Abstract
During neurogenesis, the progression from a progenitor cell to a differentiated neuron is believed to be unidirectional and irreversible. The Rb family of proteins (Rb, p107 and p130) regulates cell cycle exit and differentiation during retinogenesis. Rb and p130 are redundantly expressed in the neurons of the inner nuclear layer (INL) of the retina. We have found that in the adult Rb;p130-deficient retinae p107 compensation prevents ectopic proliferation of INL neurons. However, p107 is haploinsufficient in this process. Differentiated Rb−/−;p107+/−;p130−/− horizontal interneurons re-entered the cell cycle, clonally expanded, and formed metastatic retinoblastoma. Horizontal cells were not affected in Rb+/−;p107−/−;p130−/− or Rb−/−;p107−/−;p130+/− retinae suggesting that one copy of Rb or p130 was sufficient to prevent horizontal proliferation. This is the first demonstration that differentiated neurons can proliferate and form cancer while maintaining their differentiated state including neurites and synaptic connections.
Keywords: horizontal cell, Rb, p107, retinoblastoma, metastasis
Introduction
One of the fundamental principles in developmental biology is that cell cycle exit must precede differentiation, because the regulatory networks that drive cell proliferation are incompatible with those that direct differentiation. When differentiated cells are forced to re-enter the cell cycle, they typically undergo programmed cell death. For example, ectopic expression of cyclin D1 in differentiating retinal photoreceptors results in apoptosis (Skapek et al., 2001). In addition, programmed cell death associated with inappropriate proliferation of differentiated neurons in other regions of the central nervous system (CNS) contributes to human neurodegenerative disorders such as Alzheimer's disease (Yang et al., 2001).
Studies on progenitor cells in developing embryonic tissues have provided some key insight into the mechanism underlying the coordination of proliferation and differentiation. When differentiation is induced prematurely in proliferating progenitor cells, it is accompanied by concomitant cell cycle withdrawal. For example, in developing muscle cells, MyoD induces myocyte differentiation and binds to Rb to prevent further rounds of cell division (Gu et al., 1993). This tightly coordinated regulation of proliferation and differentiation is not limited to the developing embryo. In self-renewing adult tissues such as the hematopoietic system, cell cycle exit precedes differentiation. To retain its long-term self-renewing capability while generating terminally differentiated cell types, the hematopoietic system relies on a small number of stem cells. Hematopoietic stem cells are thought to divide asymmetrically to generate another stem cell and a daughter cell that produces differentiated hematopoietic cells. A similar mechanism is used by neural stem cells in the adult CNS in regions where neurogenesis occurs (Reynolds and Weiss, 1992). For example, neural stem cells lining the ventricles of the brain give rise to additional stem cells and committed neural precursors that migrate along the rostral migratory stream to differentiate and integrate into the olfactory bulb (Lois and Alvarez-Buylla, 1993; Luskin, 1993). Therefore, in both embryonic progenitor cells and adult stem cells, cell cycle exit precedes differentiation.
Examples of dedifferentiation and transdifferentiation have provided important insight into the reverse process. When differentiated cells re-enter the cell cycle, this event is preceded by cellular, molecular, and morphologic dedifferentiation. For example, radial glial cells in the retina (Müller glia) are capable of generating neurons in response to injury or exogenous growth factors (Fausett and Goldman, 2006; Fischer et al., 2002; Ooto et al., 2004). Neurogenesis from retinal radial glia is accompanied by dedifferentiation, proliferation, and subsequent neuronal cell fate specification and maturation. Similar dedifferentiation processes have been reported in other systems such as muscle (Odelberg et al., 2000) providing a strong foundation for the widely held belief in developmental biology that a cell must exit the cell cycle before it can differentiate and that a differentiated cell cannot proliferate while maintaining its molecular, cellular, and morphologic features.
Cancer represents one of the best examples of deregulated proliferation during development and in adults. As mentioned above, differentiated cells usually undergo programmed cell death when they re-enter the cell cycle inappropriately. Therefore, it is not surprising that genetic perturbations in genes that control cell death occur during tumorigenesis. Yet, even in cancer cells, differentiation and proliferation are, for the most part, considered incompatible. Specifically, tumor cells with molecular or histologic features of differentiated cells are generally less aggressive and less invasive than those resembling progenitor or stem cells. One of the best examples of this is chronic myeloid leukemia (CML) (Faderl et al., 1999). Early-stage CML is associated with few signs or symptoms, because the cancer cells differentiate and remain relatively benign. However, in the later stage of CML, which is called blast crisis, the cancer cells fail to differentiate and are much more aggressive and invasive. Chemotherapy that induces tumor cell differentiation has proven to be an effective treatment for CML establishing the clinical significance of the inverse correlation between tumor cell differentiation and long-term survival of patients.
Rb and its related family members (p107 and p130) lie at the heart of the cell cycle machinery that executes cell cycle exit in coordination with terminal differentiation during development. Interestingly, studies in the developing CNS and other tissues have suggested that the Rb family also regulates differentiation in some tissues. In the developing retina, the individual roles of the Rb family members and their complex genetic compensation and redundancy have been extensively studied (Chen et al., 2004; Donovan et al., 2006; MacPherson et al., 2004; Zhang et al., 2004a). In postmitotic differentiated neurons of the inner nuclear layer (INL), p130 is expressed redundantly with Rb (Donovan et al., 2006). Although proliferation is mildly deregulated in Rb;p130-deficient retinae during development (MacPherson et al., 2004), compensation by p107 facilitates relatively normal neurogenesis in the INL (Donovan et al., 2006). Additional studies have shown that p107 is haploinsufficient for proliferative control in Rb-deficient retina (Donovan et al., 2006).
In this study, we tested the effect of reduced Rb family function in the developing retina by characterizing retinal development in p107-single (Chx10-Cre;RbLox/Lox;p107+/−;p130−/−) mice. We find that horizontal interneurons in the INL of the p107-single retina differentiate normally during development and form appropriate synaptic connections, but several weeks later, they re-enter the cell cycle and clonally expand. Dividing cells maintain all of the features of differentiated neurons including neurites and synapses. This is the first example of a differentiated neuron undergoing continued cell division while maintaining the hallmarks of mature neurons. The proliferating horizontal cells form highly aggressive retinoblastomas with many of the features of differentiated horizontal cells.
Results
Horizontal Cell Expansion in Adult p107-Single Retinae
Rb and p130 are redundantly expressed in the INL neurons of the mouse retina (Donovan et al., 2006). To determine whether compensation by p107 is sufficient to generate the correct proportion of retinal neurons and glia in the INL and whether it is sufficient to prevent them from re-entering the cell cycle, we analyzed Chx10-Cre;RbLox/Lox;p130−/− retinae at postnatal day (P) 6, P12, P30, and P60. The Chx10-Cre transgene is expressed in a mosaic pattern in retinal progenitor cells during development, resulting in apical-basal stripes of cells spanning all retinal layers that lack Rb (Donovan et al., 2006; Rowan and Cepko, 2004; Zhang et al., 2004a). The advantage of this Cre transgene is that adjacent stripes lack Cre activity and provide a convenient internal genetic control (Fig. 1A). Horizontal cells, bipolar cells, amacrine cells and Müller glia differentiated normally in the absence of Rb and p130 (Fig. 1B,C and Sup. Fig. 1A-C). As expected (Donovan et al., 2006; MacPherson et al., 2004; Zhang et al., 2004a), rod photoreceptors failed to differentiate in the ONL in the absence of Rb at P12 (Sup. Fig. 1D).
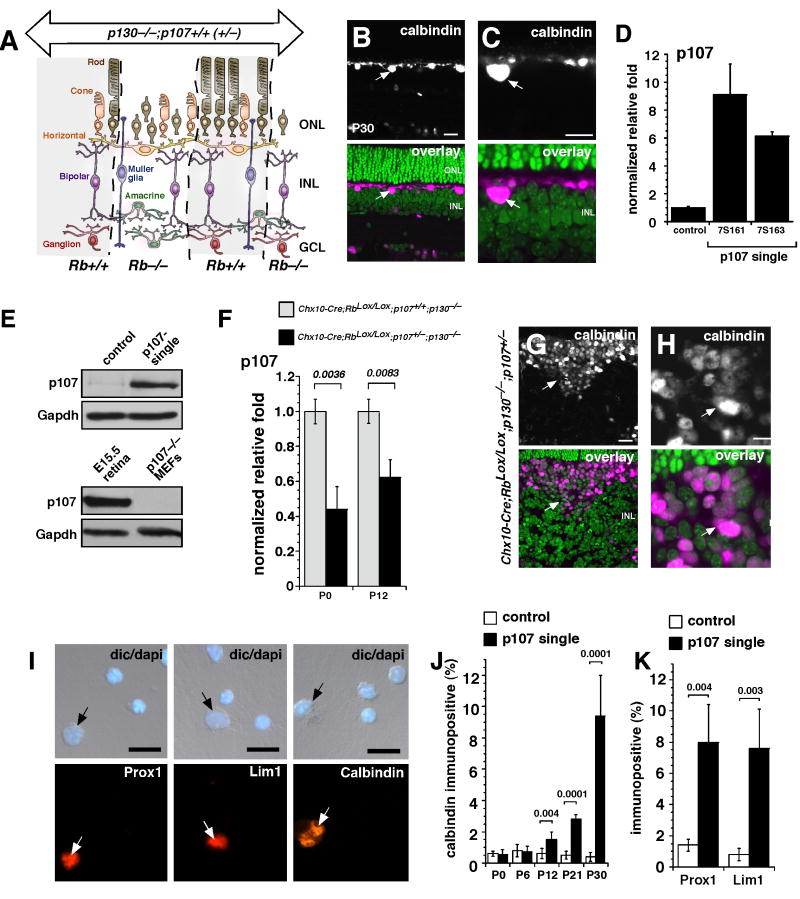
Figure 1. Horizontal cells expand in the postnatal p107-single retina.
(A) In Chx10-Cre;RbLox/Lox;p107+/−;p130−/− mice, the entire retina lacked p130 and one copy of p107, and Rb was absent in mosaic stripes, which is consistent with the expression of Cre from the Chx10 promoter. (B,C) Immunofluorescent detection of horizontal cells in Rb;p130-deficient retinae with green nuclear counterstaining. Real-time RT-PCR (D), and immunoblot (E) analysis of p107 expression in p107-single (Chx10-Cre;RbLox/Lox;p107+/−;p130−/−) and control (RbLox/Lox;p107+/−;p130−/−) retinae. (F) Real time RT-PCR for p107 expression in p107-single and control retinae at P0 and P12. (G,H) Immunofluorescent detection of horizontal cells in P30 retinal sections and in dissociated cells (I,J) for p107-single and control littermates. Two other markers of horizontal cells (Prox1 and Lim1) were also increased at P30, as measured by dissociated cell scoring (K). Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars: 10 µm.
It has been reported previously in mouse embryonic fibroblasts (Sage et al., 2003) and in the developing retina (Donovan et al., 2006; Zhang et al., 2004a) that p107 compensates for loss of Rb. To test if p107 is upregulated in Rb;p130-deficient retinae and in p107-single (Chx10-Cre;RbLox/Lox;p130−/−;p107+/−) retinae in a similar manner, we performed real-time RT-PCR (Fig. 1D), immunoblotting (Fig. 1E), and in situ hybridization (Sup. Fig. 1E) on p107-single and littermate control retinae.
In the adult p107-single retinae, p107 mRNA and protein were upregulated in the INL (Fig. 1D,E). Despite this compensatory increase in the expression of p107 in the absence of Rb, p107 was haploinsufficient to regulate proliferation of retinal progenitor cells (Donovan et al., 2006). Consistent with these previous studies, the compensatory upregulation of p107 in the the p107-single retinae was less than in the Rb;p130 double knockout retinae at P0 and P12 (Fig. 1F). To determine whether p107 compensation was haploinsufficient to maintain postmitotic, differentiated INL neurons and glia lacking Rb and p130, we analyzed the location and proportion of each retinal cell type in the INL from adult p107-single retinae. The horizontal cell population, which normally represents approximately 0.2% of the total cell population in the mouse retina, expanded in the p107-single retinae while maintaining its normal position adjacent to the outer plexiform layer (OPL) (Fig. 1G,H). Scoring of dissociated cells revealed a 50-fold expansion of horizontal cells by P30 (comprising 10% of the total cell population) (Fig. 1I-K). Expansion of the horizontal cell population was progressive in adult retinae (Fig. 1I), and the proportion of horizontal cells was not significantly increased during embryonic development (data not shown).
To confirm that these cells were indeed horizontal cells, we used three different markers: Lim1, Prox1, and calbindin (Fig. 1I-K). Real-time RT-PCR (Sup. Fig. 1G,H), dissociated cell scoring (Fig. 1I-K), and immunostaining of retinal sections (Fig. 1G,H) confirmed that the proportion of horizontal cells that were immunopositive for Prox1, Lim1, or calbindin increased in p107-single retinae. As expected, the opposite trend was seen for rod photoreceptors lacking Rb (Zhang et al., 2004a)(Sup. Fig. 1I). Similar analyses carried out on Rb-single and p130-single retinae indicated that the robust horizontal cell expansion was unique to the p107-single retinae (Sup. Figs. 2,3). Therefore, further analysis was limited to p107-single samples.
Horizontal Cell Proliferation in Adult p107-Single Retinae
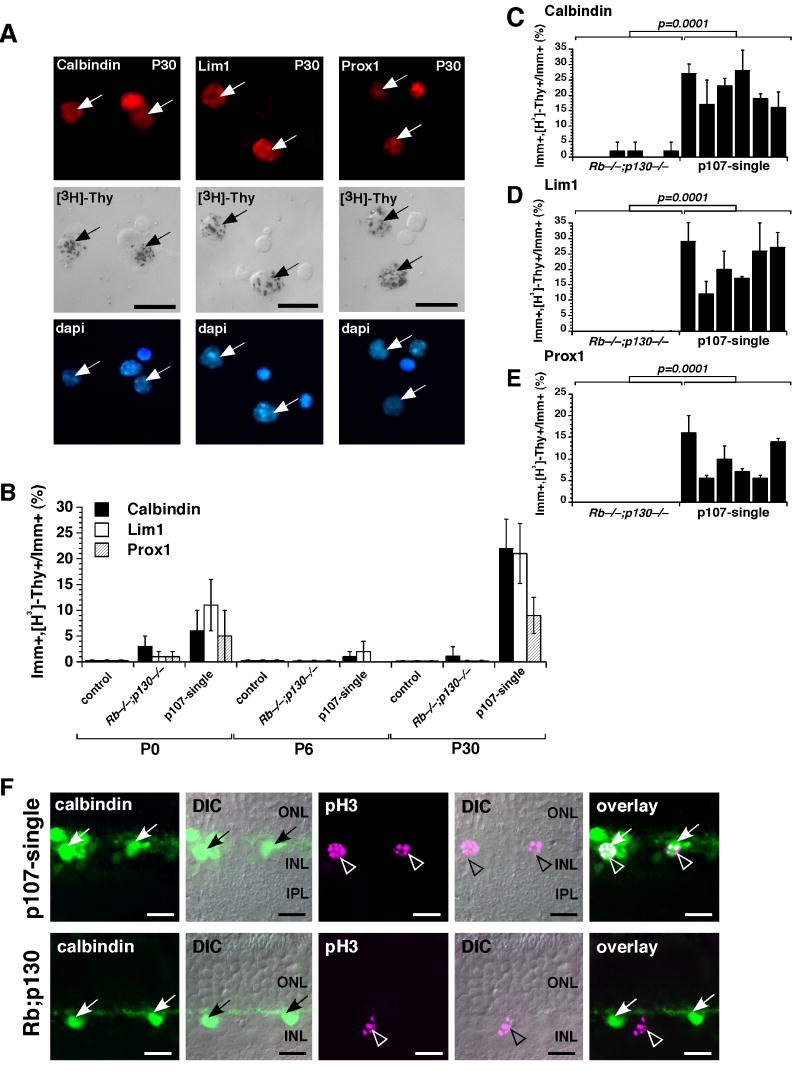
To determine if the increase in horizontal cells in the p107-single retina was due to proliferation of a retinal progenitor cell or a differentiated horizontal interneuron, we performed a [3H]-thymidine labeling experiment. Retinae from p107-single mice and their littermates were pulse-labeled with [3H]-thymidine for 1 h. The tissue was dissociated, plated on glass slides, immunostained for calbindin, Lim1, or Prox1, and overlaid with autoradiographic emulsion to detect the [3H]-thymidine (Fig. 2A). At P0 and P6, when there are still some proliferating retinal progenitor cells in the normal developing retina, there was a slightly higher proportion of proliferating cells that were immunopositive for Lim1, Prox1, or calbindin in the p107-single retinae compared to that in the Rb;p130-deficient or RbLox/Lox;p107+/−;p130−/− retinae (Fig. 2B). However, by P30, when all proliferation had ceased in the Rb;p130-deficient retinae, there was a 10- to 20-fold increase in the proportion of proliferating cells that were positive for [3H]-thymidine and Lim1, Prox1, or calbindin in the p107-single retinae (Fig. 2B-E). No Lim1, Prox1 or calbindin cells incorporated [3H]-thymidine at any stage of development in the control retinae from control (RbLox/Lox;p107+/−;p130−/−) mice (Fig. 2B and data not shown). To determine if the horizontal cells in S-phase could progress through the cell cycle to M-phase we performed co-immunolocalization with calbindin and phospho-histone H-3 (pH3) a marker of M-phase cells. In p107-single retinae, pH3/calbindin double positive cells were readily identified in clusters of horizontal cells but not in the Rb;p130-deficient retinae (Fig. 2F).
Figure 2. Horizontal cell proliferation in the postnatal mouse retina.
P30 p107-single retinae were [3H]-thymidine labeled for 1 hour and then dissociated, immunostained for Lim1, calbindin, and Prox1 expression and overlaid with autoradiographic emulsion to detect the [3H]-thymidine (A). The proportion of immunopositive cells that incorporated [3H]-thymidine was scored for each antibody at 3 stages of development (B). 6 animals from each genotype were assessed at each stage, and each sample was scored in duplicate. The controls were RbLox/Lox;p107+/−;p130−/− and Chx10-Cre;RbLox/Lox;p130−/− littermates. (C-E) Data on [3H]-thymidine labeling for individual animals at P30 is presented to illustrate the animal-to-animal variation with Chx10-Cre;RbLox/Lox;p130−/− littermates as a controls. (F) Co-immunolocalization of calbindin (green) and phospho-histone H3 (pH3) (purple) in P30 p107-single and control (Chx10-Cre;RbLox/Lox;p130−/−) littermates. Scale bars: 10 µm.
Proliferating Horizontal Cells Extend Neurites and Form Synapses in p107-Single Retinae
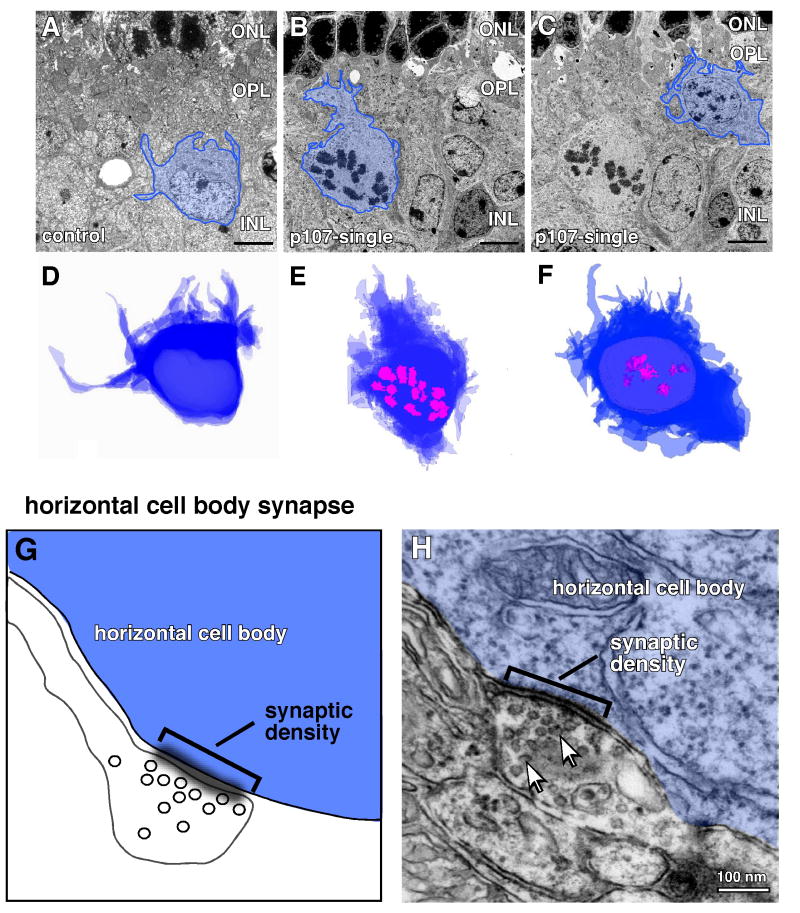
To determine whether proliferating cells in the p107-single retinae exhibit morphologic features of horizontal cells, we performed transmission electron microscopy (TEM) of adult (P30) p107-single retinae and their littermate controls. 130-150 serial 50 nm thick sections were collected, imaged, aligned and used to generate a 3-d reconstruction of control and p107-single horizontal cells (Fig. 3A-F, and Sup. Fig. 4). The cell bodies were large and adjacent to the OPL (Sup. Fig. 4B-I). The mean cross-sectional area (± SEM) of 25 p107-single horizontal cells with mitotic figures was 82.3 ± 8 µm2, and that in 25 control horizontal cells was 86.9 ± 7 µm2. In addition, triad synapses between photoreceptors, bipolar cells, and horizontal cells were adjacent to the mitotic horizontal cell bodies (Sup. Fig. 4B-E). These data demonstrate that dividing horizontal cells had morphologic features of normal horizontal cells (Fig. 3A-F). Measurements of cellular processes that projected from the cell bodies indicated that processes from both cell populations were tapered, with diameters at the base more than twice that at the tip (Sup. Fig. 4J), a unique characteristic of horizontal cells. We scored 18 p107-single horizontal cells with mitotic figures; among those cells, we observed 11 cellular processes that extended more than 3 µm from the cell body. For the 18 control horizontal cells, we observed 9 cellular processes extending more than 3 µm. The average lengths of long and short processes and the average diameters at the bases and tips were very similar for the two populations of horizontal cells (Sup. Fig. 4J).
Figure 3. Proliferating horizontal cells are differentiated.
(A-C) Representative images of the 50 nm serial sections for each horizontal cell imaged using transmission electron microscopy (TEM). Horizontal cell bodies are outlined in blue. A normal horizontal cell in a control littermate is shown in (A) and two dividing horizontal cells in M-phase are shown in (B,C) as indicated by the presence of condensed chromosomes (mitotic figures). The cell bodies were traced from images of 130-150 serial sections per horizontal cell and stacked to form a 3-d representation of the cells shown in (A-C) (D-F). The condensed chromosomes in B,C are outlined in pink in E,F. (G) Diagram of one type of conventional horizontal cell synaptic connection present on the cell body with input from a small diameter process from a neighboring horizontal cell. (H) TEM image of a typical horizontal cell body synapse on the surface of a dividing horizontal cell in the p107-single retina. The synaptic density (sd) is indicated by the bracket and the synaptic vesicles are shown by arrows. Abbreviations: ONL, outer nuclear layer; OPL, outer plexiform layer.
Next, we analyzed the pre- and post-synaptic connections on the dividing horizontal cells. There are two types of synapses that are characteristic of horizontal cells. The first are synapses from neighboring horizontal cells or interneurons on the cell body of the horizontal cell (Fig. 3G). We analyzed the serial sections of the proliferating horizontal cells and 26 other independent horizontal cells with mitotic figures. Cell body synapses were readily identified on all of these M-phase horizontal cells in the P30 p107-single retinae (Fig. 3H). The synapses have synaptic densities and synaptic vesicles characteristic normal horizontal cell body synapses.
The second type of horizontal cell synapses are part of the photoreceptor/bipolar/horizontal neuron synaptic triad. To determine if individual neurites from dividing horizontal cells contributed to outer plexiform layer synaptic triads we traced individual neurites across serial sections of dividing p107-single horizontal cells. One example is shown in Fig. 4. Three different magnifications of section #10 (Fig. 4A-C) are shown to illustrate the region of the synaptic triad (Fig. 4D). The next 50 nm section (section #11) clearly shows a neurite from the process shown in section #10 that forms a synapse with a cone pedicle containing a synaptic density and a synaptic ribbon (Fig. 4E). The horizontal cell neurite has synaptic vesicle characteristic of normal horizontal cell neurites found in synaptic triads at the OPL. These data suggest that fully differentiated horizontal cells with neurites and synapses can re-enter the cell cycle and undergo cell division.
Figure 4. Dividing horizontal cells contribute to synaptic triads.
Another type of conventional synapse made by horizontal cells is with rod and cone photoreceptor terminals via an invaginating process that is both presynaptic and postsynaptic. The horizontal cell neurite along with a bipolar cell dendrite invade the photoreceptor terminals as a bundle, termed a triad. (A-C) In the example shown here from the serial section analysis at increasing magnification, a dividing horizontal cell (blue) sends a thick process toward the outer plexiform layer consisting of photoreceptor terminals. (D) An illustration of a typical synaptic triad between horizontal neurites (blue), bipolar dendrites (yellow) and photoreceptor terminals such as a cone pedicle (shown). (E) In the section (#11) adjacent to that shown in (A-C) (#10) the neurite that extends to the outer plexiform layer forms a synaptic triad with a cone and bipolar cell. The diameter of the neurite is less than 100 nm and forms a synapse with a cone pedicle containing two synaptic ribbons in this section. The active zone of the synapse is marked by a pre-synaptic ribbon and a synaptic density. Synaptic vesicles in the horizontal cell neurite are indicated by arrows. Abbreviations: OPL, outer plexiform layer; sd, synaptic density; HC, horizontal cell; Bip, bipolar dendrite.
Clonal Expansion of p107-Single Horizontal Cells
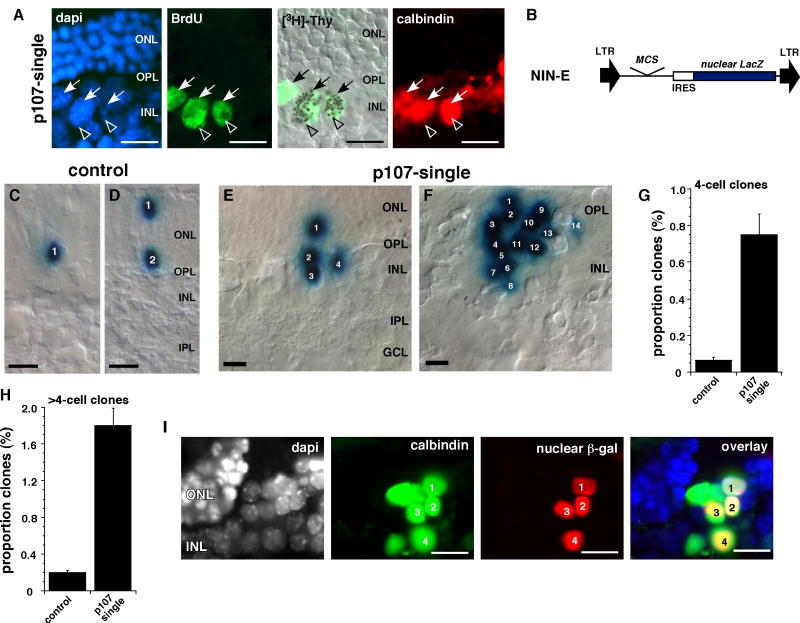
To determine whether a single horizontal cell could undergo successive rounds of cell division, we performed a double labeling experiment. P30 p107-single retinae were labeled in vivo with BrdU and 24 hours later the S-phase cells were labeled with [3H]-thymidine. The retinae were cryosectioned, immunolabeled for calbindin to identify the horizontal cells and BrdU to identify the cells that were in S-phase at the start of the experiment. Finally, the retinal sections were overlaid with autoradiographic emulsion to identify the horizontal cells that progressed through a second round of S-phase. BrdU/[3H]-thymidine double positive horizontal cells were readily identified along the apical edge of the INL where normal horizontal cells reside (Fig. 5A).
Figure 5. Clonal expansion of horizontal cells in the p107-single retina.
(A) Adult p107-single mice received a single BrdU injection followed by an injection of [3H]-thymidine 48 hours later. Co-localization of BrdU, calbindin and [3H]-thymidine demonstrated that the horizontal neurons could progress through at least two rounds of S-phase. (B) P0 retinae from p107-single mice and their control littermates were infected with a replication-incompetent retrovirus that expressed nuclear LacZ (NIN-E) and cultured for 14 days. (C-F) Representative clones from control (C,D) and p107-single (E,F) retinae. (G,H) The proportion of large clones (≥4 cells) was increased in the p107-single retinae. (I) Co-immunolocalization of a horizontal cell marker (calbindin) and the nuclear β-gal reporter protein in large clones found in the p107-single retinae. Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer;. Scale bars: 10 µm.
Next, we performed clonal analysis on adult p107-single and control retinae. For this experiment, we used a replication-incompetent retrovirus that expresses nuclear LacZ (NIN-E) (Fig. 5B) (Dyer and Cepko, 2000; Dyer and Cepko, 2001; Zhang et al., 2004a). P0 p107-single and control retinae were infected with the NIN-E retrovirus and maintained in culture as whole-tissue explants for 15 days. Because most proliferating retinal progenitor cells were in their final round of cell division in the control retinae, the clone size was primarily 1 or 2 cells (Fig. 5C,D). However, in the p107-single retinae, there were some very large clones (up to 22 cells) at the apical surface of the OPL (Fig. 5E,F). The proportion of clones that were 4 cells or larger was significantly increased in the p107-single retinae (p=0.001; Fig. 5G,H) and the β-galactosidase expressing cells in the clones also expressed calbindin (Fig. 5I). These data suggest that horizontal cells not only re-enter the cell cycle, but also clonally expand in the p107-single retina.
Live Imaging of Dividing Horizontal Cells
To visualize the proliferating horizontal cells in the p107-single retinae and analyze their morphology, we generated Gad67-GFP;Chx10-Cre;Rblox/Lox;p107+/−;p130−/− mice. In these mice, the developing and mature horizontal cells express GFP in their cell bodies and processes (Sup. Fig. 5). We isolated P30 retinae from Gad67-GFP-p107-single mice or their littermate controls and imaged the horizontal cells by confocal laser scanning microscopy. The z-series was deconvoluted and reconstructed into a 3-d image using Imaris 5.0 software. The filamentTracer feature of Imaris 5.0 was used to trace the neurites of the horizontal cells in the control (Sup. Fig. 5A and Movie 1) and the clusters of horizontal cells in the p107-single retinae (Sup. Fig. 5B and Movie 2). These data are consistent with the reconstruction of serial electron micrographs showing that the proliferating cells in p107-single retinae have morphological features of horizontal cells. To test the possibility that the proliferating cells at the apical edge of the INL were retinal progenitor cells or bipolar neurons we performed a similar series of experiments on these cell types and included analysis by transmission electron microscopy (Sup. Figs. 6,7 and Movies 3,4). Bipolar neurons were included in this characterization because they are the only other neuronal cell type found at the apical edge of the INL where horizontal cells reside. The embryonic retinal progenitor cells and the bipolar cells have none of the morphological features of the dividing cells in the p107-single retinae (Sup. Figs. 5-7).
To provide independent molecular confirmation of these data, we analyzed the gene expression profiles of purified progenitor cells, bipolar cells and horizontal cells (Sup. Fig. 5C-E) and compared them to the p107-single retinae and p107-single retinoblastomas (Sup. Fig. 5F,G, Sup. Fig. 6D,E, and Sup. Fig. 7F-H). In each of these examples, the p107-single retinae and tumors resemble horizontal cells and have little in common with retinal progenitor cells or bipolar neurons.
The Gad67-GFP;Chx10-Cre;Rblox/Lox;p107+/−;p130−/− mice also provided us with a unique opportunity to visualize horizontal cell division in the p107-single retinae using two-photon confocal time-lapse imaging of proliferating horizontal cells. Control retinae showed a regular mosaic of horizontal cells (Sup. Fig. 8) and the GAD-67-GFP;p107-single retinae showed clusters of horizontal cells. Analysis of time-lapse, 4-dimensional movies of these clusters revealed proliferating cells with morphologic features of horizontal cells (Fig. 6A-C, and Movies 5,6). To provide additional confirmation that the dividing cells in these 2-photon time-lapse experiments are horizontal cells, we performed single cell injections of individual daughter cells after cytokinesis. We used a mixture of Alexa 594 and neurobiotin for the injections (see Sup. Materials and Methods). The Alexa 594 labeled the injected horizontal cell and was traced using Imaris 5.0 FilamentTracer and the neurobiotin can difuse through gap junctions that connect adjacent horizontal cells in the retina. The daughter cells has morphological features of a horizontal neuron (Sup. Fig. 9A-C and Movie 7) and is connected by gap junctions to the other cells in the cluster and neighboring horizontal neurons. This is a unique hallmark of horizontal neurons in the retina.
Figure 6. Live imaging of proliferating horizontal cells in p107-single retinae.
2-photon live imaging of horizontal cells in P30 Gad67-GFP;Chx10-Cre;RbLox/Lox;p107+/−;p130−/− retinae. The Gad67 promoter drives GFP expression in developing and mature horizontal cells but not progenitor or stem cells. (A) An example of 2 different horizontal cells viewed from the apical surface. One of these cells underwent cytokinesis during the 12 hour culture period (upper panels) and the other cell (lower panel) was unchanged (arrow) during the same time period. A tracing of the cell body at each timepoint is shown in the lower right of each panel in green. (B,C) In order to more clearly visualize the processes we used Imaris 5.0 to trace the processes and cell body in the dividing horizontal cell. The arrows indicate a clear separation of the two cell bodies shown with different colors (yellow and green) and different colored processes. Scale bars: 5 μm.
Metastatic Retinoblastoma Forms in p107-Single Mice
Because Rb is conditionally inactivated in the p107-single retinae, these mice are viable and fertile. However, by 8-14 weeks of age, most of these mice develop aggressive and invasive bilateral retinoblastoma (Fig. 7A-F). TEM analysis revealed that the tumor cells exhibited features of differentiated horizontal cells with processes and synapses characteristic of this cell type (Fig. 7G,H) and distinct from amacrine cell synapses that have been found in other mouse models of retinoblastoma (Johnson et al., 2007). We selected 9 areas of tumor plexus from p107-single eyes and determined the average diameter of the synapses from 141 individual synapses. The synapses were 0.36±0.2 µm with a range from 0.08 to 1.0 µm. These data are consistent with normal horizontal cell synapses. In addition, we measured the density of synapses in the p107-single tumors (0.18±0.02 synapses/µm2; n=141) and found that it agreed very well with the density of synapses in the normal OPL (0.17±0.02 synapses/µm2, n=22).
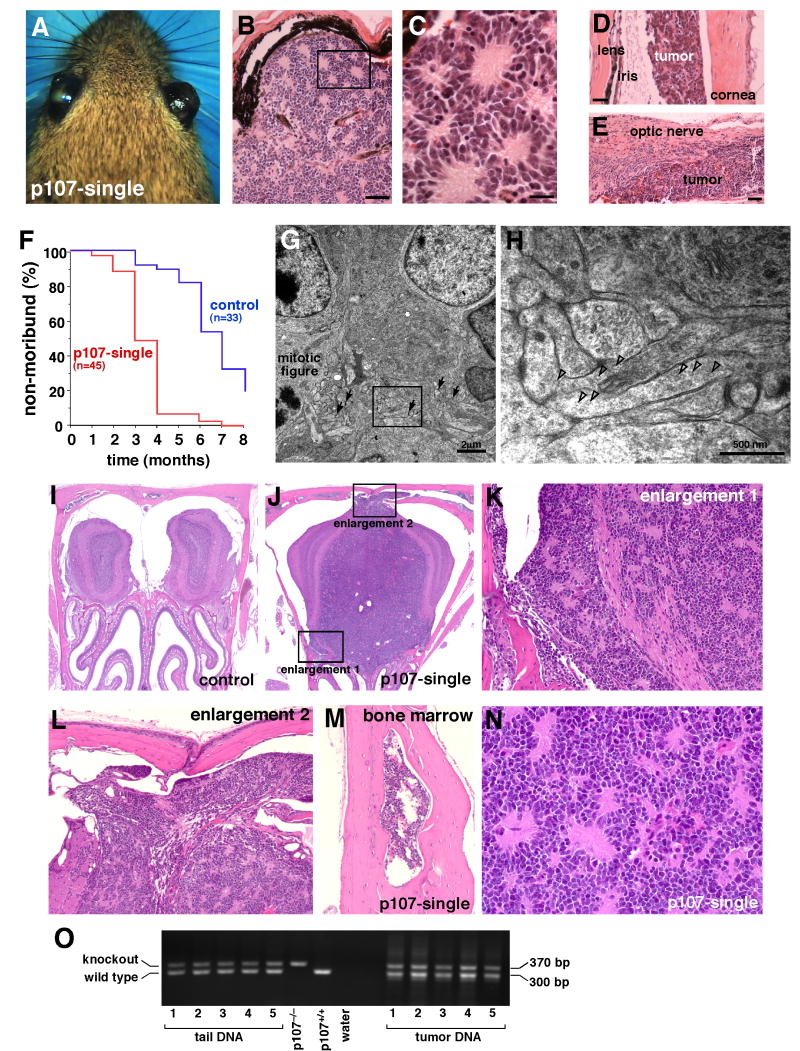
Figure 7. Metastatic retinoblastoma in p107-single mice.
(A) By several months of age, p107-single mice developed bilateral retinoblastoma with ocular hypertrophy. (B-E) Histologic analysis revealed that the eyes were filled with tumor, and the tumor cells had invaded the ocular tissue, including the anterior chamber (D) and the optic nerve (E). The image shown in (C) is the boxed region in (B), showing classic retinoblastoma histopathology. When ocular rupture was imminent the mice were considered moribund and euthanized. (F) Plot of disease progression to moribund status in p107-single retinae and Rb;p130-deficient mice. (G,H) TEM analysis revealed that the p107-single retinoblastoma cells exhibited morphologic features of differentiated horizontal cells. (G) The tumor was filled with processes and synaptic densities (arrows), even adjacent to a proliferating tumor cell with a mitotic figure. A higher magnification view of the boxed region in (G) is shown in (H) to highlight the long, tapered process with relatively sparse synaptic vesicles; these traits are characteristic of horizontal cell processes. Open arrowheads in (H) indicate synaptic vesicles. Necropsy revealed that several of the p107-single mice had metastatic retinoblastoma (I-N). An example of metastatic retinoblastoma in the olfactory bulb of p107-single mice (J) compared to the littermate control (I). The metastatic retinoblastoma had invaded the surrounding bone marrow (M), which is a hallmark of human retinoblastoma. (N) A higher magnification view of the metastatic retinoblastoma showing that the tumor has the same histopathologic features as that of the original tumor in the eye (compare N to B and C). (O) PCR analysis of the knockout and wild type p107 allele in the p107-single retinoblastomas and the corresponding tail DNA from the same animals suggests that loss of heterozygosity (LOH) at the wild type p107 locus is not required for retinoblastoma progression. Scale bars in B,E: 50 μm. Scale bars in C,D: 25 μm.
These tumors also express horizontal cell markers and have morphological features of horizontal cells as measured by Golgi-Cox staining (Sup. Fig. 10) and they lacked expression of retinal progenitor cell markers such as Chx10 (Sup. Fig. 7). As the p107-single mice aged, they developed palpable tumors in the lymph nodes and became lethargic. Necropsy revealed that the p107-single mice had metastatic retinoblastoma in the lymph nodes, brain, and bone marrow. The metastastasized tissue had histopathologic features of human metastatic retinoblastoma (Fig. 7I-N). There was no evidence for loss of heterozygosity at the wild type p107 allele in the p107-single retinoblastomas (Fig. 7O). Consistent with out previous study on human and mouse retinoblastoma, the p107-single tumor cells lose their differentiated features as they invade the optic nerve and surrounding tissue and they lose cell-cell adhesion (Sup. Fig. 11) that are characteristic of early stage tumors in both species (Johnson et al., 2007). Consistent with the finding that horizontal cells clonally expand to form retinoblastoma in the p107-single retina, we found no evidence for a retinoblastoma stem cell or expression of stem/progenitor markers in clonogenic cultures of p107-single retinoblastomas (Sup. Fig. 12).
Discussion
Proliferation and differentiation are believed to be so tightly regulated that even in tumors with deregulated proliferation and apoptosis, differentiation precludes proliferation. Tumors that exhibit features of differentiation tend to be less aggressive than undifferentiated tumors. In our efforts to elucidate the unique functions of the Rb family members in retinal development, we generated mice that express a single copy of each Rb family member in the developing retina. In p107-single retinae, horizontal neurons differentiate normally during development. Several weeks later, they re-enter the cell cycle, clonally expand, and form retinoblastoma while maintaining features of differentiated horizontal cells. These tumors rapidly progress to metastatic retinoblastoma. This is the first report of fully differentiated neurons with neurites and synapses re-entering the cell cycle and clonally expanding to form a tumor while maintaining their differentiated state. In addition, our data suggest that highly differentiated retinoblastoma can be aggressive and invasive in contrast to studies on other types of differentiated tumors.
Compensation and Redundancy Among Rb Family Members
The individual members of the Rb family are not ubiquitously expressed during retinal development. Previous studies have found that intrinsic genetic compensation and redundancy can make the interpretation of single- or compound-knockout mice difficult. For example, Rb-deficient retinoblast cells fail to progress to retinoblastoma because of intrinsic genetic compensation by p107 (Donovan et al., 2006). Beyond compensation and redundancy, haploinsufficiency can make data interpretation even more challenging in the retinae of mice with targeted deletions of one or more Rb family members. For example, p107 is haploinsufficient in retinal progenitor cells lacking Rb, but Rb is not haploinsufficient in retinal progenitor cells lacking p107 (Donovan et al., 2006). In order to elucidate the unique and overlapping function of Rb, p107 and p130 in retinogenesis, we generated mice that expressed only one copy of each family member in the developing retina. For example, based on previous studies, we predicted that a single copy of Rb would be sufficient to regulate much of retinal development including rod photoreceptor maturation. Data presented here confirmed these findings.
One question that particularly interested us was whether p107 could compensate for Rb and p130 loss in INL cells of the retina. INL neurons and glia redundantly express Rb and p130, and when Rb is inactivated, these cells upregulate p107 in a compensatory manner. There were some minor defects in retinal development in Rb,p130-deficient retinae but for the most part, INL retinogenesis proceeded normally. Analysis of p107-single retina revealed that p107 was haploinsufficient in this process. Real time RT-PCR revealed that there was less p107 expressed in the p107-single retinae compared to Rb;p130-deficient littermates. This approach unmasked a key role of the Rb family in coordinating the proliferation and differentiation of horizontal interneurons. One copy of p107 was sufficient to facilitate cell cycle exit, commitment to the horizontal cell fate, neuronal migration, neurite extension, and synaptogenesis. However, it was not sufficient to prevent horizontal cells from re-entering the cell cycle. Genomic PCR analysis showed that in the retinoblastomas derived from p107-single mice, there was no evidence for LOH at the wild type allele of p107 supporting our conclusion that horizontal cells re-enter the cell cycle and form retinoblastoma as a result of haploinsufficiency.
Neuronal cell cycle re-entry itself is not a novel finding. There are numerous reports of experimental manipulations and neurodegenerative disorders that involve ectopic neuronal proliferation (Sage et al., 2005; Skapek et al., 2001; Yang et al., 2001). Even in cases when specific subsets of neurons are increased through proliferation (Sage et al., 2005), clonal expansion of differentiated neurons was not distinguished from proliferation of a stem/progenitor cell. For example, in a previous study of p19;p27-deficient retinae Cunningham and colleagues reported that calbindin immunopositive cells incorporated BrdU in adult mice (Cunningham et al., 2002). These data support our conclusion that horizontal cells are uniquely susceptible to cell-cycle re-entry when the G1 cell cycle machinery is deregulated. However, it is not known if the cells that incorporated BrdU in the previous study were fully differentiated horizontal cells with neurites and synapses or if they were able to clonally expand. There was only a small increase in the total proportion of horizontal cells in the p19;p27-deficient retinae (<1.5-fold) and no tumors formed. The data presented here are the first example of a differentiated neuron proliferating while maintaining its differentiated features including synaptic connections and clonally expanding to form metastatic cancer.
Differentiated Horizontal Cell Proliferation
Retinal progenitor cell proliferation and histogenesis is largely complete by P7 in the mouse. Between P7 and P30, we observed a progressive, 50-fold increase in the proportion of horizontal cells from 0.2% to 10%. This increase in the proportion of horizontal cells in the postnatal retina raised two possibilities: either an immature cell in the adult retina had generated additional horizontal cells, or the horizontal cells themselves were expanding.
We favor the latter interpretation because cells that were immunopositive for Lim1, Prox1, or calbindin incorporated [3H]-thymidine immediately after a 1-h pulse in the adult retina. Indeed, there was a high proportion of dividing horizontal cells in the adult retina compared to the earlier stages of development because ∼98% of the cells at P30 were derived from the proliferative expansion of differentiated horizontal cells. In addition, mitotic figures in the TEM images were detected in differentiated horizontal cells, and lineage analysis showed a marked increase in clonal expansion of cells adjacent to the OPL that had characteristics of proliferating horizontal cells. Finally, 2-photon live imaging confirmed that the cells adjacent to the OPL that had morphologic features of horizontal cells were proliferating. However, this finding does not preclude the possibility that immature cells are also dividing in the p107-single retina. Both of these processes may occur simultaneously.
Cell-type specific roles of the Rb family in retinal development
We do not believe that p107 has a unique function in mature horizontal cells because p107 is not normally expressed in these neurons and it is only expressed when Rb is deleted due to intrinsic genetic compensation. Instead, we propose that the Rb-family is only required in horizontal cells to prevent them from re-entering the cell cycle and forming retinoblastoma. As mentioned above, horizontal cell fate specification, migration, differentiation and synaptogenesis proceeds normally in all of the combinations of Rb-family knockout mice we have analyzed to date including Rb-single, p107-single and p130-single retinae. In striking contrast, the Rb family is required for rod photoreceptor development. In the absence of Rb, rods fail to mature and eventually undergo programmed cell death (Macpherson and Dyer, 2007; Zhang et al., 2004a). It is intriguing that rod photoreceptors do not form retinoblastoma when different combinations of the Rb family members are deleted in the developing retina. One interpretation of these data is that neurons that require the Rb family for their normal development such as rods are not susceptible to tumor formation while neurons that do not require the Rb family for their normal development such as horizontal cells are susceptible to tumor formation.
The Retinoblastoma Cell-of-Origin
Our data suggest that the retinoblastoma cell of origin in the p107-single retinae is a fully differentiated horizontal cell. Previous studies (Chen et al., 2004; Donovan et al., 2006; MacPherson et al., 2004; Zhang et al., 2004a; Zhang et al., 2004b) have suggested that the retinoblastoma cell of origin may be a retinal progenitor cell or a newly postmitotic cell committed to a particular neuronal cell fate referred to as a retinal transition cell (discussed in (Dyer and Bremner, 2005). None of the previous studies suggested that a fully differentiated neuron was the cell-of-origin for retinoblastoma in mice or humans (Dyer and Bremner, 2005). Indeed, it was proposed that if a postmitotic cell was the cell-of-origin for retinoblastoma then it would be a cell that was committed to a particular cell fate but had not yet differentiated (Chen et al., 2004; Dyer and Bremner, 2005). It is difficult to recapitulate retinoblastoma tumor initiation in the human retinae and identify the cell of origin in this context. However, studies on human fetal retinae in which the RB1 gene has been inactivated by an siRNA and the p53 pathway has been inactivated by ectopic expression of MDMX indicated that the proliferating cells express the horizontal cell marker calbindin (Laurie et al., 2006).
Our data also suggest that the retinoblastomas in the p107-single mice do not expand by a stem cell mechanism but rather by the clonal expansion of a differentiated horizontal cell. Clonogenic cultures of p107-single tumors showed that spheres formed from single tumor cells contained differentiated horizontal cells and had none of the features of retinal progenitor cells, retinal stem cells or neural stem cells.
Metastatic Retinoblastoma in p107-Single Retinae
The data presented here indicated that retinoblastoma in p107-single retinae is highly differentiated. Based on the widely held belief that tumor cell differentiation is inversely correlated with metastasis and morbidity, one would predict that retinoblastoma in p107-single mice should be relatively benign. However, these were the most aggressive and invasive retinoblastomas we have characterized in mice to date. Retinoblastoma in p107-single mice always presented as bilateral disease, and the tumors rapidly invaded the optic nerve, the anterior chamber, and the subretinal space. The tumor cells then spread to the brain and bone marrow through the lymphatic system, optic nerve, or both. This model shows that tumor cell differentiation does not always inversely correlate with poor prognosis. Moreover, treatment protocols that induce tumor cell differentiation may not be useful for retinoblastomas with such extensive horizontal cell differentiation.
It is important to note that the cells that invade the optic nerve and eventually metastasize are undifferentiated. They do not express markers of horizontal cells and in TEM images they show none of the features of differentiated horizontal cells. One possible explanation is that the differentiated tumor cells rapidly expand and undergo a transition to a less differentiated state concomitant with invasion (Johnson et al., 2007). The p107-single retinoblastomas progress much more rapidly than any other genetic combination we have tested to date and this may reflect the unique status of the Rb family of genes in this tumor or an intrinsic property of the horizontal cell of origin. An alternative explanation for the rapid tumor progression and invasion is that the retinoblastomas in the p107-single mice are a heterogeneous mixture of differentiated cells with features of horizontal cells and immature cells that can metastasize. We could find no evidence of such heterogeneity but it may have been beyond the limit of our detection. Additional tumor cell fractionation and transplantation studies will be required to distinguish between these two possibilities.
Experimental Procedures
Mouse Strains
Rb+/−, and p107+/− and p130+/− mice were obtained from Dr. Tyler Jacks. RbLox/Lox mice were obtained from the National Cancer Institute and were originally made by Dr. Anton Berns. Chx10-Cre mice were obtained from Dr. Connie Cepko. mGluR6-GFP mice were provided by Dr. Rachel Wong and the Gad67-GFP mice were provided by Dr. Josh Huang.
Antibodies, Immunostaining, BrdU, [3H]-thymidine, and TUNEL Studies
We immunolabeled retinae sections cut on a vibratome and dissociated retinae (500 cells per sample, in triplicate) as previously described (Dyer and Cepko, 2000; Dyer and Cepko, 2001). The list of antibodies used is provided (Sup. Materials and Methods). To label S-phase retinal progenitor cells, we incubated freshly dissected retinae in 1 ml explant culture medium containing [3H]thy (5 μCi/ml; 89 μCi/mmol) or 10 μM BrdU for 1 h at 37 °C. Autoradiography and BrdU detection were carried out as described previously (Dyer and Cepko, 2000; Dyer and Cepko, 2001). For apoptosis analysis, we sectioned (14-μm) retinae on a cryostat. We used the colorimetric TUNEL apoptosis system (Promega, Madison, WI) per the manufacturer's instructions; however, for detection, we used tyramide-Cy3 (NEN) rather than the colorimetric substrate.
Real-Time RT-PCR
Real-time RT-PCR experiments were performed using the ABI 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA). Primers and probes were designed using Primer Express® software (Applied Biosystems). TaqMan® probes were synthesized with 5′-FAM and 3′-BHQ. RNA was prepared using Trizol, and cDNA was synthesized using the Superscript system (Invitrogen, Carlsbad, CA). Samples were analyzed in duplicate and normalized to Gapdh, Gpi1, and Mmt2 expression levels. Individual probe and primer sequences can be found in the Sup. Materials and Methods.
Microscopy
Bright-field and single-cell fluorescent images were obtained using a Zeiss Axioplan-2 fluorescent microscope with the Zeiss AxioCam digital camera. Fluorescent images of tissue sections were obtained using a Leica TCSNT confocal microscope. Details of the 2-photon imaging are provided in the Sup. Materials and Methods.
Electron Microscopy
For electron microscopy (EM), animals were anesthetized with avertin until a loss of deep tendon reflexes. Transcardial perfusion was performed with carboxygenated Ames medium supplemented with 40 mM glucose to clear the vasculature, followed by perfusion with Sorenson's phosphate buffer (pH 7.2) with 2% EM-grade paraformaldehyde and 1% EM-grade glutaraldehyde. Eyes were then harvested, a slit was made in the cornea to aid in diffusion, and the tissue was placed in 3% glutaraldehyde in Sorenson's phosphate buffer overnight. Tissue was washed with 0.2 M cacodylate buffer in 5% sucrose, postfixed in 1% OsO4, embedded, sectioned, and viewed by transmission EM (TEM).
Retroviruses and Retinal Cultures
Retroviruses and retinal culture procedures have been extensively described elsewhere (Dyer and Cepko, 2000; Dyer and Cepko, 2001; Dyer et al., 2003).
Supplementary Material
Acknowledgments
We thank Angie McArthur for editing the manuscript. We also acknowledge Marina Kedrov at the UTHSC and Libby Perry and Robert M. Smith at the Cellular Biology and Anatomy Electron Microscopy Core at the Medical College of Georgia for assistance with TEM image analysis and 3-d reconstruction of serial TEM images. We would also like to thank Abbie Hayes for assistance with in situ hybridization and Dr. Damon Reed for assistance with p107-single tumor mice. This work was supported by grants (to M.A.D.) from the National Institutes of Health, Cancer Center Support from the National Cancer Institute, the American Cancer Society, Research to Prevent Blindness, the Pearle Vision Foundation, the International Retinal Research Foundation, and the American Lebanese Syrian Associated Charities (ALSAC). M.A.D. is a Pew Scholar. This work was supported in part by grants (to S.S.Z.) from the Whitehall Foundation, NARSAD, Cancer Center Support from the NCI. S.S.Z. is a Searle Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen D, Livne-Bar I, Vanderluit JL, Slack RS, Agochiya M, Bremner R. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell. 2004;5:539–551. doi: 10.1016/j.ccr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Cunningham JJ, Levine EM, Zindy F, Goloubeva O, Roussel MF, Smeyne RJ. The cyclin-dependent kinase inhibitors p19(Ink4d) and p27(Kip1) are coexpressed in select retinal cells and act cooperatively to control cell cycle exit. Mol Cell Neurosci. 2002;19:359–374. doi: 10.1006/mcne.2001.1090. [DOI] [PubMed] [Google Scholar]
- Donovan SL, Schweers B, Martins R, Johnson D, Dyer MA. Compensation by tumor suppressor genes during retinal development in mice and humans. BMC Biol. 2006;4:14. doi: 10.1186/1741-7007-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Bremner R. The search for the retinoblastoma cell of origin. Nat Rev Cancer. 2005;5:91–101. doi: 10.1038/nrc1545. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL. p57(Kip2) regulates progenitor cell proliferation and amacrine interneuron development in the mouse retina. Development. 2000;127:3593–3605. doi: 10.1242/dev.127.16.3593. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J of Neurosci. 2001;21:4259–4271. doi: 10.1523/JNEUROSCI.21-12-04259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Livesey FJ, Cepko CL, Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002;22:9387–9398. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Schneider JW, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Zhang J, Frase S, Wilson M, Rodriguez-Galindo C, Dyer MA. Neuronal differentiation and synaptogenesis in retinoblastoma. Cancer Res. 2007;67:2701–2711. doi: 10.1158/0008-5472.CAN-06-3754. [DOI] [PubMed] [Google Scholar]
- Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C, Teunisse A, Lam S, Ramos Y, Mohan A, et al. Inactivation of the p53 pathway in retinoblastoma. Nature. 2006;444:61–66. doi: 10.1038/nature05194. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Macpherson D, Dyer MA. Retinoblastoma: from the two-hit hypothesis to targeted chemotherapy. Cancer Res. 2007;67:7547–7550. doi: 10.1158/0008-5472.CAN-07-0276. [DOI] [PubMed] [Google Scholar]
- MacPherson D, Sage J, Kim T, Ho D, McLaughlin ME, Jacks T. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 2004;18:1681–1694. doi: 10.1101/gad.1203304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odelberg SJ, Kollhoff A, Keating MT. Dedifferentiation of mammalian myotubes induced by msx1. Cell. 2000;103:1099–1109. doi: 10.1016/s0092-8674(00)00212-9. [DOI] [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rowan S, Cepko CL. Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev Biol. 2004;271:388–402. doi: 10.1016/j.ydbio.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, Garcia-Anoveros J, Hinds PW, Corwin JT, Corey DP, Chen ZY. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, Jacks T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424:223–228. doi: 10.1038/nature01764. [DOI] [PubMed] [Google Scholar]
- Skapek SX, Lin SC, Jablonski MM, McKeller RN, Tan M, Hu N, Lee EY. Persistent expression of cyclin D1 disrupts normal photoreceptor differentiation and retina development. Oncogene. 2001;20:6742–6751. doi: 10.1038/sj.onc.1204876. [DOI] [PubMed] [Google Scholar]
- Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer's disease. J Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gray J, Wu L, Leone G, Rowan S, Cepko CL, Zhu X, Craft CM, Dyer MA. Rb regulates proliferation and rod photoreceptor development in the mouse retina. Nat Genet. 2004a;36:351–360. doi: 10.1038/ng1318. [DOI] [PubMed] [Google Scholar]
- Zhang J, Schweers B, Dyer MA. The First Knockout Mouse Model of Retinoblastoma. Cell Cycle. 2004b;3:952–959. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.