Abstract
Periventricular leukomalacia is a risk factor for visual impairment in children born prematurely. The impact of diffuse white matter injury detected on magnetic resonance imaging (MRI) on early visual function is unknown. We developed two five-point visual gaze scores to analyze the association between this clinical assessment and white matter injury in 93 premature neonates less than 34 weeks gestational age at birth. Older gestational age was associated with higher values of the two gaze scores. Infants with moderate/severe white matter injury had lower scores than their peers without white matter injury (0.41 points, 95% CI 0.13 to 0.69 for visual fixation score and 0.70 points, 95% CI 0.30 to 1.10 for conjugate score, p < 0.005). Using the results from both scales, a score of nine or higher in an infant > 36 weeks gestation predicted normal white matter on magnetic resonance examination with a sensitivity of 84% and a specificity of 100%. These preliminary findings suggest that white matter injury impacts visual function even prior to term corrected gestational age.
Keywords: Premature infant, white matter injury, magnetic resonance imaging, neurological examination, brain injury, visual impairment, periventricular leukomalacia
Introduction
Infants born prematurely have an increased prevalence of visual impairment and oculomotor dysfunction [1-4]. Adverse visual outcome after premature birth is related not only to ophthalmologic defects following retinopathy of prematurity, but also to lesions of the posterior visual pathways. Intraventricular hemorrhage and white matter injury detected on neonatal ultrasound or on conventional magnetic resonance imaging (MRI) are associated with impaired visual outcome [5-8]. The impact of visual impairment is considerable; recent studies suggest poorer motor and cognitive performances in children with adverse ophthalmologic outcomes, even after adjusting for possible confounders such as birthweight, sex, ultrasound findings and socioeconomic class [9, 10].
Premature infants have rapid changes in their visual acuity, pattern preference and ability to fix and follow between the time of birth and term equivalent corrected gestational age. These changes occur in a predictable pattern in healthy premature infants that matures according to postconceptual age and is similar to term-born infants by 36 and 40 weeks corrected gestational age [11-14].
Past studies have focused on the relationship between periventricular leukomalacia and its effect on visual outcome, with the most significant impairment seen in infants with cyctic periventricular leukomalacia [15]. The incidence of cystic periventricular leukomalacia has declined significantly, and noncystic white matter injury detected on MRI is now the prevailing central nervous system lesion seen in infants born prematurely [16, 17]. The relationship between this less severe form of white matter injury and visual outcomes is unclear, though recent studies using MRI in the early neonatal period show that moderate to severe noncystic white matter injury is associated with increased risk of neurodevelopmental and neurosensory impairment (hearing or vision) when children are assessed at 18−24 months of age [18, 19].
There are no studies assessing the association between white matter injury detected by MRI in the neonatal period and early visual examination in infants born prematurely. We developed a two-part visual examination based on the known patterns of gaze maturation in premature infants to test the hypothesis that moderate or severe white matter injury has an impact on vision even before term corrected gestational age.
Methods
Subjects
We examined infants born between June 2001 and April 2005 and consecutively enrolled into a larger prospective study of the value of MRI for prediction of neurodevelopmental outcome in children born before 34 weeks gestation[20]. Infants with congenital infection, inborn error of metabolism or malformation were excluded from the larger study. Newborns with retinopathy of prematurity (ROP) grade II plus or higher were excluded to avoid confounding due to anterior visual deficits. Infants with intraventricular hemorrhage (IVH) grade III or IV (periventricular hemorrhagic infarct) were excluded in order to isolate the effect of diffuse white matter injury on visual system development. The Institutional Committee on Human Research at UCSF approved the protocol. Infants were studied after voluntary informed parental consent.
Clinical Data Collection
Clinical data were extracted prospectively from maternal and infant medical records by a team of trained neonatal research nurses. The research nurses recorded a given diagnosis based on the treating physician assessment as stated in the medical records. Perinatal variables included gestational age at birth, birthweight and maternal chorioamnionitis. Postnatal variables included patent ductus arteriosis, necrotizing enterocolitis and chronic lung disease.
Gaze Examination
We developed two clinical gaze scores based on patterns of eye movement development in premature infants[21]. A child neurologist (SPM) examined all patients within 24 hours of the MRI. The examiner was blinded to the results of the MRI scan. The first score measured visual fixation: 1) no fixation with random eye movements, 2) transient fixation with random eye movements, 3) stable fixation with rare following but no smooth pursuit, 4) stable fixation with incomplete following, 5) stable fixation with complete following. The second score measured the amount of time that eye movements were conjugate: 1) 25% conjugate, 2) 50%, 3) 75−85%, 4) 85 to 95%, 5) >95%. Infants were examined only during a state of wakefulness (eyes spontaneously open and with gross motor movements of the extremities). The examiner observed eye movements for a minimum of two consecutive minutes. Inter-rater reliability between examiners trained to perform the scoring was high, with unweighted kappa values of 0.86 for the early gaze exams (n=22) and 1.00 for the near term exam (n=7) when observers scored infants over the same two minutes.
MR imaging
Infants were evaluated with one or more MRI scans according to protocol. All scans were performed on a 1.5-T Signa EchoSpeed system (GE Medical Systems) using an isolette designed for this study. MR findings were graded using a validated score[20]. White matter was considered “normal” if there were no periventricular white matter abnormalities, “minimal” WMI for three or fewer areas of T1 signal abnormality each less than 2 mm, “moderate” for more than three areas of T1 signal abnormality or these areas measured more than 2 mm but less than 5% of the hemisphere was involved, and “severe” if more than 5% of the hemisphere was affected. The “moderate” and “severe” groups were combined to improve the power of the statistical analysis. Two pediatric neuroradiologists who were blinded to the clinical condition independently interpreted the MRIs (Kappa = 0.84). Discrepancies were resolved by consensus.
Data Analysis
Statistical analysis was performed using Stata 9.2 software (Stata Corp., College Station, Texas). ANOVA was used for continuous variables and chi square or Fisher exact tests were used for categorical variables. The relationship between age, gaze scores and WMI groups was assessed using generalized estimating equations for repeated measures and receiver operating curves (ROC)[22]. A p-value less than 0.05 was considered statistically significant.
Results
One hundred and eight premature infants had one or more gaze examinations over the study period. Fifteen subjects were excluded from the analysis for ROP grade II or higher (seven infants), IVH grade III or IV (five infants), or either no or poor quality MRI (three infants).
The ninety-three included infants had 126 gaze examinations performed within 24 hours of neuroimaging. The age at MRI and gaze examination was approximately normally distributed with at mean of 34.1 weeks corrected gestational age (standard deviation 3.0, range 27.9 to 42.4 weeks). Four infants were 30 weeks corrected gestational age or less, 95 were between 30 and 36 weeks and 27 were greater than 27 weeks at the time of the examination. Forty-three newborns (46.2%) did not have white matter injury, while 26 (28.0%) had mild injury and 24 (25.8%) had moderate or severe white matter injury. Clinical characteristics including sex, gestational age, birth weight, maternal chorioamnionitis, patent ductus arteriosis, necrotizing enterocolitis and chronic lung disease did not differ between the groups (Table 1).
Table 1.
Clinical Characteristics of 93 Premature Infants
| Degree of White Matter Injury | ||||
|---|---|---|---|---|
| None (n=43) | Mild (n=26) | Moderate/Severe (n=24) | p* | |
| Gestational age at birth (weeks) | 28.6 | 28.7 | 28.7 | > 0.9 |
| Birth weight (grams) | 1171.5 | 1076.2 | 1069.9 | > 0.9 |
| Male (%) | 55.8 | 38.5 | 70.8 | 0.07 |
| Complications (N, %) | ||||
| Chorioamnionitis | 6 (14.0) | 1 (3.9) | 0 | 0.08 |
| Patent ductus arteriosus | 17 (39.5) | 8 (30.8) | 6 (25.0) | 0.5 |
| Necrotizing enterocolitis | 6 (14.0) | 2 (7.7) | 0 | 0.2 |
| Chronic lung disease |
7 (16.3) |
9 (34.6) |
5 (20.8) |
0.3 |
ANOVA for continuous variables and chi square or Fisher exact tests for dichotomous and ordinal variables
Gaze scores improve with increasing corrected gestational age
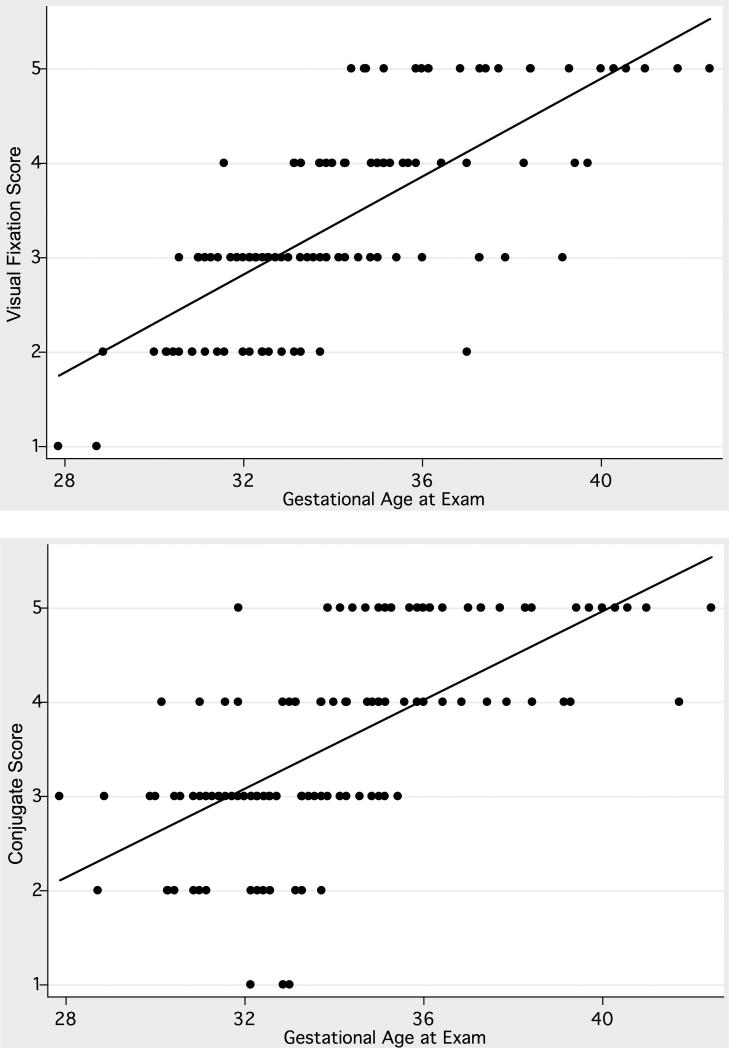
In an unadjusted analysis of the entire cohort, older gestational age was associated with higher values of the visual fixation score (0.26 points/week; 95% CI, 0.22 to 0.30, p < 0.0005) and the conjugate score (0.24 points/week; 95% CI 0.19 to 0.28, p < 0.0005, Figure 1). The gestational age accounted for 55% and 43% of the variability in the visual fixation and conjugate scores respectively.
Figure 1.
Visual fixation (top) and conjugate (bottom) gaze scores in 93 infants with and without white matter injury. Gaze scores are higher in infants with older corrected gestational age.
Infants with white matter injury have lower gaze scores
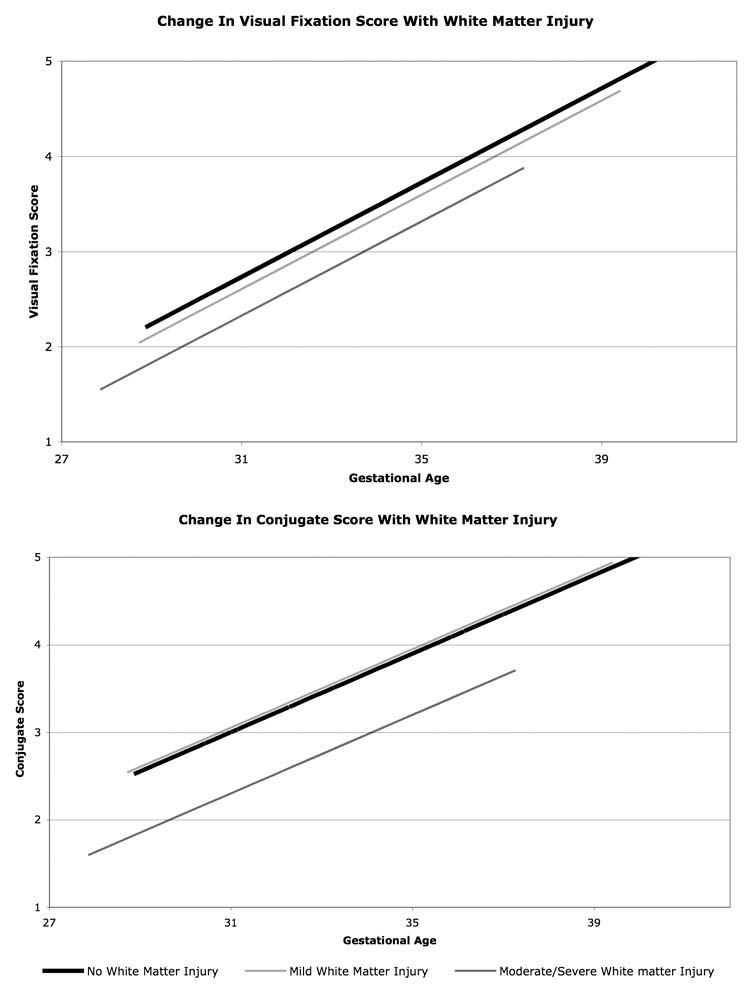
Infants with white matter injury had lower visual fixation and conjugate scores than their peers without injury (Figure 2). The difference in scores between infants with mild and no white matter injury was not statistically significant. However, infants with moderate/severe white matter injury had significantly lower gaze scores than infants without white matter injury. For visual fixation, infants with moderate/severe white matter injury had a score that was, on average, 0.41 points lower (95% CI 0.13 to 0.69, p = 0.005) than that of their peers without injury. Similarly, for conjugate gaze, infants with moderate/severe injury had a score that was, on average, 0.70 points lower (95% CI 0.30 to 1.10, p = 0.001) than that of their peers without white matter injury.
Figure 2.
Change in visual fixation (top) and conjugate (bottom) scores with gestational age according to a fitted linear model. Infants with white matter injury have lower predicted gaze scores.
Not only did infants with moderate/severe white matter injury have lower scores than infants without white matter injury, these infants also failed to achieve maximal scores. None of the 24 infants with moderate/severe white matter injury achieved the maximal score for visual fixation. Only a single infant achieved the maximal score for the conjugate gaze exam.
According to the linear model, the average predicted age at which infants without white matter injury should achieve a visual fixation score of 5 is 40.2 weeks (95% CI 38.9 to 41.4 weeks), whereas infants with moderate/severe injury are predicted to have a score of 5 at only 41.8 weeks (95% CI 40.1 to 43.5, p < 0.0005). Similarly, infants without injury are predicted to have a conjugate score of 5 at 39.9 weeks (95% CI 38.7 to 41.1 weeks), whereas infants with moderate/severe injury, only at 43.0 weeks (95% CI 40.7 to 45.4, p < 0.0005). The gaze scores increased at a similar rate for different degrees of white matter injury.
[0]Sensitivity and specificity of the gaze examination
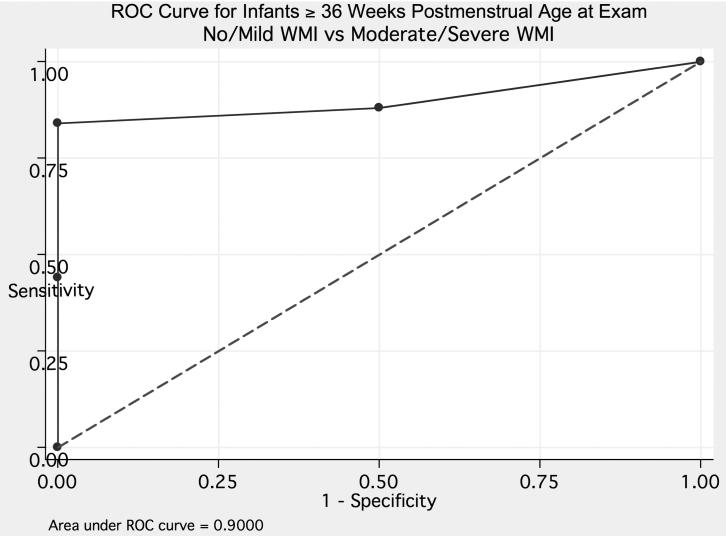
Using the results from the 27 examinations performed at greater than or equal to 36 weeks corrected gestational age, combined visual fixation and conjugate gaze scores were predictive of normal white matter on MRI (Figure 3). Using a combined score of nine or higher, the sensitivity was 84% and specificity was 100%.
Figure 3.
ROC curve for combined visual fixation and conjugate gaze scores and normal magnetic resonance imaging in 27 infants greater than 36 weeks corrected gestational age at the time of examination.
Discussion
We developed a clinical gaze assessment (visual fixation and conjugate scores) that is based on the patterns of visual fixation and conjugate eye movement maturation seen in healthy premature infants [21]. We used these scores to quantify visual development and assess the impact of MRI-detected white matter injury in a cohort of 93 infants born at 34 weeks gestational age or less. Both the visual fixation and conjugate gaze scores increased reliably with increasing corrected gestational age (p < 0.0005), indicating that they measure an aspect of visual system maturation. Infants with white matter injury had lower gaze scores, and infants with moderate/severe injury were more impaired than those with mild injury (P = 0.0001). When combined, the high scores provided a good sensitivity and specificity for predicting a normal MRI in infants over 36 weeks corrected gestational age.
These preliminary findings suggest that there is an association between white matter injury detected on conventional T1-weighted imaging and early gaze maturation in premature infants. It is unclear whether the dysfunction seen in infants with moderate/severe white matter disease is due to direct injury to the optic radiations, or whether injury to other structures impairs alertness and attention in these infants, which would tend to reduce fixation and conjugate eye movements. Alternatively, the white matter injury may be a marker for injury to other visual system structures (such as the lateral geniculate body, calcarine cortex or visual association cortex) involved in cerebral visual impairment. We excluded infants with clinically significant retinopathy of prematurity, therefore confounding due to visual loss by retinopathy is no a factor. Finally, the association between white matter injury and gaze examination could also be related to an unmeasured confounder, such as medication or infection.
This association between gaze and MRI-detected white matter injury is in keeping with past studies that show a relationship between visual abnormalities and white matter-injury in premature infants. Several studies show that visual evoked potentials performed prior to term adjusted age may be absent or abnormal in premature infants with cystic periventricular leukomalacia [5, 23-25]. Furthermore, there is a strong association between periventricular leukomalacia detected by ultrasound or MRI and childhood visual and oculomotor abnormalities (reviewed in [7]). Finally, children with periventricular leukomalacia have high rates of visual-perceptual impairment [26, 27]. Shah et al recently showed a relationship between early neuroimaging and later visual outcome. In a cohort of 68 children born at less than 33 weeks gestational age, those with specific oculomotor abnormalities such as impaired saccades at age two years had reduced volume in the inferior occipital brain region on MRI performed at term equivalent corrected gestational age [28].
The small number of infants examined over a wide range of gestational ages limits our ability to suggest normative scores that could be applied in the intensive care nursery. Further studies will be necessary to verify the results and apply them to a wider group of infants to develop normal expected values at each gestational age. However, when we looked at infants greater than 36 weeks gestational age, a perfect or near perfect score (nine or higher on the combined score) predicted children with normal MRI scan (no or mild white matter injury) with good sensitivity and specificity. The gaze examinations assess a spectrum of visual and higher order tasks, including afferent vision, supranuclear and nuclear oculomotor control, as well as attention and maintenance of an awake state. Infant state significantly affects eye movements, with a tendency toward dysconjugate gaze in somnolent infants. In this study, we avoided confounding due to infant state as much as possible by examining the children only when they were awake, with spontaneous eye-opening and limb movement. Examining a premature infant while fully awake can be a challenge, and, in several cases, we required several trips to the bedside to ensure that the child could be appropriately examined.
The gaze scores provide a simple, quick and informative bedside tool that can be added to the neurological assessment of the premature infant. Lower gaze scores in infants with moderate/severe white matter injury suggest that these lesions can have influence neurodevelopment as early as the neonatal period and prior to term corrected gestational age. The impact of the differences seen on gaze examination is unknown. Past studies suggest that an infant's ability to fix and follow predicts good neurodevelpmental outcome, while absence of fixation and following is not predictive [11]. This historical information, combined with the good sensitivity and specificity of the combined visual fixation and conjugate gaze scores for normal MRI suggests that premature infants with fixation, following and conjugate eye movements are at lower risk for adverse neurodevelopmental outcome. Careful longitudinal follow-up of this cohort will be necessary to further assess the clinical utility of the gaze examination in predicting later neurodevelopment.
Acknowledgements
The authors thank Dr. William Good for his expertise in the development of the gaze scores and the nurses of the Neonatal Clinical Research Center (NCRC) for patient recruitment and scanning. This research is supported by the UCSF PCRC (NIH RR01271) and the National Institutes of Health NS40117, NS35902, and NS40382. SPM is a Canadian Institutes of Health Research Clinician Scientist and Michael Smith Foundation for Health Research Scholar. HCG is supported by the NINDS Neurological Sciences Academic Development Award (NS01692).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keith CG, Kitchen WH. Ocular morbidity in infants of very low birth weight. Br J Ophthalmol. 1983;67(5):302–5. doi: 10.1136/bjo.67.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibson NA, et al. Ophthalmic findings in infants of very low birthweight. Dev Med Child Neurol. 1990;32(1):7–13. doi: 10.1111/j.1469-8749.1990.tb08461.x. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor AR, et al. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109(1):12–8. doi: 10.1542/peds.109.1.12. [DOI] [PubMed] [Google Scholar]
- 4.Larsson EK, Rydberg AC, Holmstrom GE. A population-based study on the visual outcome in 10-year-old preterm and full-term children. Arch Ophthalmol. 2005;123(6):825–32. doi: 10.1001/archopht.123.6.825. [DOI] [PubMed] [Google Scholar]
- 5.Pike MG, et al. Patterns of visual impairment associated with lesions of the preterm infant brain. Dev Med Child Neurol. 1994;36(10):849–62. doi: 10.1111/j.1469-8749.1994.tb11776.x. [DOI] [PubMed] [Google Scholar]
- 6.Cioni G, et al. Cerebral visual impairment in preterm infants with periventricular leukomalacia. Pediatr Neurol. 1997;17(4):331–8. doi: 10.1016/s0887-8994(97)00152-5. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson LK, Dutton GN. Periventricular leukomalacia: an important cause of visual and ocular motility dysfunction in children. Surv Ophthalmol. 2000;45(1):1–13. doi: 10.1016/s0039-6257(00)00134-x. [DOI] [PubMed] [Google Scholar]
- 8.Kok JH, et al. Visual function at 11 years of age in preterm-born children with and without fetal brain sparing. Pediatrics. 2007;119(6):e1342–50. doi: 10.1542/peds.2005-2857. [DOI] [PubMed] [Google Scholar]
- 9.Cioni G, et al. Correlation between visual function, neurodevelopmental outcome, and magnetic resonance imaging findings in infants with periventricular leucomalacia. Arch Dis Child Fetal Neonatal Ed. 2000;82(2):F134–40. doi: 10.1136/fn.82.2.F134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephenson T, et al. Children born weighing less than 1701 g: visual and cognitive outcomes at 11−14 years. Arch Dis Child Fetal Neonatal Ed. 2007;92(4):F265–70. doi: 10.1136/adc.2006.104000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brazelton TB, Scholl ML, Robey JS. Visual responses in the newborn. Pediatrics. 1966;37(2):284–90. [PubMed] [Google Scholar]
- 12.Dubowitz LM, Dubowitz V, Morante A. Visual function in the newborn: a study of preterm and full-term infants. Brain Dev. 1980;2(1):15–29. doi: 10.1016/s0387-7604(80)80004-0. [DOI] [PubMed] [Google Scholar]
- 13.Dobson V, Mayer DL, Lee CP. Visual acuity screening of preterm infants. Invest Ophthalmol Vis Sci. 1980;19(12):1498–505. [PubMed] [Google Scholar]
- 14.Morante A, et al. The development of visual function in normal and neurologically abnormal preterm and fullterm infants. Dev Med Child Neurol. 1982;24(6):771–84. doi: 10.1111/j.1469-8749.1982.tb13698.x. [DOI] [PubMed] [Google Scholar]
- 15.Scher MS, et al. Visual and neurological outcome of infants with periventricular leukomalacia. Dev Med Child Neurol. 1989;31(3):353–65. doi: 10.1111/j.1469-8749.1989.tb04004.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamrick SE, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr. 2004;145(5):593–9. doi: 10.1016/j.jpeds.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12(2):129–40. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 18.Woodward LJ, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 19.Miller SP, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147(5):609–16. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Miller SP, et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging. 2002;16(6):621–32. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 21.Volpe J. Neurology of the Newborn. 4th ed. WB Saunders; Philadelphia: 2001. [Google Scholar]
- 22.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–60. [PubMed] [Google Scholar]
- 23.De Vries LS, et al. Neurological, electrophysiological and MRI abnormalities in infants with extensive cystic leukomalacia. Neuropediatrics. 1987;18(2):61–6. doi: 10.1055/s-2008-1052453. [DOI] [PubMed] [Google Scholar]
- 24.Ekert PG, et al. Visual evoked potentials for prediction of neurodevelopmental outcome in preterm infants. Biol Neonate. 1997;71(3):148–55. doi: 10.1159/000244410. [DOI] [PubMed] [Google Scholar]
- 25.Kato T, et al. The evolutionary change of flash visual evoked potentials in preterm infants with periventricular leukomalacia. Clin Neurophysiol. 2005;116(3):690–5. doi: 10.1016/j.clinph.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 26.Fazzi E, et al. Visual-perceptual impairment in children with periventricular leukomalacia. Brain Dev. 2004;26(8):506–12. doi: 10.1016/j.braindev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 27.van den Hout BM, et al. Visual perceptual impairment in children at 5 years of age with perinatal haemorrhagic or ischaemic brain damage in relation to cerebral magnetic resonance imaging. Brain Dev. 2004;26(4):251–61. doi: 10.1016/S0387-7604(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 28.Shah DK, et al. Reduced occipital regional volumes at term predict impaired visual function in early childhood in very low birth weight infants. Invest Ophthalmol Vis Sci. 2006;47(8):3366–73. doi: 10.1167/iovs.05-0811. [DOI] [PubMed] [Google Scholar]