Abstract
Repetition of visually common objects was examined in relation to prior intentional learning and memory status using a delayed match-to-sample task. Both response time and two temporally separate ERP components indexed repetition. The early repetition effect (~200 –550 ms) evoked more ERP responses for repeated visual objects, and was diminished by prior intentional learning (old / new) or being maintained in working memory (targets / distracters). In contrast, the late repetition effect (after ~550 ms) evoked reduced ERP activation for repeated items, and was not affected by prior learning nor working memory status. Our source localization results indicate that the late and posterior repetition effect in visual cortex is consistent with repetition suppression results reported in monkey physiology and human fMRI studies. Meanwhile, the early and anterior repetition effect, in temporal pole and frontal cortices, is modulated by explicit memory mechanisms.
Keywords: implicit, explicit learning, EEG/ERPs, fMRI, working memory, priming, temporal pole, LORETA
Introduction
Repetition effects represent a common form of memory and learning where repeated encounters with an item result in faster and more efficient processing of the item. The effect is also known as repetition priming or adaptation, which is preserved even among amnesia patients. Thus, repetition priming seems to be a more automatic process, which is in stark contrast to explicit memory recall.
Single-cell studies in monkeys have revealed reduced activation when presented with repeated stimuli, which may reflect neurons’ tuning to a specific stimulus (e.g., Desimone 1996; Miller & Desimone, 1994). This effect is referred to as repetition suppression, and fMRI studies have confirmed this reduction in neural activation to repeated stimuli compared with new (e.g., Bucker et al., 1998; Grill-Spector & Malach, 2001; van Turennout, Ellmore, & Martin, 2000) or studied non-repeated stimuli (Jiang, Haxby, Martin, Ungerleider, & Parasuraman, 2000). The underlying brain mechanisms of repetition suppression, however, are still under debate and can not be clearly explained by a single neural model (for reviews see Grill-Spector, Henson, & Martin, 2006; Schacter & Buckner, 1998). Furthermore, recent evidence suggests that repetition suppression can be moderated by both perceptual and response-related processes including exposure duration (Zago, Fenske, Aminoff, & Bar, 2005), attention (e.g., Vuilleumier, Schwartz, Duhoux, Dolan, & Driver, 2005; Yi & Chun, 2005), lag time (e.g., Henson, Rylands, Ross, Vuilleumeir, & Rugg, 2004; Wagner, Maril, & Schacter, 2000), emotion (Bentley, Vuilleumier, Thiel, Driver, & Dolan, 2003; Ishai, Pessoa, Bikle, & Ungerleider, 2004), and task performance (e.g., Jiang et al., 2000; Sawamura, Orban & Vohels, 2006).
Changes in brain activation reflecting repetition effects have also been indexed with event-related potentials (ERPs), distinguished for their high temporal resolution. Some researchers have reported a repetition effect beginning as early as 160 ms when no intervening stimuli are present (Henson et al., 2003). This early effect has been shown to occur with the repetition of both words and non-words (Rugg, 1987), and may reflect more efficient perceptual processing from the immediate repetition of stimuli (Nagy & Rugg, 1989).
A second, ERP repetition effect beginning around 300 ms has been more readily observed. This component is related to lexical memory (Rugg & Nagy, 1987) and is modulated by several factors including semantic (e.g., Pickering & Schweinberger, 2003) and decision processes (e.g., Bentin & McCarty, 1994). Rugg (1990) suggested that this positive-going repetition effect is actually composed of two ERP components: a decrease in N400 activation and an increase in P300 (P3b) activation. The N400 is elicited by stimuli that are incongruent with the current semantic context (for review see Fabiani, Gratton, & Coles, 2000). The P300 is associated with several distinct neural processes, but most often interpreted in relation to brain mechanisms involved in processing capacity and working memory (WM; for review see Kok, 2001).
While ERP repetition effects using words are typically observed as positive shifts, ERPs for repeated pictorial stimuli have been reported as both positive and negative deflections, depending on the task demands and lag between initial and repeated presentations (e.g., Van Petten & Senkfor, 1996; Zhang, Begleiter, Porjesz, Wang, & Litke, 1995).
Using novel objects without any semantic meaning, Rugg, Soardi & Doyle (1995) indexed repetition priming by virtue of less positive shifts at parietal sites between 200 and 400 ms and at all midline sites between 400 and 900 ms. Consistent with previous accounts (e.g., Bentin & McCarthy, 1994), Rugg and colleagues suggested that their ERP repetition effects reflected a diminished need for stimulus analysis processes. Penney, Mecklinger & Nessler (2001) extended this parietal repetition effect to possible and impossible objects, although their repetition effect occurred 100 ms later (i.e., 300 – 600 ms interval). Because objects processed at an identification level have more positive waveforms than objects processed at a post-identification level (Viggiano & Kutas, 1998), Penney and colleagues stated that such a reduction in ERP activation for repeated objects may reflect more efficient identification of those objects.
In addition to the study of repetition effects, ERPs have been used to distinguish brain mechanisms of intentional learning (for reviews see Friedman & Johnson, 2000; Paller, 2001). ERP studies of intentional learning have typically focused on old-new effects in which participants perform a recognition task requiring discrimination between previously studied (i.e., old) and previously non-studied (i.e., new) items. Generally, these studies have shown old/new effects between 400 and 800 ms with old items having more positive amplitudes than new items. This body of research has revealed two robust mechanisms of learning. The first mechanism, termed the early frontal old/new effect, indexes automatic, familiarity processes that do not include information related to its source (e.g., where or when the memory was formed). A second memory mechanism, termed the late posterior old/new effect, has been shown to index more attention-controlled, recollective processes that include source and contextual information.
The current study extended this line of research by examining how repetition priming influenced and interacted with prior intentional learning and WM processes using ERPs. The principle question addressed by the present study was whether changes in prior learning and /or working memory status during a matching task moderated brain responses to repetition.
A second question addressed was whether the distinct repetition effects indexed by ERPs are functionally consistent with the automatic repetition reduction indicated by BOLD fMRI signals and single-cell recordings. Researchers have recognized a dissociation between ERP and fMRI data in some repetition priming tasks (Grill-Spector et al., 2006; Wiggs & Martin, 1998). While fMRI and monkey physiology data showed repetition suppression in mostly posterior regions of the brain, EEG/ERP results showed repetition-related increases in ERP waveforms (e.g. Rugg et al., 1995). Such dissociations vary depending on familiarity and repetition lags (e.g., Fiebach, Gruber, & Supp, 2005; Henson, 2003). Given the sensitivity of ERPs in distinguishing brain mechanisms involved in repetition and prior intentional learning, we hypothesized that repetition would not be indexed simply as an unitary process, but would include multiple effects, and at least partially influenced by explicit mechanisms.
Experimental Procedures
Participants
Fourteen students (five male and nine female, M age = 22.3 years) from the University of Kentucky participated in the experiment and received monetary compensation. Informed consent, approved by the Institutional Review Board, was obtained from each participant. Inclusion criteria included normal or corrected 20/40 vision, right hand dominance, between 18 and 28 years of age and English as their first language.
Visual Stimuli
Stimuli consisted of 240 two dimensional pictures of common objects taken from Sondgrass and Vanderwart (1980). Each object was presented in white-black within a rectangular area of approximately, 8.3 by 5.8 cm, with a 65 cm viewing distance, and at a visual angle of approximately 7 degrees. Also, target objects were presented with a 6 mm green border at the beginning of a trial. The 240 object stimuli were divided into 2 groups with 60 “old” objects being initially studied by participants and 180 “new” objects not previously studied. The 60 studied objects were used as both studied targets and studied distracters. The 180 new objects were subdivided into 60 objects that served as new targets, and 120 objects that served as new distracters. Each object group was normed for familiarity and complexity.
Working Memory Task
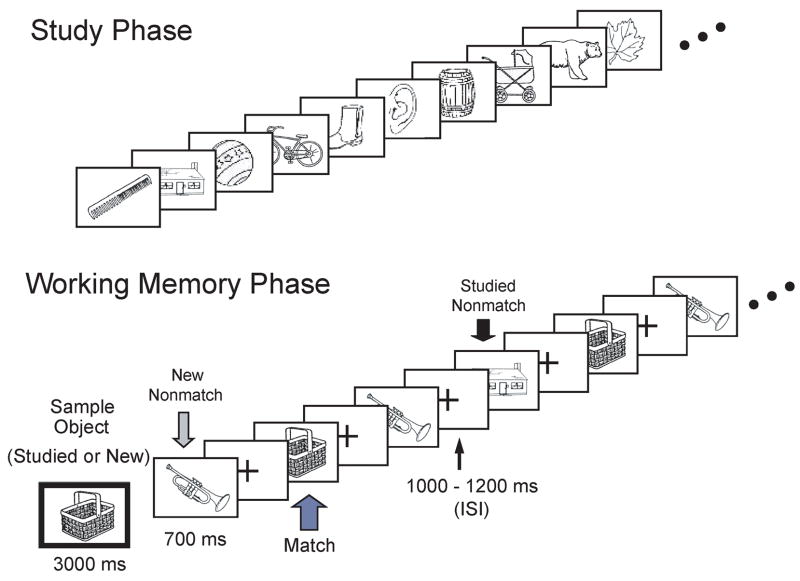
The short-term memory task consisted of 120 trials separated into 12 blocks of 10 trials each. Each trial began with the presentation of the sample target object (for 3000 ms) distinguished by having a green border (see Figure 1). A single tone presented at the onset of the sample target further distinguished it from subsequent test objects. The sample target was followed by 9 successive test objects with an ISI of 700 ms per object. All objects were divided by a fixation cross with an ISI of 1100± 100 ms. Each trial lasted approximately 21 seconds.
Figure 1. The modified delayed match-to-sample task.
The memory task included a sample target object followed by 9 successive test picture that did (targets) or did not (distracters) match the sample object. Both targets and distracters included prior studied (old) and new objects, and each object was presented up to 4 times in a trial.
The 120 trials included 60 trials having a studied target and 60 trials having a new target. The order of studied and new target trials was balanced in a pseudo-random sequence that was consistent across subjects. Within trials, test objects were classified into one of three groups: (a) targets, (b) studied distracters, and (c) new distracters. Each of the studied objects served as a studied target in one trial and as a studied distracter in a later trial. New objects, whether serving as a target or distracter, were not used in any subsequent trials. The test portion of each trial contained a pseudo-random presentation of targets, studied distracters, and new distracters, with each being repeated one to three times, making up a total of nine test objects per trial.
Procedure
Participants were instructed to study and memorize 60 objects by performing a computerized naming task that lasted approximately 10 minutes. Subjects also continued to study these objects in paper form during the placement of an EEG cap which lasted about 20 minutes. Subjects were told to relate the objects to personal experiences and that they would be tested after placement of the EEG cap. The subsequent recognition test included the 60 studied objects and 60 new objects that were not used further in the study. All participants performed well on the recognition test with accuracy no less than 96% (mean accuracy = 98.2%).
For the working memory task, participants were shown a sample target object to hold in mind and were directed to indicate whether the following 9 test objects were the same or different from the sample target by pressing one of two buttons with their right or left hand. Assignment of hands to indicate a target versus distracter was counterbalanced across subjects. Participants were instructed to forget the previous sample target object when a new sample target appeared, indicating the beginning of a new trial. All participants performed at least 10 practice trials prior to data collection and the working memory task lasted approximately 60 minutes overall.
ERP Recordings
ERP recordings were obtained from 62 scalp sites using Ag/AgCI electrodes embedded in an elastic cap at locations from the extended International 10–20 System. These electrodes were referenced to the right mastoid during recording and re-referenced to the average of the right and left mastoid potentials offline. Two additional channels were used for monitoring horizontal and vertical electrooculographic (EOG) recordings. Impedance was reduced below 5KΩ. EEG signals were filtered with a band-pass of 0.05 40 Hz and sampled at a rate of 500 Hz. Each epoch lasted 1000 ms, including 100 ms prior to stimulus onset. Trials with a voltage, relative to the 100-ms baseline, exceeding ± 75μV at any electrode were excluded from analysis, as were trials with artifacts in the EOG channels.
ERP source analysis (LORETA)
An intracranial source analysis was calculated for each time point between 200 and 550 ms, as well as at the time point with the maximal signal strength as estimated by mean global field power (MGFP). Analyses were conducted for several maximal MGFP of difference waves (i.e., repeated minus first for target pictures, distracter pictures, new pictures and studied pictures). We used the LORETA method (Low Resolution Electromagnetic Tomography, via Curry V5.0), a new method for localizing electrical activity in the brain (Anderer et al., 1998), which uses a Laplacian model term. The Laplacian measures the second derivative of source strengths. Current Density Reconstructions (CDRs) assume simultaneous activity at a large number of possible source locations. Prior results have shown that LORETA produces blurred but accurate localizations of point sources (Pascual-Marqui et al., 2002; Guo et al., 2006, 2007). The procedure used a realistic volume conductor model derived using a boundary element method with three layers [skin (10mm), skull (9mm), and brain (7mm), with conductivities of 0.3300, 0.0042 and 0.3300, respectively].
Statistical Analyses
Behavioral effects were indexed using mean response times (RT) of correct responses and response accuracy data for each condition. ERPs were averaged correct responses elicited by each target or distracter condition recorded during the working memory task. Also, preliminary topographic analyses indicated that the midline sites Fz, FCz, Cz, CPz, Pz, and Oz provided a good index of neural activation from all 64 scalp locations.
Based on the visual inspection of repetition effects for each condition, ERP mean amplitude data were gathered at the time segments 200–400, 400–550 and 550–850 ms relative to the mean amplitude of the pre-stimulus baseline (−100—0 ms set to 0 μV). For both target and distracter objects, the initial time interval indexed N2 activation and the latter two intervals indexed P3 activation.
Preliminary examination of repeated items for each condition revealed that 2nd, 3rd, and 4th, presentations did not noticeably differ, and thus, were grouped together for significance testing. All ANOVAs had a level of significance set to 0.05 and were supplemented with Bonferroni pairwise comparisons or simple main effects comparisons when appropriate. Greenhouse-Geisser corrections were reported with all effects having two or more degrees of freedom in the numerator.
Results
Accuracy and Response Time
Response accuracy was high for recognizing targets and rejecting distracters (typically greater than 90%, see Table 1). Three-way repeated-measures ANOVAs, i.e., memory status (target, distracter), study type (new, studied), and repetition type (1st, repeated), were conducted for percent correct responses. A main effect of memory status, F(1,13) = 45.65, p < .001, indicated that responses to targets were overall less accurate than responses to distracters. An interaction of memory status by repetition, F(1,13) =11.58, p = .005, also indicated that repetition led to an increase in accuracy for targets, F(1,13) = 9.04, p = .01, but led to a decrease in accuracy for distracters, F(1,13) = 8.13, p = .01.
Table 1. Reaction Times and Accuracy Performance.
Performance measures across targets and distracters
| Memory Status | Reaction Time
|
% Accuracy
|
||
|---|---|---|---|---|
| Mean(ms) | (S.E.) | Mean(%) | (S.E.) | |
| Studied targets | ||||
| First presentation | 509 | (15) | 89.6 | (1.48) |
| Second presentation | 475 | (14) | 91.3 | (1.21) |
|
| ||||
| New targets | ||||
| First presentation | 504 | (16) | 90.0 | (1.39) |
| Second presentation | 473 | (16) | 91.7 | (1.32) |
|
| ||||
| Studied distracters | ||||
| First presentation | 480 | (13) | 98.1 | (0.45) |
| Second presentation | 452 | (11) | 97.1 | (0.40) |
|
| ||||
| New distracters | ||||
| First presentation | 489 | (12) | 98.5 | (0.42) |
| Second presentation | 453 | (11) | 97.9 | (0.36) |
Response times (RT) of correct responses were examined using a three-way repeated-measures ANOVA. A main effect of memory status, F(1, 13) = 9.12, p = .01, indicated that responses to distracters were overall faster than those to targets. A main effect of repetition, F(1, 13) = 60.38, p < .001, also indicated that responses to repeated objects were faster than their initial presentation. No interaction effects were found.
ERP Results
To examine repetition effects of studied and new targets and distracters at different time intervals, a five-way repeated-measures ANOVA for memory status (target, distracter), study type (new, studied), repetition (1st, repeated), time interval (200–400ms, 400–550ms, 550–850ms) and electrode site (Fz, FCz, Cz, CPz, Pz, Oz), was conducted for ERP mean amplitude data. A main effect of memory status was significant, F(1,13) = 182.31, p < .001, along with a five-way interaction, F(10,130) =3.29, p = .036. We, therefore, examined targets and distracters separately using four-way repeated-measure ANOVAs.
1. Repetition Effects for Distracters
A four-way repeated-measures ANOVA, i.e. study type (new, studied), repetition type (1st, repeated), time interval (200–400ms, 400–550ms, 550–850ms) and electrode site (Fz, FCz, Cz, CPz, Pz, Oz; for ERP analyses), was conducted. The main effects of study type, F(1,13) = 4.65, p < .05, and time interval, F(2,26) =24.66, p < .001, were significant. In addition, a three-way interaction of study type × repetition × time interval, F(2,26) = 22.48, p < .001, was significant,. Simple effects of the interaction are described below.
Repetition Effects for New Distracters
From 200– 400ms, there was a significant main effect of repetition F(1,13) = 51.73, p < .001, and interaction effect of repetition × electrode site, F(5,65) = 28.32, p < .001. ERPs of the initial presentation of new distracters were smaller and less positive-going than subsequent repetitions. This repetition effect was significant at Fz, FCz, Cz, CPz and Pz (p < .001), but not at Oz (p = .27). From 400–550 ms, a main effect of repetition, F(1,13) = 6.58, p = .024, and an interaction effect of repetition × electrode site, F(5,65) = 19.46, p < .001, were significant. Similar to the 200–400 ms interval, the initial presentation of new distracters evoked smaller and less positive-going ERPs than repeated presentations at frontal-central sites, (all p < .001), but not at other electrodes (p > .05). For the 550–850 ms interval, a main effect of repetition F(1,13) = 27.01, p < .001, revealed that the initial presentation of new distracters evoked larger and more positive ERPs than subsequent repetitions (Figure 2A, Figure 4B).
Figure 2. The repetition effects of Distracters.
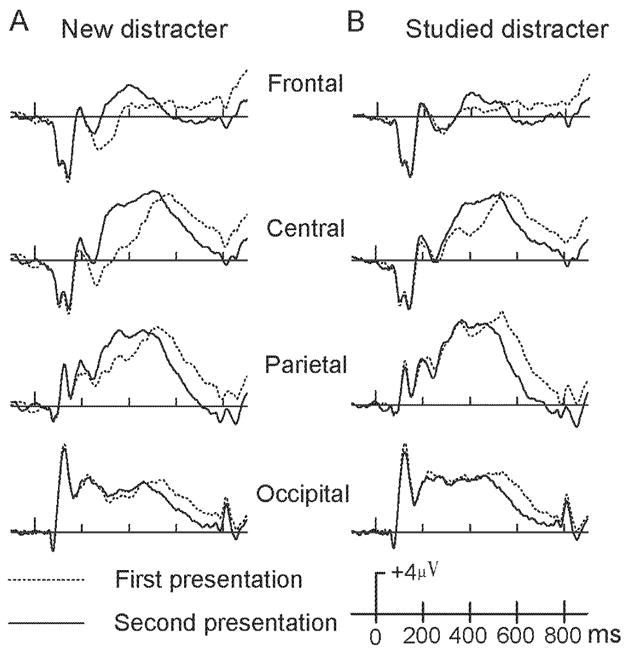
Averaged ERPs for the initial (dash lines) and repeated (solid lines) presentations of new (panel A) and studied (panel B) distracters at four midline scalp locations (Fz, Cz, Pz, and Oz).
Figure 4. Difference waves for old/new and repetition effects of distracters.
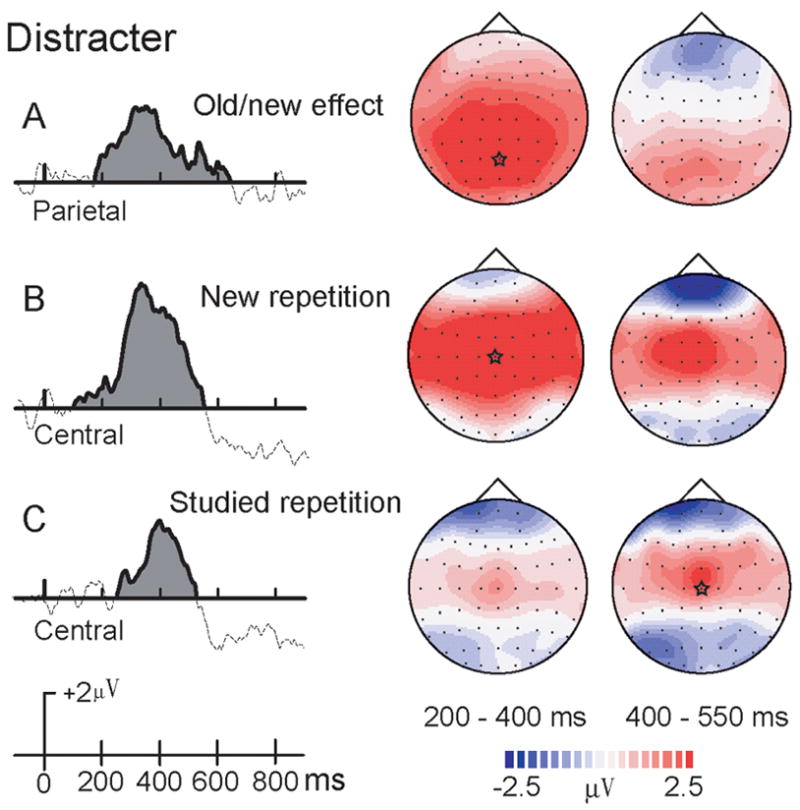
A. ERP old/new difference waves (studied minus new) for distracters at Pz.
B. Early ERP repetition effect (repeated minus initial presentations) for new distracters at Cz.
C. Early ERP repetition effect (repeated minus initial presentations) for studied distracters at Cz. Corresponding topographical maps are shown on the right. A star in each topographic map indicates the cortical location of the largest difference waves.
Repetition Effects for Studied Distracters
In contrast to new distracters, no repetition effects were found for studied distracters from 200–400 ms. At 400–550 ms, a repetition × electrode site interaction was significant, F(5,65) = 18.35, p < .001, indicating that repeated studied distracters were more positive than their initial presentation and this effect occurred over central sites (i.e., FCz, Cz, all p < .02). From 550–850 ms, a main effect of repetition, F(1,13) = 41.46, p < .001, indicated that ERP activation was larger and more positive for the initial presentation than subsequent repetitions (Figure 2B, Figure 4C).
Old-New Repetition Effects for Distracters
A study type × repetition interaction, F(1,13) = 28.34, p < .001, was significant for 200–400 ms. Pair-wise comparisons revealed that during the initial presentation, studied objects had greater positive activation than new objects at all sites. A similar result was found at 400–550 ms interval, except that on sites Pz and Oz (Figure 4A). At 550–850 ms, no significant effects involving study type were found.
2. Repetition Effects for Targets
A four-way repeated-measures ANOVA, i.e. study type, repetition, time interval and electrode site was conducted. Significant interactions of study type × repetition × electrode site, F(5, 65) = 3.47, p < .05, and study type × time interval, F(2,26) =5.75, p < .01 were further examined using simple effects reported below.
Repetition Effects for New Targets
From 200–400 ms, a main effect of repetition type, F(1,13) = 5.30, p = .038, and a repetition type × electrode site interaction, F(5,65) = 8.83, p = .001, indicated more positive-going activation for repeated new targets than their initial presentation at central sites (all p < .03). No differences relating to repetition were found for the 400–550 ms interval. From 550–850 ms, a significant main effect of repetition, F(1,13) = 19.53, p = .001, indicated that repeated presentations evoked less positive-going activation than the initial presentation (Figure 3A, figure 5B). This is an opposite repetition trend from earlier time intervals.
Figure 3. Target repetition effects.
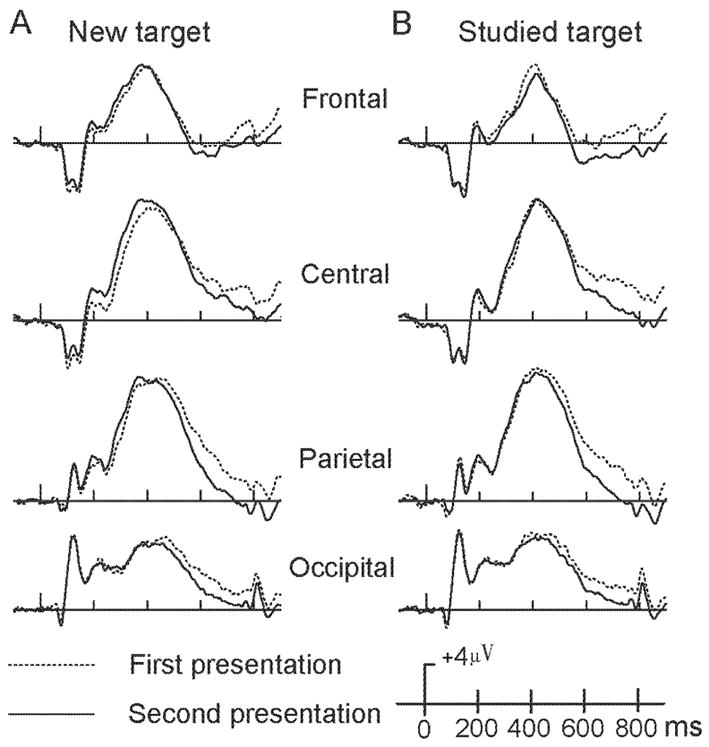
Averaged ERPs for the initial (dash lines) and repeated (solid lines) presentations of new (panel A) and studied (panel B) targets at four midline scalp locations (Fz, Cz, Pz, and Oz).
Figure 5. Difference waves for old/new and repetition effects of targets.
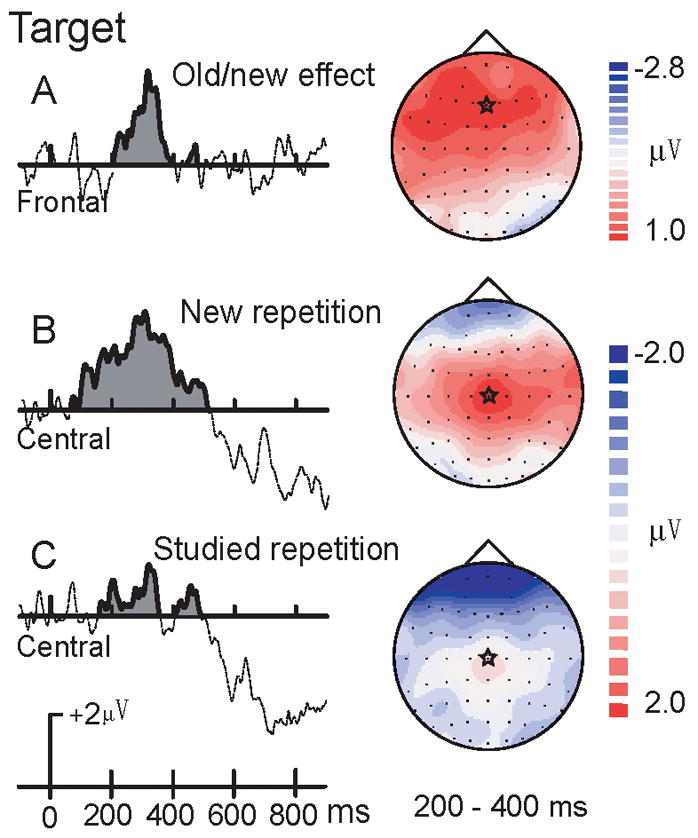
A. ERP old/new difference waves (new minus studied) for targets at Fz. B. Early ERP repetition effect (repeated minus initial presentations) for new targets at Cz. C. Early ERP repetition effect (repeated minus initial presentations) for studied targets at Cz. Corresponding topographical maps are shown on the right. A star in each topographic map indicates the cortical location of the largest difference waves.
Repetition Effects for Studied Targets
For both 200–400 and 400–550 ms intervals, no significant differences were found in repetition of studied targets. From 550–850 ms, a significant main effect of repetition type, F(1,13) = 19.55, p = .001, indicated that repeated presentations evoked less positive-going activation than their initial presentation (Figure 3B, 5C).
Old-new Effects for Targets
From 200–400 ms, a main effect of study type, F(1,13) = 9.34, p = .009, and a study type × electrode interaction, F(5,65) = 20.51, p < .001, revealed that studied targets were more negative than new targets at frontal-central sites (all p < 0.05). The study type × repetition, F(1,13) = 3.35, p = .09, was marginally significant for target objects. Study type × repetition effects were not significant at the 400–550 and 550–850 ms intervals (Figure 5A).
3. Dissociation of Early and Late Repetition Effects
In order to isolate early and late repetition effects, the two earlier intervals were combined in the following analysis. A five-way repeated-measures ANOVA was performed with only two time windows reflecting early (200–550ms) and late time (550–850ms) intervals. A significant interaction of repetition x study type x time interval, F(1,13)=15.04, p < 0.01, was found. Pair-wise comparisons revealed that during the early time interval, only new objects were affected by repetition (p < .002). Both new and studied objects, however, had repetition effects during the late time interval (p < .001). Also, a three-way interaction of repetition × memory status × time interval approached significance, F(1,13)=4.24, p = 0.06. Pair-wise comparisons revealed that during the early time interval, repetition of distracter objects was significant (p = .001), repetition of targets was not (p > .70). Target and distracter objects all revealed greater repetition effects during the late time interval (p < .001, see Figure 6 left panel).
Figure 6. Difference waves for the early repetition effect.
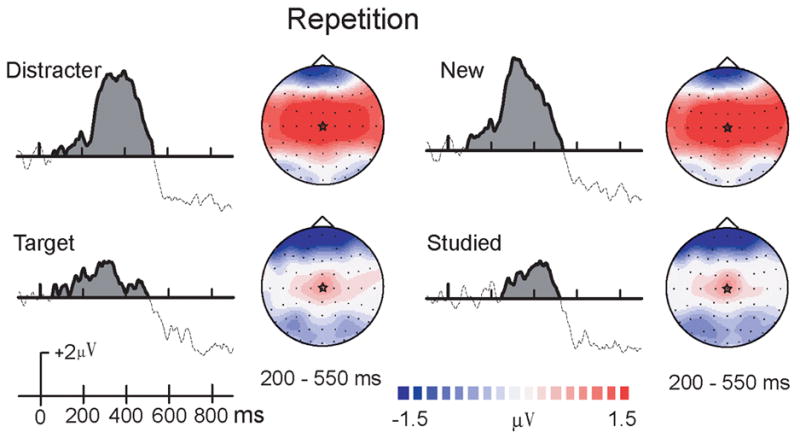
Early repetition effects (repeated minus initial presentations) of Targets and Distracters (combined new and studied), and New versus studied objects (combined target and distracters). A star in each topographic map indicates the central maximum of this repetition effect.
Repetition Reduction
To examine differences in the magnitude of repetition effects, we examined amplitude subtraction ERPs between the second and first presentation. A three-way repeated-measures ANOVA, i.e. stimulus type (new target, studied target, new distracter, studied distracter), time interval (200–550ms, 550–850ms) and electrode site (Fz, FCz, Cz, CPz, Pz, Oz), was conducted. The three-way interaction was significant, F(15,195) = 4.54, p = .006, confirming that repetition effects differed by study type and memory status at the early (p < .005) but not the late (p = .69) time interval (Figure 7).
Figure 7. Difference waves for the late repetition effect.
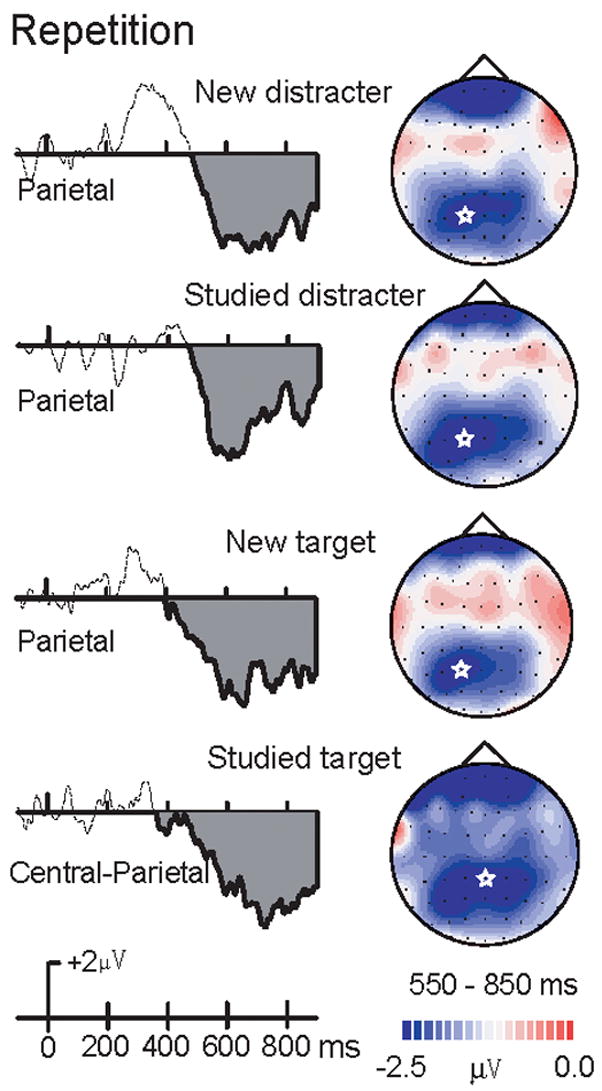
Late repetition effects (repeated minus initial presentations) of new and studied targets and distracters. A star in each topographic map indicates the posterior maximum of this repetition effect. Repetition effects of new versus studied distracter activation (Top graph) and new versus studied target activation (Bottom graph).
4. LORETA Source Localization
To examine the source of the early repetition effect, we were interested in locating the brain regions that evoked response difference between the first and the second presentations of the same visual object. LORETA method was used to yield images of standardized current density associated with repeated visual experience. We examined the source of ERP responses differences by subtracting the ERPs of the second presentation from those of the first presentation. Note that there is always a potential problem using difference wave, because it could potentially create artificial sources. It will be more convincing if LORETA of original ERP waveforms reveal the identical sources. Thus, LORETA source localizations on the two original ERP waves at the time-point of the largest difference were also conducted. Seeing an object for the first time evoked the network of visual areas including occipital, temporal visual cortex, cerebellum and parietal cortices. Seeing the same object for the second time revealed the same network of brain regions and additional activation at inferior frontal area. The LORETA of difference EEG waveforms confirmed the repetition enhancement at the frontal region. We found that the source patterns of the two raw waveforms and the sources for the difference waveform are consistent but with different source strength. Therefore, the LORETA analyses support the existence of the anterior source for early repetition effect.
Results of repetition of targets, distracter, new and studied objects from intracranial source analyses calculated using the LORETA method are shown in Figure 8. The figure illustrates the largest difference of evoked potentials during the interval of 200 to 550 ms in the scalp topographic. The sources of the ERP responses correspond to the early effect at the central locations illustrated in Figures 6. In each case, LORETA source analyses localized the source as ventral prefrontal cortex, which showed similar patterns across all repetitions. In addition, only repetitions of new objects revealed extra source of left temporal pole.
Figure 8. Intracranial source analysis for ERP comparisons.
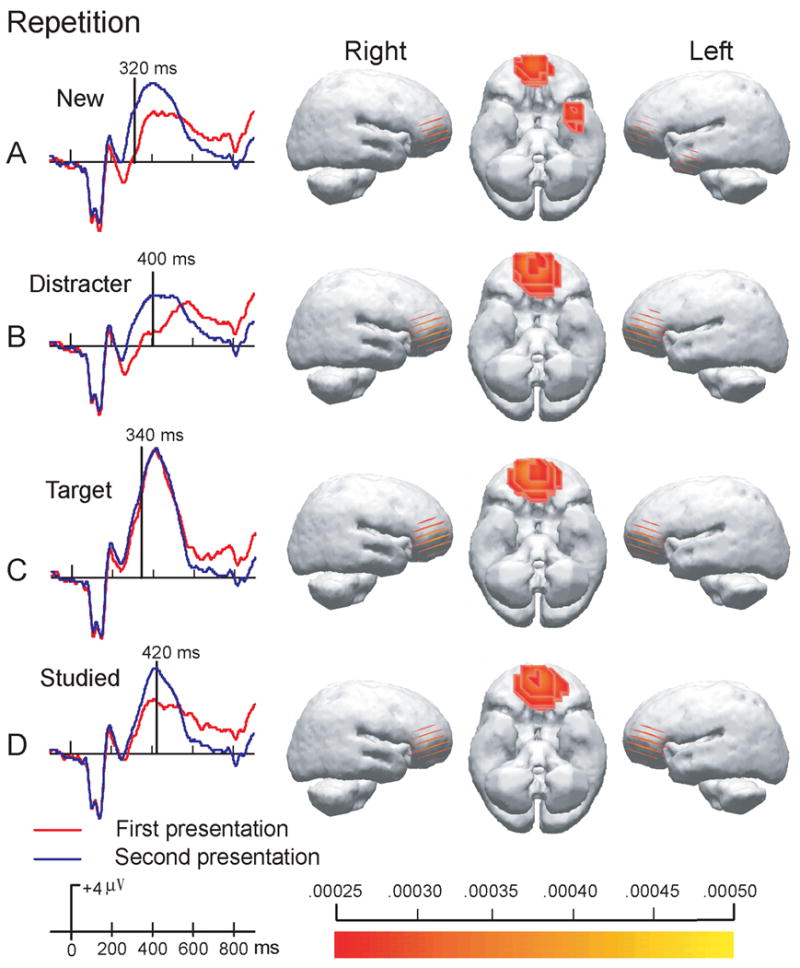
Low-resolution current density reconstructions based on the LORETA model are shown via a color scale for current density reconstructions (CDRs) as computed at the designated time point superimposed on left and right hemispheres. The left column shows the time point with the largest difference, i.e. the largest MGFP functions (Mean Global Field Power), between ERPs of the first and the second presentations.
Discussion
Utilizing a matching task in which participants determined whether successive test objects matched a target held in mind, the present study examined how repetition was influenced by and interacted with prior intentional learning and current working memory processes. Behavioral results revealed a typical repetition effect with faster responses to repeated objects than their initial presentations. Also, when objects were held in working memory as targets, repetition increased accuracy. When objects served as distracters, however, repetition decreased performance accuracy. Caggiano, Jiang and Parasuraman (2006) used a similar working memory task involving the matching of studied faces that repeated up to five times. While the performance for targets showed the trend of improvement with repetition, accuracy declined linearly as distracters were repeated. These behavioral results indicate the disassociation of repetition of distracters and targets. In addition, they support the notion that some frontal conflict monitoring and manipulation of remembered information are involved during this working memory task. fMRI data (Jiang et al, 2000) revealed the anterior activation of inferior frontal/insula, dorsal lateral frontal cortex, and cingulate during this type of working memory task. These cortical regions are often involved in the network that includes working memory, high-level executive function, attention control and conflict monitoring (e.g. Wagner 1999). As we know, the frontal lobe function decays in older adults (Reuter-Lorenz & Sylvester, 2005). This may explain why repetition reduces the accuracy of distracters more in elderly than in the young. The current ERP data provide further evidence that repetition effects can be modulated by high-level executive functions in the frontal cortices.
For ERP results, we initially checked the repetition effect of ERPs before 200 ms by dividing this epoch into two time windows reflecting, 0–200 ms and 100–200 ms. From 0–200 ms, a four-way repeated-measures ANOVA was conducted. The main effects of repetition type were not significant. The repetition effect was significant only with new targets at FCz and Cz (p < .05). From 100–200 ms, the same patterns of results were found. That is, the repetition effect was significant only with new targets at FCz and Cz (p < .05), and ERPs of the initial presentation of new targets were more positive-going than subsequent repetitions, but not at any other electrodes in the other conditions. Since this significant results were 1/10 of all electrodes for four conditions (new distracter, studied distracter, new target and studied target), we focused on the contrast between the repetition effect before 200 ms and the repetition effect after 550 ms. Our ERP results demonstrate the presence of two dissociable repetition effects (summarized in Figure 9). The early ERP repetition effect (200 –500 ms) was centered over central sites with more positive-going and larger potentials to repeated items than their initial presentations. In contrast, the late posterior ERP repetition effect, however, was centered over parietal sites and began after 550 ms with less positive and smaller activation to repeated items than their initial presentations.
Figure 9. Summary of repetition effects for new distracters, studied distracters, new targets and studied targets.
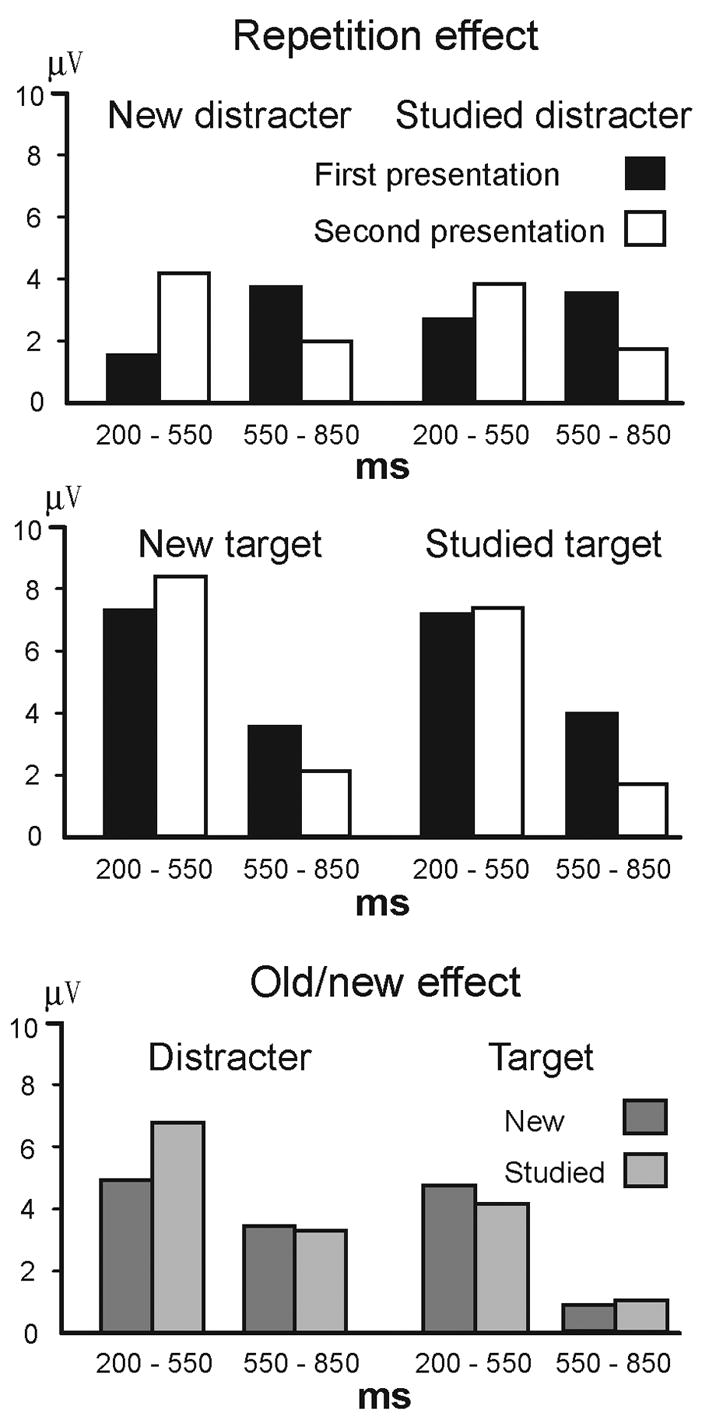
The largest averaged voltages in middle electrodes are seen at Cz for all repetition effects, Pz for the old/new effect of distracters, and Fz for the old/new effect of targets.
The early ERP repetition effect was modulated by both intentional prior learning and working memory processes. This early effect was smaller overall for studied objects than for new objects, and was also visibly smaller for targets than distracters (Figure 6). For new distracters, the early effect was larger and occurred almost 150 msec earlier than for studied distracters. For targets, the early repetition effect occurred only with new targets (Figure 5). As mentioned in the introduction, some previous studies have reported repetition effects at even earlier latencies (e.g., 160 ms, Henson et al., 2003). The long-term repetition (repetition interval was longer than 8 min) of faces and shapes was found to be as early as 50–300 ms for faces or shapes, and as late as 450–650 ms regardless of stimulus meaning (George et al. 1997). Our early repetition effect is consistent with robust ERP old/new effects that differentiated old from new items by virtue of more positive going activation (Friedman & Johnson, 2000; Paller, Hutson, Miller, & Boehm, 2003). ERP studies of explicit learning have revealed similar early ERP components that reflect automatic, familiarity driven (i.e., semantic) memory processes (Paller, 2001). Familiarity processes are distinguished from recollective processes in that familiarity does not include contextual details related to when or how the memory trace is formed. In accord with this familiarity interpretation, our early repetition effect was diminished by both the prior study of ‘old’ objects and responding to a target that was held in mind. Both intentional learning and WM conditions required participants to perceive the object, and thus, would be expected to activate such an automative familiarity mechanism.
While the early repetition effect may be a function of stimulus familiarity, several concerns with such an interpretation persist. First, the comparatively early onset (200 ms earlier than the typical N400 semantic component) and centralized distribution of our early repetition effect may reflect differential neural mechanisms from those comprising the typical early old/new effect (Paller, 2001). Second, all objects used in the study were common objects, and thus, were familiar to participants prior to beginning the study. Third, it seems plausible that if only familiarity mechanisms were responsible for our early repetition effect, then prior elaborative study of ‘old’ objects should, by itself, account for this repetition effect. Prior intentional learning did not, by itself eliminate the early repetition effect (Figures 4 & 5). Only when a test object was both intentionally studied and held in WM (i.e., old target), did the early repetition effect extinguish.
Importantly, the late repetition effect was not affected by whether the repeated item was old or new nor was visibly altered by its status as a target or distracter (Figure 7). The lack of influence from intentional prior learning processes and working memory status, along with its posterior distribution, suggest that this is a “universal” effect and likely reflects an automatic process. In comparison, changes in the early repetition effect due to intentional learning and WM processes, along with its more centralized activation, suggest that this effect may not reflect automatic processing, but contaminated with more frontal, intentionally driven mechanisms.
It should be noted that there was an important difference between the early and late ERP components is that occurrence before or after a subject’s behavioral response. Could the early and late effect be contaminated by motor- or somatosensory-related ERPs? The early repetition effect consistently reflected processing before subjects’ motor responses and the late repetition effect consistently occurred after the motor response. Since neither repetition effect occurred as a function of motor response changes, the comparison between early and late repetition effects should also not reflect differences in motor activity. The early and late effects reflected changes in repetition and not the ERP component overall, so we assert that motor-related activity does not diminish the integrity of our findings.
Similar ERP repetition effects between 400 and 900 ms have been found using both novel and familiar objects (Rugg et al., 1995; Penney et al. 2001). Rugg and colleagues suggested that reduced stimulus analysis processes most likely accounted for their late repetition effect (see also Bentin & McCarthy, 1994). Alternatively, Penney et al. (2001) suggested that a reduction in ERP positive activation for repeated objects may be due to more efficient identification of those objects. Given the indifference of our late ERP effect to prior learning and WM status, however, a repetition suppression interpretation consistent with single-cell and fMRI studies seems most fitting. Previous monkey physiology and human brain imaging evidence has demonstrated the co-existence of two temporary memory mechanisms, i.e. target enhancement in working memory and repetition suppression. The DMS task used in the present study was highly similar to the DMS task used in monkey single-cell recording studies (e.g. Miller & Desimone 1994; Desimone, 1996). The present ERP results indicate that the interaction between these two memory mechanisms occurs before 500 ms, which is beyond the current temporal resolution of human functional MRI. Also using a DMS task, Jiang et al. (2000) showed repetition suppression fMRI effects using familiar faces at occipital, temporal, and parietal lobes. Since hemodynamic effects of repetition suppression are the integration of several seconds of neural activity (Henson & Rugg, 2003; Logothetis et al., 2001), the late and persistent repetition effect showed reduced ERP responses to repeated presentations, i.e. repetition suppression. The early and more transit repetition effect showed the opposite trend. Although inferences of localization from electrode activation are problematic, the posterior distribution of our late repetition component (after 550 ms) is consistent with studies (e.g., Schacter & Buckner; 1998; Fiebach et al., 2005) that have localized repetition reductions to posterior areas of the brain.
In contrast, further evidence of distinct early repetition effect came from ERP localization analysis using LORETA (Figure 8). Three different repetition effects (Figure 7 B, C, and D) revealed identical source - the anterior and early ERP responses were generated from mid prefrontal cortex, which is related with working memory function. To capture the point of ERP responses that differentiate the first and the second viewing of an object, LORETA method of source activation at the largest mean global field power between repetition was used. The results provide further evidence of explicit contamination in the early processing at frontal cortex. Interestingly, response differences of repeated new objects has an extra source, i.e. at left temporal pole (figure 8A). Previous research had showed that anterior temporal or temporal pole function is important in memory and the mnemonic functions of matching and learning (Dupont, 2002). It is possible that the temporal pole responses are linked to learning and repetition of new visual stimuli. Though the exact role of temporal pole in the repetition effect of visual objects is not yet clear, it may reflect the activity of making semantic encoding of the new pictures.
Even though repetition effects have been examined using psychophysics, functional MRI, EEG/ERP, and single-cell recordings methods, the neural underpinnings of repetition are still under debate. For instance, repetition effects are often linked to behavioral priming in humans (e.g. Wiggs & Martin, 1998; Wig, Grafton, Demos, Kelley, 2005). However, such linkage of performance and “repetition suppression” in human fMRI results has been questioned (e.g., Sayres & Grill-Spector, 2006). Recent studies have reported a more complicated picture with fMRI results, for which repetition has not necessarily lead to the suppression of BOLD signals (e.g., Henson et al., 2000; Vuilleumieretal et al., 2005). Three neural models, i.e., fatigue model (less overall activation for repeated presentations), sharpening model (fewer neurons for repeated presentations), and facilitation model (less processing time for repeated presentations), have been proposed (Grill-Spector, Henson, & Martin, 2006). These models were derived from different repetition tasks or methods of measurements. It is likely that the early and late repetition effects reported here can be explained by a combination of models. Comparing results measured by different methods are key to gain understanding of the underlying mechanism of adaptation, but should be done with great caution (Boynton et al., 2003; Krekelberg, Boynton, & Wezel, 2006).
The current study provides direct evidence that repetition effects are not simply the product of a uniform system, but composed of two distinct components. The early repetition effect allows interaction with working memory/attention and long-term memory mechanisms to improve performance efficiency that is related to the memory task. This interaction may allow for a more efficient recognition of targets and rejection of distracters. Meanwhile, the more posterior, late repetition effect appears to be immune from more intentional memory mechanisms, which is consistent with the repetition suppression for objects in occipital, temporal, and parietal lobes as reported in human functional MRI and monkey single-cell recording studies. Evidence of the early frontal repetition and late posterior repetition mechanisms are emerging (e.g. Dale et al., 2000; Gilbert et al., 2005; Grill-Spector et al., 2006). Interestingly, Lawson, Guo & Jiang (2007) reported the significant age-related differences on the early anterior repetition effect but not the late posterior effect using the same paradigm. In addition, combined fMRI and transcranial magnetic stimulation (TMS) evidence supports the idea of separable frontal conceptual and posterior perceptual priming (Wig et al., 2005). Future studies linking these temporally dissociable effects with their anatomical networks should be conducted to test such an hypothesis.
Conclusion
Using a modified DMS task, we examined the influence of prior intentional learning and working memory mechanisms on repetition priming. Two separate priming ERP components were distinguishable in time course, amplitude, and source of the responses. The early repetition effect (200 – 550 ms, anterior-central distribution, i.e. frontal-temporal responses) distinguished repeated items with larger responses and was diminished when objects were prior learned or maintained in memory. Indeed, the early repetition effect was eliminated for targets objects that were prior studied. The more late repetition effect (550 – 900 ms, posterior distribution in visual cortices), however, indexed repeated items with smaller responses and was not affected by neither prior learning nor matching targets. While the late repetition effect may reflect repetition suppression in posterior occipital, temporal and parietal cortices, our ERP results suggest an early repetition effect that can be modulated by explicit memory mechanisms in frontal cortex and temporal pole.
Acknowledgments
The present study was supported by NIH grant AG00986, a pilot grant as part of NIH grant P50 AG05144-21 (YJ), Chinese Ministry of Education 20040028001, Beijing Key Laboratory PHR(IHLB), and National Natural Science Foundation of China 30170322 & 30570603 (CG). The authors thank Q. Zhang for assistance in data collection, and two anonymous reviewers for insightful suggestions. Address correspondence to yjiang@uky.edu (Y. Jiang) or to guocy@mail.cnu.edu.cn (C. Guo).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderer P, Pascual-Marqui RD, Semlitsch HV, Saletu B. Differential effects of normal aging on sources of standard N1, target N1 and target P300 auditory event-related brain potentials revealed by low resolution electromagnetic tomography (LORETA) Evoked Potentials-Electroencephalogr Clin Neurophysiol. 1998;108(2):160–174. doi: 10.1016/s0168-5597(97)00080-4. [DOI] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Effects of attention and emotion on repetition priming and their modulation by cholinergic enhancement. J of Neurophysio. 2003;90:1171–81. doi: 10.1152/jn.00776.2002. [DOI] [PubMed] [Google Scholar]
- Benton S, McCarthy G. The effects of immediate stimulus repetition on reaction time and event-related potentials in tasks of different complexity. J of Exp Psych: Learn, Mem, & Cogn. 1994;20:130–149. [Google Scholar]
- Boynton GM, Finney EM. Orientation-specific adaptation in human visual cortex. J Neurosci. 2003;23:8781–8787. doi: 10.1523/JNEUROSCI.23-25-08781.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Caggiano DM, Jiang Y, Parasuraman R. Aging and repetition priming for targets and distracters in a working memory task. Aging, Neuropsychology and Cognition. 2006;13:552–573. doi: 10.1080/138255890969555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, et al. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93:13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S. Investigating temporal pole function by functional imaging. Epileptic Disord. 2002 Sep;4(Suppl 1):S17–22. [PubMed] [Google Scholar]
- Fabiani M, Gratton G, Coles MGH. Event-related brain potentials. Handbook of Psychophysiology. 2000;2:53–84. [Google Scholar]
- Fiebach CJ, Gruber T, Supp GG. Neuronal mechanisms of repetition priming in occipitotemporal cortex: spatiotemporal evidence from functional magnetic resonance imaging and electroencephalography. J of Neurosci. 2005;25:3414–3422. doi: 10.1523/JNEUROSCI.4107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Johnson R., Jr Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microscopy Res and Techniq. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Guo C, Duan L, Li W, Paller KA. Distinguishing source memory and item memory: brain potentials at encoding and retrieval. Brain Res. 2006;1118:142–154. doi: 10.1016/j.brainres.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Guo C, Lawson AL, Zhang Q, Jiang Y. Brain potentials distinguish new and studied objects during working memory. Human Brain Mapping. 2007 doi: 10.1002/hbm.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George N, Jemel B, Fiori N, Renault B. Face and shape repetition effects in humans: a spatio-temporal ERP study. NeuroReport. 1997;8:1417–1423. doi: 10.1097/00001756-199704140-00019. [DOI] [PubMed] [Google Scholar]
- Gilbert J, Holroyd T, Carver FW, Bellgowan P, Martin A. Top-down modulation during object priming: Evidence from MEG. Proc Cogn Neurosci Soc. 2005:132. [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMRI-adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst) 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Gruber T, Muller MM. Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cereb Cortex. 2005;15:109–116. doi: 10.1093/cercor/bhh113. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Henson R. Neuroimaging studies of priming. Progress in Neurobio. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson R, Goshen-Gottstein Y, Ganel T, Otten LJ, Quayle A, Rugg MD. Electrophysiological and haemodynamic correlates of face perception, recognition, and priming. Cerebral Cortex. 2003;13:793–805. doi: 10.1093/cercor/13.7.793. [DOI] [PubMed] [Google Scholar]
- Henson R, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioral priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Henson R, Rylands A, Ross E, Vuilleumeir P, Rugg MD. The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. NeuroImage. 2004;21:1674–1689. doi: 10.1016/j.neuroimage.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci U S A. 2004;101:9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking items in human working memory. Science. 2000;287:643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- Krekelberg B, Boynton GM, van Wezel R. Adaptation: from single cells to BOLD signals. TRENDS in Neurosciences. 2006;29(5):250–256. doi: 10.1016/j.tins.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Lawson AL, Guo C, Jiang Y. Age effects on brain activity during repetition priming of targets and distracters. Neuropsychologia. 2007;45:1223–1231. doi: 10.1016/j.neuropsychologia.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–523. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- Nagy ME, Rugg MD. Modulation of event-related brain potentials by word repetition: The effects of inter-item lag. Psychophysiology. 1989;26:431–436. doi: 10.1111/j.1469-8986.1989.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Paller KA. Neurocognitive foundations of human memory. In: Medin D, editor. The Psychology of Learning and Motivation: Advances in Research and Theory. Vol. 40. 2001. pp. 121–145. [Google Scholar]
- Paller KA, Hutson CA, Miller BB, Boehm SG. Neural manifestations of memory with and without awareness. Neuron. 2003;38:507–516. doi: 10.1016/s0896-6273(03)00198-3. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Esslen M, Kochi K, Lehmann D. Functional imaging with low resolution brain electromagnetic tomography (LORETA): review, new comparisons, and new validation. Japanese J Clin Neurophysiol. 2002;30:81–94. [PubMed] [Google Scholar]
- Penney TB, Mecklinger A, Nessler D. Repetition related ERP effects in a visual object target detection task. Brain Res Cogn Brain Res. 2001;10:239–250. doi: 10.1016/s0926-6410(00)00041-0. [DOI] [PubMed] [Google Scholar]
- Pickering EC, Schweinberger SR. N200, N250r, and N400 event-related brain potentials reveal three loci of repetition priming for familiar names. J of Exp Psych: Learn, Mem, and Cogn. 2003;29:1298–1311. doi: 10.1037/0278-7393.29.6.1298. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Sylvester CC. The cognitive neuroscience of working memory and aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York, NY: Oxford University Press; 2005. pp. 186–217. [Google Scholar]
- Rugg MD. Dissociation of semantic priming, word and non-word repetition effects by event-related potentials. Quart J of Exp Psych: Human Exp Psych. 1987;39:123–148. [Google Scholar]
- Rugg MD. Event-related potentials dissociate repetition effects of high and low frequency words. Mem & Cogn. 1990;18:367–379. doi: 10.3758/bf03197126. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Nagy ME. Lexical contribution to nonword-repetition effects: Evidence from event-related potentials. Mem and Cogn. 1987;15:473–481. doi: 10.3758/bf03198381. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Soardi M, Doyle MC. Modulation of event-related potentials by the repetition of drawings of novel objects. Brain Res Cogn Brain Res. 1995;3:17–24. doi: 10.1016/0926-6410(95)00014-3. [DOI] [PubMed] [Google Scholar]
- Sayres R, Grill-Spector K. Object-selective cortex exhibits performance-independent repetition suppression. J Neurophysiol. 2006;95:995–1007. doi: 10.1152/jn.00500.2005. [DOI] [PubMed] [Google Scholar]
- Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: A single-cell study of the fMRI adaptation paradigm. Neuron. 2006;49:307–318. doi: 10.1016/j.neuron.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 objects: Norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psych. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Van Turennout MV, Ellmore T, Martin A. Long-lasting cortical plasticity in the object naming system. Nature Neurosci. 2000;3:1329–1334. doi: 10.1038/81873. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Senkfor AJ. Memory for words and novel visual patterns: Repetition, recognition, and encoding effects in the event-related brain potential. Psychophysiology. 1996;33:491–506. doi: 10.1111/j.1469-8986.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Viggiano MP, Kutas M. Overt and covert identification of fragmented objects inferred from performance and electrophysiological measures. J of Exp Psych: Gen. 1998;129:107–125. doi: 10.1037//0096-3445.129.1.107. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S, Duhoux S, Dolan RJ, Driver J. Selective attention modulates neural substrates of repetition priming and “implicit” visual memory: Suppressions and enhancements revealed by fMRI. J of Cognitive Neurosci. 2005;17(8):1245–1260. doi: 10.1162/0898929055002409. [DOI] [PubMed] [Google Scholar]
- Wagner AD. Working memory contributions to human learning and remembering. Neuron. 1999;22:19–22. doi: 10.1016/s0896-6273(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Schacter DL. Interactions between forms of memory: When priming hinders new episodic learning. J of Cognitive Neurosci. 2000;12(2):52–60. doi: 10.1162/089892900564064. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nature Neuroscience. 2005:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiol. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Yi DJ, Chun MM. Attentional modulation of learning-related repetition attenuation effects in human parahippocampal cortex. J Neurosci. 2005;25:3593–3600. doi: 10.1523/JNEUROSCI.4677-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago L, Fenske MJ, Aminoff E, Bar M. The rise and fall of priming: How visual exposure shapes cortical representations of objects. Cereb Cortex. 2005;15:1655–1666. doi: 10.1093/cercor/bhi060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Begleiter H, Porjesz B, Wang W, Litke A. Event related potentials during object recognition tasks. Brain Res Bulletin. 1995;38:531–538. doi: 10.1016/0361-9230(95)02023-5. [DOI] [PubMed] [Google Scholar]