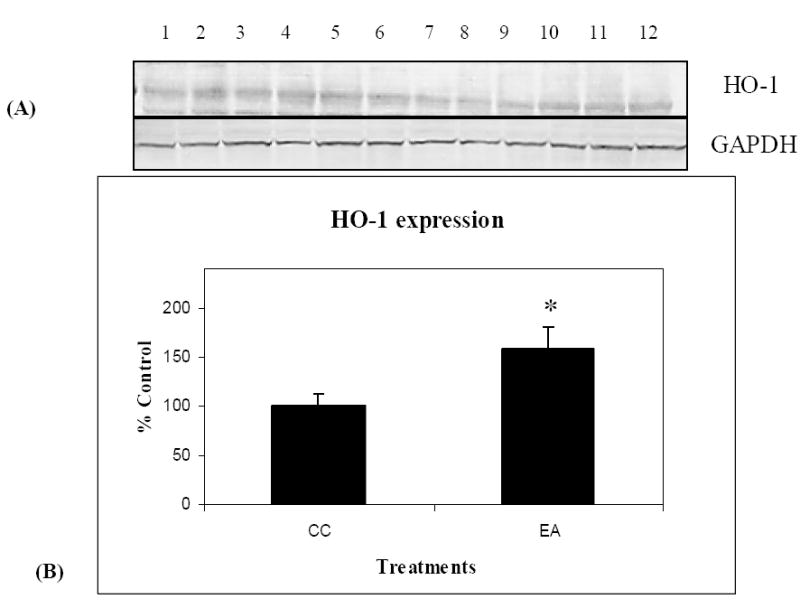
HO-1 protein levels increase in response to treatment in aged canines. Western immunoblot analysis and quantification of canine brain homogenates samples containing 50μg of protein loaded onto 10% SDS-PAGE gels were completed using an anti-HO-1 antibody. A representative immunoblot (A) with lanes (1-6) representing the treatment group EA, and lanes 7-12 representing the control group CC is shown. GAPDH was used as a control for equal loading of protein. Densitometric values are plotted as a function of treatment group (B) showing a significant increase in HO-1 expression following the combined treatment of enriched environment and antioxidant-fortified food (EA) compared to control (CC). GAPDH densitometric data are represented as % control; ± SEM for each group. (n=6), * p <0.05.
3.6 Correlation among protein expression levels, oxidative damage, and antioxidant status with cognitive function
To determine if error scores on individual cognitive tasks were associated with increased protein expression of CuZnSOD, FBP, CK, GLUD or GAPDH a correlational analysis was used. CuZnSOD protein level was negatively correlated with error scores on a black/white reversal task (Figure 8A) and on a spatial memory task (Figure 8B) with SOD levels, i.e. higher antioxidant protein level, being associated with lower error scores (improved cognition). Figure 8 shows the linear association between CuZnSOD protein and cognitive ability. Because age at death may also be a contributor to either increased error scores or increased protein expression, correlations were also computed and corrected for age. The significant association between CuZnSOD and cognition remained. Other protein measures (FBP, CK, GLUD, and GAPDH) did not correlate with cognitive scores.
Figure 8.
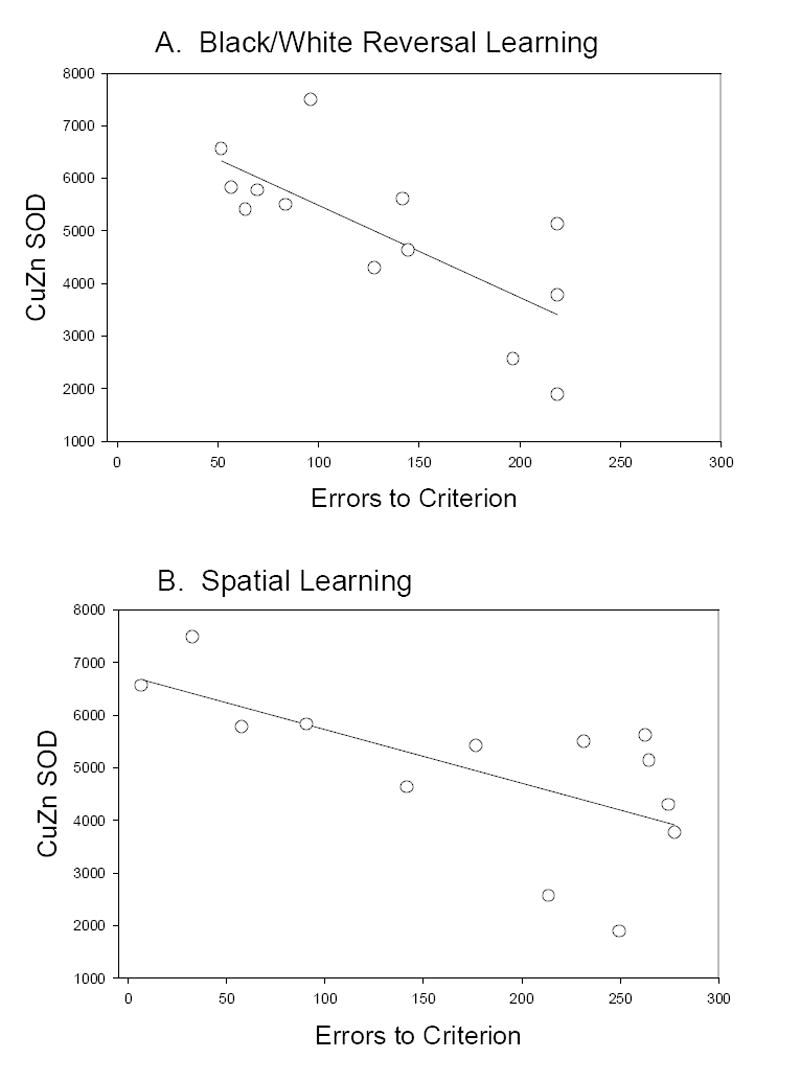
Individual error scores are plotted as a function of CuZnSOD protein levels in the parietal cortex. A. Black/white reversal learning was poorer in animals with lower levels of SOD. B. Spatial learning was also impaired in animals with lower SOD protein levels. Line represents the results of a linear regression analysis.
To determine if error scores on individual cognitive tasks were associated with reduced oxidative damage or increased antioxidant enzyme/protein levels a correlational analysis was used. Table 6 shows that, generally, higher error scores (i.e. poorer cognition) on tests of black/white discrimination, black/white reversal and spatial memory were associated with higher levels of oxidative damage. Correlations were significant for black/white reversal and 3-NT (Fig 9 A) and for 3-NT and spatial memory (Fig. 9 B). Overall, higher levels of antioxidant enzyme activity (SOD, GST) or higher protein levels of HO-1 were generally associated with lower error scores on all the tasks. These were statistically significant for GST and HO-1 (Fig 10 C, D) but not SOD, although all showed the same inverse relationship. Because age at death may also be a contributor to both increased error scores and increased oxidative damage, correlations were computed that corrected for age. The correlation between GST activity and black/white discrimination was significant (r=-0.81 p=0.05) and between black/white reversal learning and HO-1 protein levels was significant (r=-0.81 p=0.05).
Table 6.
Correlations between Cognition, Oxidative Damage and Antioxidant Status
| Cognitive Task | Protein Carbonyls | 3-NT | HNE | GR | SOD | GST | HO-1 |
|---|---|---|---|---|---|---|---|
| Black/White Discrimination | r=0.30 p=0.19, n=21 | r=0.38 p=0.09 n=21 | r=0.08 p=0.73 n=21 | r=-0.30 p=0.48 n=8 | r=-0.04 p=0.92 n=8 | r=-0.67 p=0.07 n=10 | r=-0.37 p=0.30 n=10 |
| Black/White Reversal | r=0.40 p=0.18 n=20 | r=0.43 p=0.05 n=21 | r=0.42 p=0.06 n=21 | r=-0.22 p=0.59 n=8 | r=-0.34 p=0.40 n=8 | r=-0.65 p=0.08 n=8 | r=-0.75 p=0.01 n=10 |
| Spatial Memory | r=0.31 p=0.18 n=20 | r=0.44 p=0.05 n=20 | r=0.32 p=0.17 n=20 | r=-0.26 p=0.53 n=8 | r=-0.20 p=0.64 n=8 | r=-0.16 p=0.71 n=8 | r=-0.61 p=0.06 n=10 |
Figure 9.
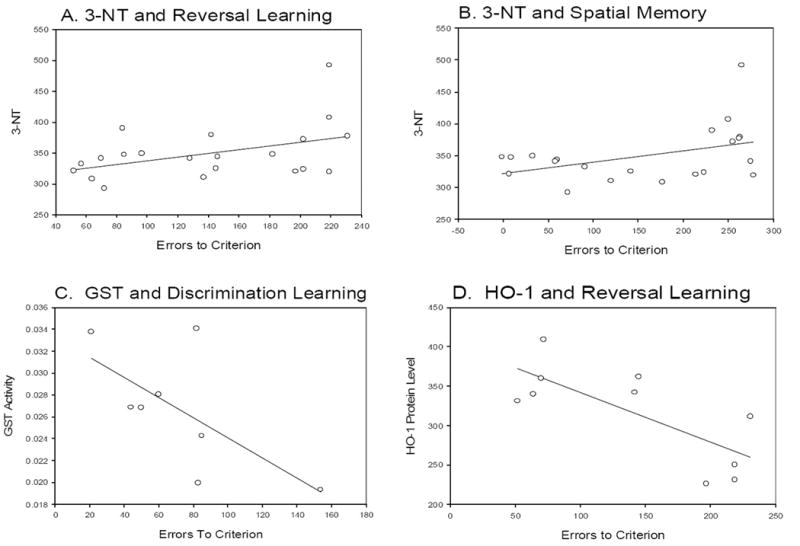
Association between cognitive test scores and measures of oxidative damage in treated animals. Shows error scores on individual cognitive tasks associated with reduced oxidative damage or increased antioxidant enzyme/protein levels. Higher error scores on tests of black/white discrimination, black/white reversal and spatial memory were associated with higher levels of oxidative damage. Higher error scores on a reversal learning task (A) and on a visuospatial memory task (B) were correlated with 3-NT. Discrimination learning ability was inversely associated with GST activity (C). Reversal learning error scores were also negatively associated with HO-1, with higher levels of HO-1 associated with better cognition (D).
Figure 10.
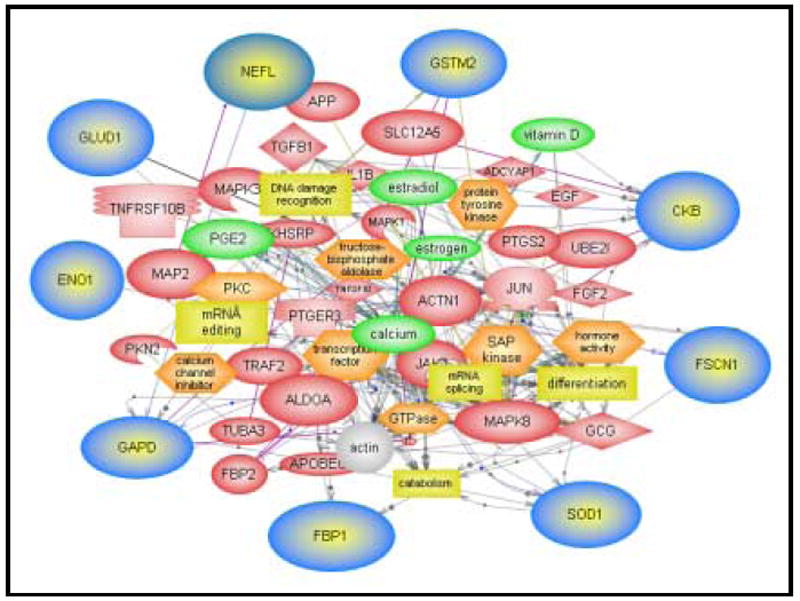
Schematic diagram of a functional interacteome of all parietal cortex proteins identified to be significantly less oxidatively modified following the combined treatment of the enriched environment and antioxidant-fortified food (EA). This diagram was generated by the interaction explorer ™ Pathway Module (Stratagene), indicating that all the proteins directly or indirectly is associated with cellular process shown.
A multiple stepwise regression was used to determine which measures of oxidative damage or antioxidant status best predicted cognitive dysfunction. Age at death was also included in the analysis. The best predictor of error scores on black/white discrimination learning was GST activity (F (1, 6) =14.31 p=0.013 r2=0.74), on black/white reversal learning was HO-1 (F (1, 6) =11.54 p=0.019 r2=0.70) and on spatial memory was age at death (F (1, 6)-7.22 p=0.044 r2=0.59). Thus, at least one significant explanatory variable for error scores on tasks administered within 1 year of euthanasia was antioxidant enzyme function.
3.8 Protein Interactome
Fig 10 shows the protein interactome of proteomics-identified proteins with decreased oxidation in response to the various intervention paradigms are illustrated by using Interaction Explorer Software PathwayAssist (Stratagene) software. The proteins identified in this study are related to hormone activities, transcription and regulation of signal transduction among others. As a result, the present findings continue to confirm and support previous findings [86, 89] that antioxidants and a program of behavioral enrichment provide beneficial effect of protection and improvement in cognitive functions and memory through the deceased oxidation and increased activity of key proteins
4.0 Discussion
Oxidative stress may be involved in the development of pathology leading to decline in memory and cognitive functions observed in AD and in other age-related neurodegenerative disorders [16-18, 56]. However, interventions with antioxidants delays age-related cognitive decline and improves performance in animal models of AD and other age-related neurodegenerative disorders [10, 47, 65]. The present study investigated the effect of an antioxidant-fortified diet and a program of behavioral enrichment on the levels of oxidative damage and in restoring antioxidant reserve systems in the aging canine brain. Four different treatments were compared (CC, CE, CA and EA) in 23 age-matched beagle dogs for a period of 2.8 yrs and markers of oxidative stress in the parietal cortex were analyzed. There was a reduction in the levels of brain 3NT and protein carbonyls assayed with all treatments, but only those in the combined treatment EA showed a significant reduction when compared to control. The levels of brain lipid peroxidation as measured by HNE were marginally reduced in all treatments, but none was significantly reduced compared to control. We also used redox proteomics to show that following the combined treatment EA, the aging canine shows less oxidation and increased expression of key brain proteins involved in energy metabolism, antioxidant systems, and in maintenance and stabilization of cell structure. In addition, there is a significant increase in the activity of antioxidant enzymes GST and SOD in the combined treatment EA when compared to control, and a significant increase in the expression of HO-1 protein, an important defense system in neurons under oxidative stress [27]. The significant decrease in oxidation and expression of some of these key brain proteins was also shown to correlate with improved cognitive function in the aged canines undergoing these interventions. These findings suggest possible mechanisms for the improved memory and cognitive function previous reported in the canine model of human aging [40, 79] and are discussed herein with relevance to AD.
In AD, the Aβ peptide plays a central role in the generation of free radicals and oxidative stress [16, 18, 55, 56]. In the aging canine, no significant correlation between the levels of Aβ deposition in brain and oxidative damage is observed [54], however, since the aging canine deposits the more toxic form of Aβ 1-42 as that seen in human aging [19, 74] and since Aβ load and decline in cognitive function events develop in parallel, Aβ could still play a significant role in the mechanism of oxidative stress observed in the aging canine [42, 43, 52]. In the peptide sequence of Aβ (1-42), there is a methionine-35 residue that our laboratory has shown to play a critical role in Aβ induced oxidative stress and neurotoxicity observed in AD [24]. We have proposed that the Aβ1-42 peptide, as a small oligomer, intercalates in the lipid bilayer in an alpha helix conformation. A one-electron oxidation of methionine forms the methionine sulfuranyl radical, which can then abstract a labile hydrogen atom from neighboring unsaturated lipids forming a carbon-centered lipid radical (L·). This radical, in turn, can react with molecular oxygen to from a peroxyl radical (LOO·). This peroxyl radical can abstract hydrogen from a neighboring lipid to form the lipid hydroperoxide LOOH and a carbon centered radical L·, which propagates the free radical chain reaction [24, 116]. It is this mechanism of free radical generation in the aging canine brain that we believe contributes to the increased levels of oxidative stress, leading to neurodegeneration and a decline in memory and cognitive function previously observed in the aging canine [54,77].
The use of dietary intervention with anti-oxidants or free radical quenchers and a regular program of behavioral enrichment (social, cognitive, environmental and physical exercise) is protective against oxidative damage, reduces oxidative stress, protects neurons and consequently improves cognitive function in human aging and in animal models [3, 10 27, 46, 65, 78]. In the present study, the fortified antioxidant diet included vitamin E and vitamin C, both well-known free radical quenchers. Vitamin E is lipid soluble, hence protects cell membranes from oxidative insults, while vitamin C protects the soluble phase of the cell and also regenerates the vitamin E from the vitamin E free radical [20]. However, recent studies in which vitamin C was not included, reported vitamin E did not inhibit the conversion of patients with mild cognitive impairment to AD [84]. As a result, the ability of vitamin E in protecting cell membranes provides one possible mechanism through which the fortified diet given to the aging canines provides protection from oxidative damage as seen by the decreased levels of lipid peroxidation assayed by HNE and previously seen to have been elevated as measured by malondialdehyde [54]. In addition, the inclusion of fruits and vegetables rich in flavonoids and carotenoids, could help in quenching the possible free radicals generated by Aβ, which as noted here is deposited in the aging canine brain [43, 53], leading to the low levels of protein oxidation as measured by protein carbonyls and 3NT observed in the present study. Further, there was a significant correlation between 3NT and spatial memory and black/white reversal learning indicating that there is a correlation between improved cognition and reduction in oxidative damage in the aging canine brain following the combined treatment with a diet fortified with antioxidants and a program of behavioral enrichment.
The behavioral enrichment program used in this study involved a regimen of extra physical exercise, enhanced environmental and social stimulation and cognitive training leading to cognitive improvement [80]. Exercise is reported to improve cognitive function, reduce the risk of developing cognitive impairment and reduce neuropathology in humans or in animal models [3,61, 69,115]. In aging dogs, behavioral enrichment leads to significant improvements in visual discrimination learning and frontal-dependent reversal learning [80]. The mechanism by which behavioral enrichment provides protection against oxidative damage is still unknown, but the current study provides new insights. Aging usually lowers the expression of antioxidant enzymes and stress protein expression. This loss can be modulated through interventions with diet or exercise [58, 62, 118]. One effect of the combined treatment EA was a significant increase in the expression of inducible heme oxygenase (HO-1) also known as HSP32. The heme oxygenase pathway is an important neuronal defense system in conditions of oxidative stress [37] and has been reported to be involved in oxidative stress-related neurodegenerative disorders, including AD [107]. In AD, for example, the expression of HO-1 is significantly altered and is up-regulated during oxidative stress, as well as by GSH depletion [28, 113]. In the same fashion, since the aging canine brain is under significant oxidative stress, we believe that this in itself could trigger a stress response leading to the altered transcription of key proteins or enzymes such as HO-1, which are involved in mechanisms for protection against oxidative damage [27]. Moreover, the use of an antioxidant fortified diet and a program of behavioral enrichment could also trigger this response thereby providing an additive effect. The induction of HO-1 catabolizes heme forming carbon monoxide (CO) and biliverdin and subsequently bilirubin, a potent antioxidant and anti-inflammatory agent [28]. In the aging canine increased oxidative stress and depletion of GSH is observed [54] and with interventions with an antioxidant diet and a program of behavioral enrichment, a perfect environment is created for the induction of HO-1 and other neuroprotective proteins. This in effect could provide an additional antioxidant, i.e. bilirubin, contributing to the decreased levels of oxidative damage and improvement in memory and cognitive function in the aging canine. The higher protein levels of HO-1 were also associated with lower error scores on individual cognitive tasks. This correlation was statistically significant even after correction for age at death. As a result HO-1 was one of the best predictors of error scores on black/white reversal learning, i.e., higher HO-1 protein levels were associated with improved cognitive function.
Supplementation of the diet in the present study with mitochondrial co-factors, could lead to more efficiently functioning mitochondria. A by-product of mitochondrial respiration is the generation of superoxide, which leaks from the mitochondria inducing more oxidative stress and damage. In the present study, we have shown that there is increased activity of total SOD, which would then provide protection against an increase in the production of superoxide, leading to a reduction in oxidative damage. On looking at the correlation between increased enzymatic activity and cognition, we found that though high levels of antioxidant activity were associated with lower error scores, though this correlation was not significant for SOD.
Using proteomics in the current study, we were also able to identify key brain proteins whose expression levels were increased and others that showed a significant reduction in the levels of oxidative damage following the combined treatment EA. These identified proteins were related to energy metabolism, antioxidant systems, and in the maintenance and stabilization of cell structure. We therefore believe that these proteins may be playing a significant role in the improved cognitive function observed in the aging canine undergoing this intervention.
Energy metabolism
Alpha enolase (ENO1) is a glycolytic enzyme that interconverts 2-phosphoglycerate to phosphoenolpyruvate and is one of the proteins recently identified to be significantly oxidatively modified in individuals with mild cognitive impairment (MCI) [25], which to some extent, the aged canine models [40, 44]. We have also shown that α-enolase is oxidatively modified in AD and in various models of neurodegenerative disorders [32, 82, 83, 90], indicating that this key protein is involved in several age-related neurodegenerative disorders. In addition, we have also shown that following caloric restriction in aging rats [93] and after treatment with lipoic acid in the SAMP8 mice [89], the specific carbonyl levels of α-enolase are significantly decreased leading us to believe that this protein may play a key role in the restoration of cognitive function. Consistent with this idea, our present findings show that following treatment with antioxidants and mitochondrial co-factors (including lipoic acid) and a program of behavioral enrichment in the aging canine, the specific carbonyl levels of α-enolase are significantly reduced.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is another glycolytic enzyme that catalyzes the oxidation of glyceraldehyde-3-phosphate to 1, 3-bisphosphoglycerate and NADH [38]. GAPDH can also act as a sensor for nitrosative stress [50]. Our laboratory has shown that GAPDH undergoes significant nitration, another form of oxidative modification, in the hippocampus of AD patients [106] and also in rats after intracerebral injection with Aβ (1-42) [13]. Interestingly, we have also shown that the use of gamma-glutamylcysteine ethyl ester (GCEE), a compound that leads to increased synthesis of glutathione in neuronal cell culture treated with Aβ (1-42), protects GAPDH against Aβ (1-42)-mediated protein oxidation [12]. In the present study we have also identified GAPDH as one of the proteins that is protected from oxidative damage and whose expression was significantly increased following a program of enriched environment and a diet of antioxidants following the combined treatment with antioxidants and behavioral enrichment. As a result, the decreased oxidation of GAPDH and α-enolase could lead to improved glycolytic function and increased ATP production and possible neuronal recovery and improved cognitive function as seen in the canine model of human aging.
Fructose bisphosphate aldolase C (FBP) is a glycolytic enzyme that catalyses the reversible aldol cleavage or condensation of fructose-1, 6-bisphosphate into dihydroxyacetone-phosphate and glyceraldehyde 3-phosphate [81]. In vertebrates, three forms of this enzyme are found: aldolase A is expressed in muscle, aldolase B in liver, kidney, stomach and intestine, and aldolase C in brain, heart and ovary. The different isozymes have different catalytic functions: aldolases A and C are mainly involved in glycolysis, while aldolase B is involved in both glycolysis and gluconeogenesis [81].
The creatine kinase (CK) system is the most important immediate energy buffering and transport system especially in muscle and neuronal tissue [117]. CK consists of a cytosolic and a mitochondrial isoform (MtCK) with their substrates creatine and phosphocreatine. Creatine is typically phosphorylated to phosphocreatine in the intermembrane space of mitochondria where mitochondrial CK is located and is then transported into the cytosol [95]. In the cytosol, the energy pool can be regenerated by transphosphorylation of phosphocreatine to ATP, which is catalyzed by cytosolic CK in close proximity to cellular ATPases. Moreover, the mitochondrial synthesis of creatine phosphate is restricted to uMiCK expressing neurons, suggesting uMiCK protects neurons under situations of compromised cellular energy state, which are often linked to oxidative stress and calcium overload through compensatory up-regulation of gene expression [11]. CKs are prime targets of oxidative damage. MtCK in particular is a principal target of such damage, not only because if its sensitivity [66, 100], but also due to its mitochondrial localization. Our laboratory has shown that though there is increased expression of CK in AD, it is significantly oxidized and its activity significantly reduced [7, 33]. In the brain of old brown Norway rats, CK is oxidatively modified and its activity significantly decreased [4]. Also in aging neuronal cultures, there is a gradual increase in CK content but decreased activity of the enzyme. These changes in CK expression have been considered to be an early indicator of oxidative stress in aging neurons [6]. In the present study, following exposure of the aging beagle dogs to a program of environmental enrichment and a diet fortified with anti-oxidants, we observed a significant increase in the expression of CK. This is in agreement with a previous study from our laboratory that showed a significant increase in the expression of CK in the senescence accelerated prone mouse strain 8 (SAMP8) mice after intervention with alpha-lipoic acid, a mitochondrial co-factor and antioxidant in the diet used in the present study [89]. This increased expression we posit is a compensatory mechanism for restoration of ATP production in the aging canine.
Maintenance and stabilization of the integrity of the cell structure
Neurofilament triplet L protein also known as NF68/NF-L is a subunit of neurofilaments (NFs), which give axons their structure and diameter [60]. In addition NFs are involved in cytoskeleton organization, neurogenesis and supports the neuronal architecture in the brain [92]. The protein levels of NF-L in brains of AD, Down syndrome, and ALS patients is signsificantly decreased [8, 9], suggesting that normal NF-L expression could be critical to central nervous system (CNS) function. Oxidation or nitration of neurofilament (NF) proteins transform the α-helix secondary structure to β-sheet and random coil conformations, destabilizing the interactions between the NF proteins and resulting in axonal damage [41] and CNS dysfunction. We have previously shown that NF68 was significantly oxidized in the brain of the gracile axonal dystrophy (gad) mouse [35]. NF-66 (α-internexin) another family of the NF’s is also significantly oxidized in the brains of old versus young mice [92]. In the SAMP 8 mice, following treatment with alpha lipoic acid, we have observed a significant increase in the expression of NF-68, and since alpha lipoic acid treated-SAMP8 aged mice have improved learning and memory, this protein could be important for brain function [89]. In the present study we established that the levels of protein oxidation for neurofilament triplet L protein were decreased following interventions with antioxidants and a program of behavioral enrichment in aging dogs.
Another cytoskeleton related protein identified to be less oxidized in this study is Fascin. Fascin, a 55kD globular protein, is an actin bundling protein responsible for organizing F-actin into well-ordered, tightly packed parallel bundles in vitro and in cells [1]. It is also known to be one of the core actin bundling protein of dendrites among other structures [2]. Fascins function in the organization of two major forms of actin-based structures: dynamic, cortical cell protrusions and cytoplasmic microfilament bundles [67]. Cell protrusions in the plasma membrane sense the cellular environment, provide cell adhesion in the extracellular matrix and act in cellular migration [1]. These cell protrusions usually require a rigid cytoskeleton to support the localized extension of the plasma membrane. Formation of these structures is highly regulated by extracellular and intracellular signals, with a key point of regulation being the binding of fascin to filamentous actin (F-actin) [1]. Alterations in the expression of fascin are associated with disorders such as cardiovascular diseases and in various carcinomas among others, [1, 2, 67]. Fascin was one of the brain proteins identified in the aged canine undergoing treatment with an antioxidant diet and a program of behavioral to be less oxidized. As a result, the identification of NFL and fascin as less oxidized following the combined treatment would possibly lead to a decrease in axonal dystrophy [87], increased cellular migration, cell adhesion and communication, leading to improved neuronal communication and survival and particularly possibly leading to improved memory and cognitive function previously seen in the aging canine. However since the role of fascin in aging or neurodegenerative disorders is not known, the beneficial role of its reduced oxidation and the role it plays in cognitive function remain speculative.
Antioxidant and Cellular detoxification
Cu/Zn Superoxide dismutase (CuZnSOD, SOD1 protein) is an abundant copper- and zinc-containing protein that is present in the cytosol, nucleus, peroxisomes, and mitochondrial intermembrane space of human cells and acts as an antioxidant enzyme by lowering the steady-state concentration of superoxide [97]. When mutated, SOD can also cause disease as in the case of the neurodegenerative disorder, familial amyotrophic lateral sclerosis (fALS) [97]. The toxic gain of function of mutant SOD (mSOD) leads to the generation of reactive oxygen/nitrogen species [83, 91, 114]. Some researchers believe that the elevated oxidative activity associated with mSOD occurs by enzymes acting as peroxidases [114] or as superoxide reductases [70] or by producing O2·− to form peroxynitrite [94]. In the wild type form, SOD dismutates superoxide to oxygen and water, hence reducing the levels of oxidative stress and protecting proteins, lipid and DNA from the toxic superoxide molecule [48]. In the present study a significant increase in the expression of SOD1 and significant increase in SOD enzymatic activity in the brain from canines that had undergone a combination of both treatment with antioxidant diet and a program of behavioral enrichment compared to age matched controls were found
Glutathione-S-transferase (GST) catalyzes the conjugation of a number of exogenous and endogenous compounds such as 4-hydroxynonenal (HNE) or malondialdehyde (MDA) with glutathione inactivating the toxic products of oxygen metabolism [98]. Hence, GST plays a critical role in cellular protection against oxidative stress. There is a significant decline in the activity of GST in the amygdala, hippocampus and inferior parietal lobule of patients with AD [72], contributing to the accumulation of toxic effects of HNE and related compounds. Our laboratory has previously shown that in the AD brain, GST and multidrug resistant protein MRP1 are oxidatively modified leading to an impairment of detoxification mechanisms causing increased oxidative stress consistent with elevated HNE in AD [104]. In the aged canine, there is a overall decrease in GSH content and a significant increase in the lipid peroxidation product, MDA [54]. In the present study we show that following the combined treatments of an antioxidant fortified diet and a program of behavioral enrichment, GST was less oxidized. In addition, we also show that the activity of GST is significantly increased. This would potentially enhance the clearance of toxic aldehydes leading to improved memory and cognitive function in aging dogs. The higher activity of GST was also associated with lower error scores on individual cognitive tasks. This correlation was statistically significant even after correction for age at death. Further, increased GST activity was the best predictor of error scores on black/white discrimination learning, thus providing a possible mechanism underlying improved cognitive function following treatment in the aging canine with a diet fortified with antioxidants and a program of behavioral enrichment.
Glutamate dehydrogenase (GDH) is an enzyme located in the mitochondrial matrix that acts in both catabolic and metabolic pathways. GDH can catalyze the reductive amination of α-ketoglutarate with NADPH to yield glutamate in the metabolic pathway and can also catalyze the formation of α-ketoglutarate from glutamate with NAD+ and ammonium ion in the catabolic pathway [14]. The latter pathway is particularly important in eliminating the excitotoxin glutamate. Excess glutamate can stimulate NMDA receptors leading to an increase in Ca2+ influx and altered calcium homeostasis, which would lead to alteration in long-term potentiation (LTP) and consequently, learning and memory deficits as seen in AD [14]. We have shown in the present study that following treatment with antioxidants and a program of behavioral enrichment in the canine model of human aging, there is a decrease in the specific carbonyl levels of GDH. This would possibly lead to an increase in its activity, and more importantly its metabolic activity thereby helping to clear excess glutamate in the synaptic cleft. Consequently, this may lead to controlled Ca2+ homeostasis, improved LTP, and eventually improvement in cognitive function as observed in the canine model of human aging following interventions with antioxidants and a program of behavioral enrichment.
The present study provides additional evidence that oxidative stress may be a key mechanisms contributing to decline in memory and cognitive function with age. We have shown that a diet fortified with antioxidants in combination with a program of behavioral enrichment is capable of reducing the levels of oxidative damage, and increasing the activity and expression of key endogenous antioxidant enzymes in the aging canine brain. Increased protein expression does not necessarily directly translate to increased enzyme activity as reported for creatine kinase in AD [5]; however, we have shown here both an increase in expression and activity of Cu/Zn SOD in response to treatment. In addition, this increase in protein levels was found to be a good predictor of frontal cortex-dependent learning and a measure of spatial memory. Using the Interaction Explorer Software PathwayAssist (Stratagene) to analyze our current results as shown in Fig 10, the proteins identified in this study can be divided into three functional categories: those related to energy metabolism, antioxidant systems and maintenance, and stabilization of cell structure. The present findings therefore provide a neurobiological basis for improved neuronal function and cognition in canines treated with either or both an antioxidant enriched diet and behavioral enrichment. The inclusion of vitamin E, alpha-lipoic acid L-carnitine flavanoids in the diet not only provide improvements in antioxidant reserves but also plays a role in increasing the expression of key energy metabolism proteins that help in the maintenance of ATP levels, maintenance of cellular pathways and functions dependent on ATP eventually leading to an improvement in cognitive function. As a result of the reduction in the levels of oxidative stress/damage following this intervention, we have also established that key brain proteins associated with energy metabolism, antioxidant systems, and with the maintenance and stabilization of cell structure are protected from oxidative damage. This we believe would lead to improved activity or function consequently leading to the improved memory and cognitive function observed in the aging canine. Further we have also shown that there is a strong correlation between the increased in expression/activity of some of the identified proteins and improved cognitive function. Therefore the present study provides possible mechanisms through which the aging canine, provided with the combined intervention of an antioxidant fortified diet and a program of behavioral enrichment, shows improvements in cognitive function [78, 80]. Further, the increased expression of HO-1, increased activity of GST and SOD together could all have synergistic effects in the reduction of oxidative damage and protection of key proteins from oxidative damage observed in this study. Results from the current study in aging canines may be translatable to humans, providing a possible intervention for Aβ induced cognitive decline observed in AD.
Acknowledgments
This work was supported in part by grants from NIH to D.A.B. [AG-05119; AG-10836], and NIH to C.W.C [AG12694]. We thank Ms. Mollie Fraim for assistance in preparation of this manuscript.
Footnotes
Disclosure Statement: All authors have no conflicts of interests; neither the University of Kentucky nor the University of California at Irvine, the University of Toronto, the University of Louisville, or the Lovelace RR Institute have contracts on this research through which either institution can financially benefit; there are no agreements of any of the authors or their institutions that could be construed as a conflict of interest. Funding for this research was provided by NIH to D.A.B. [AG-05119; AG-10836] and to C.W.C. [AG12694]. Data in this submission have not been published or submitted elsewhere. All authors have reviewed the contents of this MS, approve of its contents, and validate the accuracy of the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams JC. Fascin protrusions in cell interactions. Trends Cardiovasc Med. 2004;14(6):221–6. doi: 10.1016/j.tcm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Adams JC. Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol. 2004;16(5):590–6. doi: 10.1016/j.ceb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25(17):4217–21. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aksenova MV, Aksenov MY, Carney JM, Butterfield DA. Protein oxidation and enzyme activity decline in old brown Norway rats are reduced by dietary restriction. Mech Ageing Dev. 1998;100(2):157–68. doi: 10.1016/s0047-6374(97)00133-4. [DOI] [PubMed] [Google Scholar]
- 5.Aksenov MY, Aksenova MV, Markesbery WR, Butterfield DA. Amyloid beta-peptide (1-40)-mediated oxidative stress in cultured hippocampal neurons. Protein carbonyl formation, CK BB expression, and the level of Cu, Zn, and Mn SOD mRNA. J Mol Neurosci. 1998;10(3):181–92. doi: 10.1007/BF02761773. [DOI] [PubMed] [Google Scholar]
- 6.Aksenova MV, Aksenov MY, Payne RM, Trojanowski JQ, Schmidt ML, Carney JM, Butterfield DA, Markesbery WR. Oxidation of cytosolic proteins and expression of creatine kinase BB in frontal lobe in different neurodegenerative disorders. Dement Geriatr Cogn Disord. 1999;10(2):158–65. doi: 10.1159/000017098. [DOI] [PubMed] [Google Scholar]
- 7.Aksenov M, Aksenova M, Butterfield DA, Markesbery WR. Oxidative modification of creatine kinase BB in Alzheimer’s disease brain. J Neurochem. 2000;74(6):2520–7. doi: 10.1046/j.1471-4159.2000.0742520.x. [DOI] [PubMed] [Google Scholar]
- 8.Bajo M, Yoo BC, Cairns N, Gratzer M, Lubec G. Neurofilament proteins NF-L, NF-M and NF-H in brain of patients with Down syndrome and Alzheimer’s disease. Amino Acids. 2001;21(3):293–301. doi: 10.1007/s007260170015. [DOI] [PubMed] [Google Scholar]
- 9.Bergeron C, Beric-Maskarel K, Muntasser S, Weyer L, Somerville MJ, Percy ME. Neurofilament light and polyadenylated mRNA levels are decreased in amyotrophic lateral sclerosis motor neurons. J Neuropathol Exp Neurol. 1994;53(3):221–30. doi: 10.1097/00005072-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Bickford PC, Gould T, Briederick L, Chadman K, Pollock A, Young D, Shukitt-Hale B, Joseph J. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000;866(12):211–7. doi: 10.1016/s0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- 11.Boero J, Qin W, Cheng J, Woolsey TA, Strauss AW, Khuchua Z. Restricted neuronal expression of ubiquitous mitochondrial creatine kinase: changing patterns in development and with increased activity. Mol Cell Biochem. 2003;244(12):69–76. [PubMed] [Google Scholar]
- 12.Boyd-Kimball D, Sultana R, Poon HF, Mohmmad-Abdul H, Lynn BC, Klein JB, Butterfield DA. Gamma-glutamylcysteine ethyl ester protection of proteins from Abeta(1-42)-mediated oxidative stress in neuronal cell culture: a proteomics approach. J Neurosci Res. 2005;79(5):707–13. doi: 10.1002/jnr.20393. [DOI] [PubMed] [Google Scholar]
- 13.Boyd-Kimball D, Sultana R, Poon HF, Lynn BC, Casamenti F, Pepeu G, Klein JB, Butterfield DA. Proteomic identification of proteins specifically oxidized by intracerebral injection of amyloid beta-peptide (1-42) into rat brain: implications for Alzheimer’s disease. Neuroscience. 2005;132(2):313–24. doi: 10.1016/j.neuroscience.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Boyd-Kimball D, Castegna A, Sultana R, Poon HF, Petroze R, Lynn BC, Klein JB, Butterfield DA. Proteomic identification of proteins oxidized by Abeta(1-42) in synaptosomes: implications for Alzheimer’s disease. Brain Res. 2005;1044(2):206–15. doi: 10.1016/j.brainres.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 15.Butterfield DA, Stadtman ER. Protein oxidation processes in aging brain. Adv Cell Aging Gerontol. 1997;2:161–191. [Google Scholar]
- 16.Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7(12):548–54. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 17.Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122(9):945–62. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 18.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32(11):1050–60. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 19.Butterfield DA, Griffin S, Munch G, Pasinetti GM. Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer’s disease brain exists. J Alzheimers Dis. 2002;4(3):193–201. doi: 10.3233/jad-2002-4309. [DOI] [PubMed] [Google Scholar]
- 20.Butterfield DA, Castegna A, Drake J, Scapagnini G, Calabrese V. Vitamin E and neurodegenerative disorders associated with oxidative stress. Nutr Neurosci. 2002;5(4):229–39. doi: 10.1080/10284150290028954. [DOI] [PubMed] [Google Scholar]
- 21.Butterfield DA, Boyd-Kimball D, Castegna A. Proteomics in Alzheimer’s disease: insights into potential mechanisms of neurodegeneration. J Neurochem. 2003;86(6):1313–27. doi: 10.1046/j.1471-4159.2003.01948.x. [DOI] [PubMed] [Google Scholar]
- 22.Butterfield DA. Proteomics: a new approach to investigate oxidative stress in Alzheimer’s disease brain. Brain Res. 2004;1000(12):1–7. doi: 10.1016/j.brainres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Butterfield DA, Boyd-Kimball D. Amyloid beta-peptide(1-42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol. 2004;14(4):426–32. doi: 10.1111/j.1750-3639.2004.tb00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butterfield DA, Boyd-Kimball D. The critical role of methionine 35 in Alzheimer’s amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity. Biochim Biophys Acta. 2005;1703(2):149–56. doi: 10.1016/j.bbapap.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: Insights into the development of Alzheimer’s disease. Neurobiol Dis. 2006;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Calabrese V, Scapagnini G, Ravagna A, Colombrita C, Spadaro F, Butterfield DA, Giuffrida Stella AM. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech Ageing Dev. 2004;125(4):325–35. doi: 10.1016/j.mad.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Calabrese V, Butterfield DA, Stella AM. Nutritional antioxidants and the heme oxygenase pathway of stress tolerance: novel targets for neuroprotection in Alzheimer’s disease. Ital J Biochem. 2003;52(4):177–81. [PubMed] [Google Scholar]
- 28.Calabrese V, Stella AM, Butterfield DA, Scapagnini G. Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brain stress tolerance. Antioxid Redox Signal. 2004;6(5):895–913. doi: 10.1089/ars.2004.6.895. [DOI] [PubMed] [Google Scholar]
- 29.Calabrese V, Giuffrida Stella AM, Calvani M, Butterfield DA. Acetylcarnitine and cellular stress response: roles in nutritional redox homeostasis and regulation of longevity genes. J Nutr Biochem. 2006;17(2):73–88. doi: 10.1016/j.jnutbio.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Callahan H, Ikeda-Douglas C, Head E, Cotman CW, Milgram NW. Development of a protocol for studying object recognition memory in the dog. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2000;24(5):693–707. doi: 10.1016/s0278-5846(00)00102-0. [DOI] [PubMed] [Google Scholar]
- 31.Cao G, Verdon CP, Wu AH, Wang H, Prior RL. Automated assay of oxygen radical absorbance capacity with the COBAS FARA II. Clin Chem. 1995;41(12 Pt 1):1738–44. [PubMed] [Google Scholar]
- 32.Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002;82(6):1524–32. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 33.Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002;33(4):562–71. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- 34.Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J Neurochem. 2003;85(6):1394–401. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 35.Castegna A, Thongboonkerd V, Klein J, Lynn BC, Wang YL, Osaka H, Wada K, Butterfield DA. Proteomic analysis of brain proteins in the gracile axonal dystrophy (gad) mouse, a syndrome that emanates from dysfunctional ubiquitin carboxyl-terminal hydrolase L-1, reveals oxidation of key proteins. J Neurochem. 2004;88(6):1540–6. doi: 10.1046/j.1471-4159.2003.02288.x. [DOI] [PubMed] [Google Scholar]
- 36.Chan AD, Nippak PM, Murphey H, Ikeda-Douglas CJ, Muggenburg B, Head E, Cotman CW, Milgram NW. Visuospatial impairments in aged canines (Canis familiaris): the role of cognitive-behavioral flexibility. Behav Neurosci. 2002;116(3):443–54. [PubMed] [Google Scholar]
- 37.Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75(1):304–13. doi: 10.1046/j.1471-4159.2000.0750304.x. [DOI] [PubMed] [Google Scholar]
- 38.Chuang DM, Hough C, Senatorov VV. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2005;45:269–90. doi: 10.1146/annurev.pharmtox.45.120403.095902. [DOI] [PubMed] [Google Scholar]
- 39.Conrad CC, Choi J, Malakowsky CA, Talent JM, Dai R, Marshall P, Gracy RW. Identification of protein carbonyls after two-dimensional electrophoresis. Proteomics. 2001;1(7):829–34. doi: 10.1002/1615-9861(200107)1:7<829::AID-PROT829>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 40.Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging. 2002;23(5):809–18. doi: 10.1016/s0197-4580(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 41.Crow JP, Ye YZ, Strong M, Kirk M, Barnes S, Beckman JS. Superoxide dismutase catalyzes nitration of tyrosines by peroxynitrite in the rod and head domains of neurofilament-L. J Neurochem. 1997;69(5):1945–53. doi: 10.1046/j.1471-4159.1997.69051945.x. [DOI] [PubMed] [Google Scholar]
- 42.Cummings BJ, Su JH, Cotman CW, White R, Russell MJ. Beta-amyloid accumulation in aged canine brain: a model of early plaque formation in Alzheimer’s disease. Neurobiol Aging. 1993;14(6):547–60. doi: 10.1016/0197-4580(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 43.Cummings BJ, Head E, Afagh AJ, Milgram NW, Cotman CW. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learn Mem. 1996;66(1):11–23. doi: 10.1006/nlme.1996.0039. [DOI] [PubMed] [Google Scholar]
- 44.Cummings BJ, Head E, Ruehl W, Milgram NW, Cotman CW. The canine as an animal model of human aging and dementia. Neurobiol Aging. 1996;17(2):259–68. doi: 10.1016/0197-4580(95)02060-8. [DOI] [PubMed] [Google Scholar]
- 45.Drake J, Sultana R, Aksenova M, Calabrese V, Butterfield DA. Elevation of mitochondrial glutathione by gamma-glutamylcysteine ethyl ester protects mitochondria against peroxynitrite-induced oxidative stress. J Neurosci Res. 2003;74(6):917–27. doi: 10.1002/jnr.10810. [DOI] [PubMed] [Google Scholar]
- 46.Eckles-Smith K, Clayton D, Bickford P, Browning MD. Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain Res Mol Brain Res. 2000;78(12):154–62. doi: 10.1016/s0169-328x(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 47.Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84(5):1173–83. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 48.Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci. 2000;899:136–47. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 49.Habig WH, Jakoby WB. Glutathione S-transferases (rat and human) Methods Enzymol. 1981;77:218–31. doi: 10.1016/s0076-6879(81)77029-0. [DOI] [PubMed] [Google Scholar]
- 50.Hara MR, Cascio MB, Sawa A. GAPDH as a sensor of NO stress. Biochim Biophys Acta. 2006;1762(5):502–9. doi: 10.1016/j.bbadis.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 1998;19(5):415–25. doi: 10.1016/s0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 52.Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 2000;21(1):89–96. doi: 10.1016/s0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- 53.Head E, Torp R. Insights into Abeta and presenilin from a canine model of human brain aging. Neurobiol Dis. 2002;9(1):1–10. doi: 10.1006/nbdi.2002.0476. [DOI] [PubMed] [Google Scholar]
- 54.Head E, Liu J, Hagen TM, Muggenburg BA, Milgram NW, Ames BN, Cotman CW. Oxidative damage increases with age in a canine model of human brain aging. J Neurochem. 2002;82(2):375–81. doi: 10.1046/j.1471-4159.2002.00969.x. [DOI] [PubMed] [Google Scholar]
- 55.Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, Butterfield DA. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91(8):3270–4. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Adsenova M, Gabbita SP, Wu JF, Carney JM, Lovell M, Markesbery WR, Butterfield DA. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 57.Hensley K, Butterfield DA, Hall N, Cole P, Subramaniam R, Mark R, Mattson MP, Markesbery WR, Harris ME, Aksenov M, et al. Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer’s disease-associated amyloid beta peptide. Ann N Y Acad Sci. 1996;786:120–34. doi: 10.1111/j.1749-6632.1996.tb39057.x. [DOI] [PubMed] [Google Scholar]
- 58.Heydari AR, Wu B, Takahashi R, Strong R, Richardson A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol Cell Biol. 1993;13(5):2909–18. doi: 10.1128/mcb.13.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodges PE, Carrico PM, Hogan JD, O’Neill KE, Owen JJ, Mangan M, Davis BP, Brooks JE, Garrels JI. Annotating the human proteome: the Human Proteome Survey Database (HumanPSD) and an in-depth target database for G protein-coupled receptors (GPCR-PD) from Incyte Genomics. Nucleic Acids Res. 2002;30(1):137–41. doi: 10.1093/nar/30.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A. 1987;84(10):3472–6. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging. 1999;14(2):245–63. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- 62.Ji LL. Exercise-induced modulation of antioxidant defense. Ann N Y Acad Sci. 2002;959:82–92. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 63.Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP. Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res. 1991;10(4):299–305. doi: 10.1016/0169-328x(91)90088-f. [DOI] [PubMed] [Google Scholar]
- 64.Joseph JA, Denisova N, Fisher D, Bickford P, Prior R, Cao G. Age-related neurodegeneration and oxidative stress: putative nutritional intervention. Neurol Clin. 1998;16(3):747–55. doi: 10.1016/s0733-8619(05)70092-x. [DOI] [PubMed] [Google Scholar]
- 65.Joseph JA, Shukitt-Hale B, Denisova NA, Prior RL, Cao G, Martin A, Taglialatela G, Bickford PC. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J Neurosci. 1998;18(19):8047–55. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koufen P, Stark G. Free radical induced inactivation of creatine kinase: sites of interaction, protection, and recovery. Biochim Biophys Acta. 2000;1501(1):44–50. doi: 10.1016/s0925-4439(00)00005-3. [DOI] [PubMed] [Google Scholar]
- 67.Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function. Bioessays. 2002;24(4):350–61. doi: 10.1002/bies.10070. [DOI] [PubMed] [Google Scholar]
- 68.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1-42. J Neurochem. 2001;78(2):413–6. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 69.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 70.Liochev SI, Fridovich I. Copper- and zinc-containing superoxide dismutase can act as a superoxide reductase and a superoxide oxidase. J Biol Chem. 2000;275(49):38482–5. doi: 10.1074/jbc.M007891200. [DOI] [PubMed] [Google Scholar]
- 71.Lovell MA, Ehmann WD, Butler SM, Markesbery WR. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology. 1995;45(8):1594–601. doi: 10.1212/wnl.45.8.1594. [DOI] [PubMed] [Google Scholar]
- 72.Lovell MA, Xie C, Markesbery WR. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer’s disease. Neurology. 1998;51(6):1562–6. doi: 10.1212/wnl.51.6.1562. [DOI] [PubMed] [Google Scholar]
- 73.Lovell MA, Gabbita SP, Markesbery WR. Increased DNA oxidation and decreased levels of repair products in Alzheimer’s disease ventricular CSF. J Neurochem. 1999;72(2):771–6. doi: 10.1046/j.1471-4159.1999.0720771.x. [DOI] [PubMed] [Google Scholar]
- 74.Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23(1):134–47. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 75.Maurer MH, Feldmann RE, Jr, Bromme JO, Kalenka A. Comparison of statistical approaches for the analysis of proteome expression data of differentiating neural stem cells. J Proteome Res. 2005;4(1):96–100. doi: 10.1021/pr049841l. [DOI] [PubMed] [Google Scholar]
- 76.Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Shoffner JM, Wallace DC, Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993;34(4):609–16. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- 77.Milgram NW, Adams B, Callahan H, Head E, Mackay B, Thirlwell C, Cotman CW. Landmark discrimination learning in the dog. Learn Mem. 1999;6(1):54–61. [PMC free article] [PubMed] [Google Scholar]
- 78.Milgram NW, Zicker SC, Head E, Muggenburg BA, Murphey H, Ikeda-Douglas C, Cotman CW. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiology of Aging. 2002;23:737–745. doi: 10.1016/s0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- 79.Milgram NW, Head E, Zicker SC, Ikeda-Douglas C, Murphey H, Muggenberg BA, Siwak CT, Tapp PD, Lowry SR, Cotman CW. Long-term treatment with antioxidants and a program of behavioral enrichment reduces age-dependent impairment in discrimination and reversal learning in beagle dogs. Exp Gerontol. 2004;39(5):753–65. doi: 10.1016/j.exger.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 80.Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, Muggenburg B, Siwak C, Tapp D, Cotman CW. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol Aging. 2005;26(1):77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 81.Perham RN. The fructose-1,6-bisphosphate aldolases: same reaction, different enzymes. Biochem Soc Trans. 1990;18(2):185–7. doi: 10.1042/bst0180185. [DOI] [PubMed] [Google Scholar]
- 82.Perluigi M, Poon HF, Maragos W, Pierce WM, Klein JB, Calabrese V, Cini C, De Marco C, Butterfield DA. Proteomic analysis of protein expression and oxidative modification in r6/2 transgenic mice: a model of Huntington disease. Mol Cell Proteomics. 2005;4(12):1849–61. doi: 10.1074/mcp.M500090-MCP200. [DOI] [PubMed] [Google Scholar]
- 83.Perluigi M, Fai Poon H, Hensley K, Pierce WM, Klein JB, Calabrese V, De Marco C, Butterfield DA. Proteomic analysis of 4-hydroxy-2-nonenal-modified proteins in G93A-SOD1 transgenic mice--a model of familial amyotrophic lateral sclerosis. Free Radic Biol Med. 2005;38(7):960–8. doi: 10.1016/j.freeradbiomed.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 84.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 85.Pocernich CB, Butterfield DA. Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer’s disease. Neurotox Res. 2003;5(7):515–20. doi: 10.1007/BF03033161. [DOI] [PubMed] [Google Scholar]
- 86.Poon HF, Joshi G, Sultana R, Farr SA, Banks WA, Morley JE, Calabrese V, Butterfield DA. Antisense directed at the Abeta region of APP decreases brain oxidative markers in aged senescence accelerated mice. Brain Res. 2004;1018(1):86–96. doi: 10.1016/j.brainres.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 87.Poon HF, Castegna A, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, Morley JE, Klein JB, Butterfield DA. Quantitative proteomics analysis of specific protein expression and oxidative modification in aged senescence-accelerated-prone 8 mice brain. Neuroscience. 2004;126(4):915–26. doi: 10.1016/j.neuroscience.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 88.Poon HF, Vaishnav RA, Butterfield DA, Getchell ML, Getchell TV. Proteomic identification of differentially expressed proteins in the aging murine olfactory system and transcriptional analysis of the associated genes. J Neurochem. 2005;94(2):380–92. doi: 10.1111/j.1471-4159.2005.03215.x. [DOI] [PubMed] [Google Scholar]
- 89.Poon HF, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, Morley JE, Klein JB, Butterfield DA. Proteomic analysis of specific brain proteins in aged SAMP8 mice treated with alpha-lipoic acid: implications for aging and age-related neurodegenerative disorders. Neurochem Int. 2005;46(2):159–68. doi: 10.1016/j.neuint.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Poon HF, Frasier M, Shreve N, Calabrese V, Wolozin B, Butterfield DA. Mitochondrial associated metabolic proteins are selectively oxidized in A30P alpha-synuclein transgenic mice--a model of familial Parkinson’s disease. Neurobiol Dis. 2005;18(3):492–8. doi: 10.1016/j.nbd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 91.Poon HF, Hensley K, Thongboonkerd V, Merchant ML, Lynn BC, Pierce WM, Klein JB, Calabrese V, Butterfield DA. Redox proteomics analysis of oxidatively modified proteins in G93A-SOD1 transgenic mice--a model of familial amyotrophic lateral sclerosis. Free Radic Biol Med. 2005;39(4):453–62. doi: 10.1016/j.freeradbiomed.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 92.Poon HF, Vaishnav RA, Getchell TV, Getchell ML, Butterfield DA. Quantitative proteomics analysis of differential protein expression and oxidative modification of specific proteins in the brains of old mice. Neurobiol Aging. 2006;27:1010–1019. doi: 10.1016/j.neurobiolaging.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 93.Poon HF, Shepherd HM, Reed TT, Calabrese V, Stella AM, Pennisi G, Cai J, Pierce WM, Klein JB, Butterfield DA. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: Mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol Aging. 2006;27:1020–1034. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 94.Rakhit R, Cunningham P, Furtos-Matei A, Dahan S, Qi XF, Crow JP, Cashman NR, Kondejewski LH, Chakrabartty A. Oxidation-induced misfolding and aggregation of superoxide dismutase and its implications for amyotrophic lateral sclerosis. J Biol Chem. 2002;277(49):47551–6. doi: 10.1074/jbc.M207356200. [DOI] [PubMed] [Google Scholar]
- 95.Schlattner U, Forstner M, Eder M, Stachowiak O, Fritz-Wolf K, Wallimann T. Functional aspects of the X-ray structure of mitochondrial creatine kinase: a molecular physiology approach. Mol Cell Biochem. 1998;184(12):125–40. [PubMed] [Google Scholar]
- 96.Selkoe DJ, Bell DS, Podlisny MB, Price DL, Cork LC. Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science. 1987;235(4791):873–7. doi: 10.1126/science.3544219. [DOI] [PubMed] [Google Scholar]
- 97.Selverstone Valentine J, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–93. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 98.Singh SP, Janecki AJ, Srivastava SK, Awasthi S, Awasthi YC, Xia SJ, Zimniak P. Membrane association of glutathione S-transferase mGSTA4-4, an enzyme that metabolizes lipid peroxidation products. J Biol Chem. 2002;277(6):4232–9. doi: 10.1074/jbc.M109678200. [DOI] [PubMed] [Google Scholar]
- 99.Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88(23):10540–3. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stachowiak O, Dolder M, Wallimann T, Richter C. Mitochondrial creatine kinase is a prime target of peroxynitrite-induced modification and inactivation. J Biol Chem. 1998;273(27):16694–9. doi: 10.1074/jbc.273.27.16694. [DOI] [PubMed] [Google Scholar]
- 101.Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49(6):1006–15. doi: 10.2337/diabetes.49.6.1006. [DOI] [PubMed] [Google Scholar]
- 102.Studzinski CM, Christie LA, Araujo JA, Burnham WM, Head E, Cotman CW, Milgram NW. Visuospatial function in the beagle dog: An early marker of cognitive decline in a model of human aging and dementia. Neurobiol Learn Mem. 2006 doi: 10.1016/j.nlm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 103.Su MY, Head E, Brooks WM, Wang Z, Muggenburg BA, Adam GE, Sutherland R, Cotman CW, Nalcioglu O. Magnetic resonance imaging of anatomic and vascular characteristics in a canine model of human aging. Neurobiol Aging. 1998;19(5):479–85. doi: 10.1016/s0197-4580(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 104.Sultana R, Butterfield DA. Oxidatively modified GST and MRP1 in Alzheimer’s disease brain: implications for accumulation of reactive lipid peroxidation products. Neurochem Res. 2004;29(12):2215–20. doi: 10.1007/s11064-004-7028-0. [DOI] [PubMed] [Google Scholar]
- 105.Sultana R, Ravagna A, Mohmmad-Abdul H, Calabrese V, Butterfield DA. Ferulic acid ethyl ester protects neurons against amyloid beta- peptide(1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92(4):749–58. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- 106.Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiol Dis. 2006;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 107.Takahashi M, Dore S, Ferris CD, Tomita T, Sawa A, Wolosker H, Borchelt DR, Iwatsubo T, Kim SH, Thinakaran G, Sisodia SS, Snyder SH. Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer’s disease. Neuron. 2000;28(2):461–73. doi: 10.1016/s0896-6273(00)00125-2. [DOI] [PubMed] [Google Scholar]
- 108.Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, Milgram NW. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn Mem. 2003;10(1):64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tapp D, Siwak CT, Zicker SC, Head E, Muggenburg BA, Cotman CW, Murphey HL, Ikeda-Douglas CJ, Milgram NW. An Antioxidant Enriched Diet Improves Concept Learning in Aged Dogs. Society for Neuroscience Abstracts. 2003;(Abstract 836):12. [Google Scholar]
- 110.Tapp PD, Siwak CT, Gao FQ, Chiou JY, Black SE, Head E, Muggenburg BA, Cotman CW, Milgram NW, Su MY. Frontal lobe volume, function, and beta-amyloid pathology in a canine model of aging. J Neurosci. 2004;24(38):8205–13. doi: 10.1523/JNEUROSCI.1339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tapp PD, Head K, Head E, Milgram NW, Muggenburg BA, Su MY. Application of an automated voxel-based morphometry technique to assess regional gray and white matter brain atrophy in a canine model of aging. Neuroimage. 2006;29(1):234–44. doi: 10.1016/j.neuroimage.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 112.Thongboonkerd V, McLeish KR, Arthur JM, Klein JB. Proteomic analysis of normal human urinary proteins isolated by acetone precipitation or ultracentrifugation. Kidney Int. 2002;62(4):1461–9. doi: 10.1111/j.1523-1755.2002.kid565.x. [DOI] [PubMed] [Google Scholar]
- 113.Tyrrell R. Redox regulation and oxidant activation of heme oxygenase-1. Free Radic Res. 1999;31(4):335–40. doi: 10.1080/10715769900300901. [DOI] [PubMed] [Google Scholar]
- 114.Valentine JS. Do oxidatively modified proteins cause ALS? Free Radic Biol Med. 2002;33(10):1314–20. doi: 10.1016/s0891-5849(02)01080-8. [DOI] [PubMed] [Google Scholar]
- 115.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 116.Varadarajan S, Kanski J, Aksenova M, Lauderback C, Butterfield DA. Different mechanisms of oxidative stress and neurotoxicity for Alzheimer’s A beta(1--42) and A beta(25--35) J Am Chem Soc. 2001;123(24):5625–31. doi: 10.1021/ja010452r. [DOI] [PubMed] [Google Scholar]
- 117.Wallimann T, Dolder M, Schlattner U, Eder M, Hornemann T, O’Gorman E, Ruck A, Brdiczka D. Some new aspects of creatine kinase (CK): compartmentation, structure, function and regulation for cellular and mitochondrial bioenergetics and physiology. Biofactors. 1998;8(34):229–34. doi: 10.1002/biof.5520080310. [DOI] [PubMed] [Google Scholar]
- 118.Wu B, Gu MJ, Heydari AR, Richardson A. The effect of age on the synthesis of two heat shock proteins in the hsp70 family. J Gerontol. 1993;48(2):B50–6. doi: 10.1093/geronj/48.2.b50. [DOI] [PubMed] [Google Scholar]


