Abstract
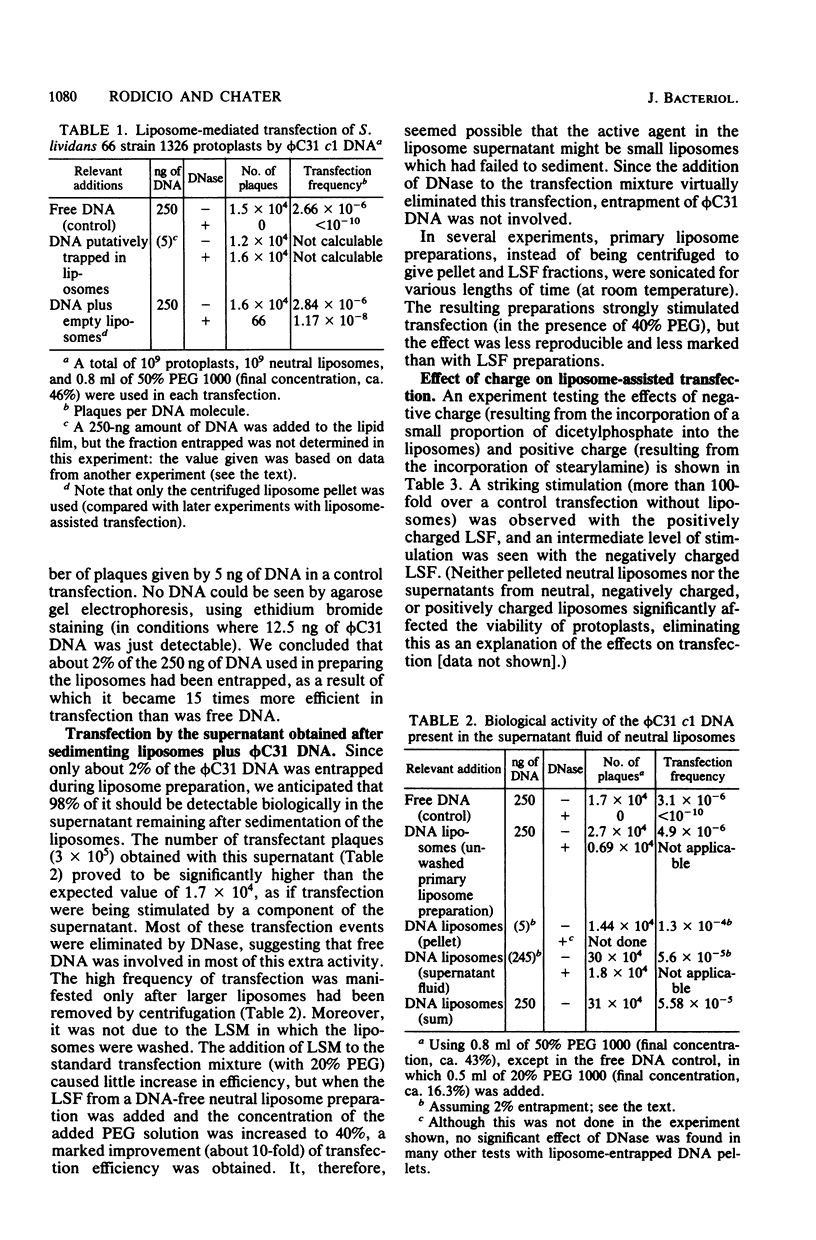
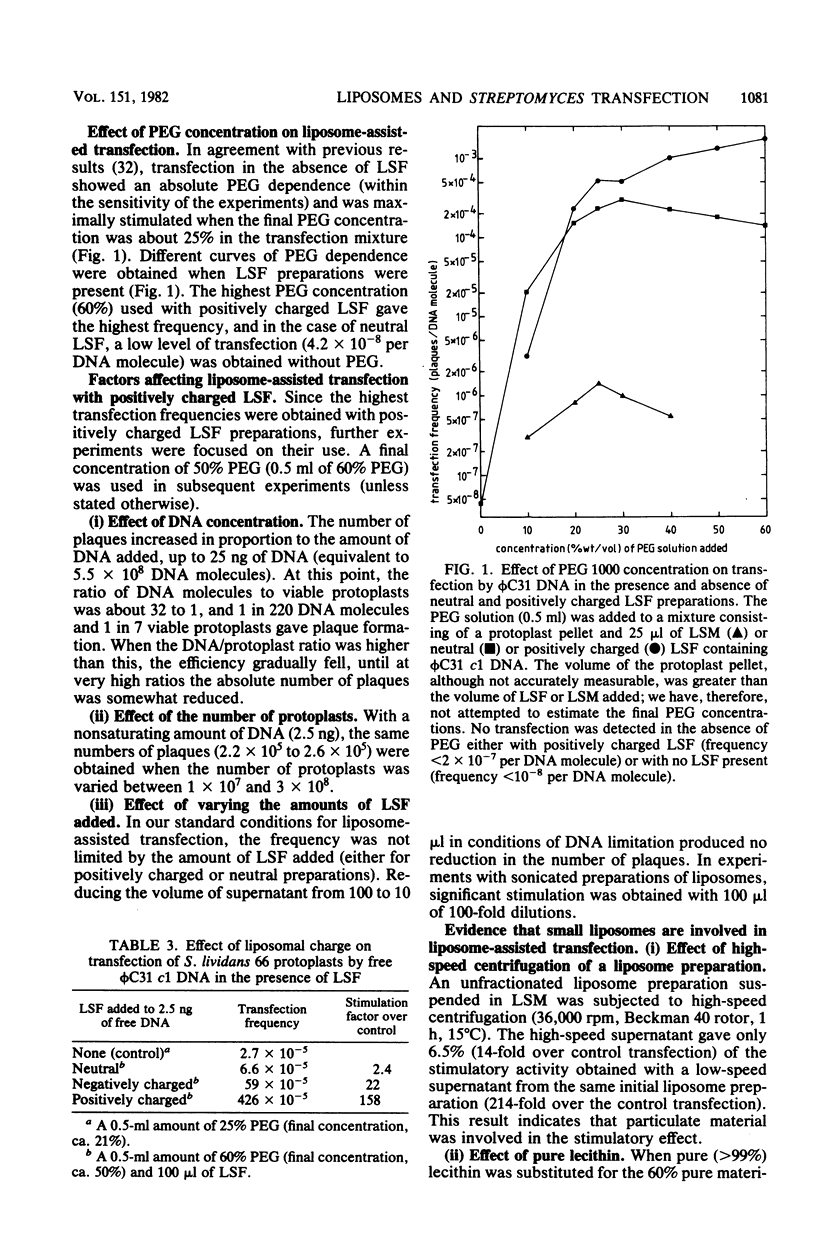
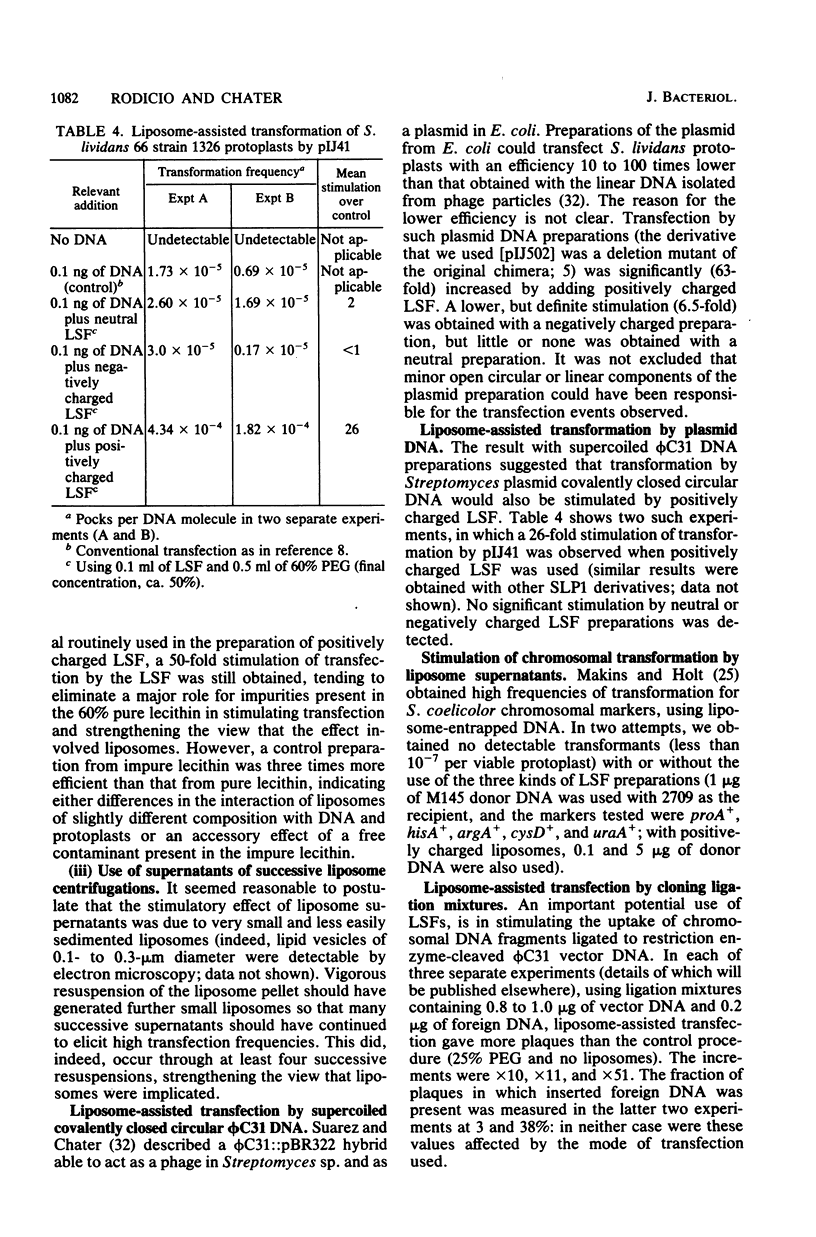
DNA of the bacteriophage phi C31 was rendered DNase resistant by entrapment in liposomes. Liposome-entrapped phi C31 DNA transfected Streptomyces protoplasts in the presence of 50% polyethylene glycol (PEG), providing a potential alternative route to conventional PEG-mediated transfection of protoplasts. However, probably partially because of low entrapment of DNA, this system did not result in an effective increase in transfection efficiency over the conventional transfection procedure. A more effective use of liposomes for stimulating transfection was provided by the discovery that supernatants obtained during the washing of DNA-free liposome preparations stimulated PEG-mediated transfection of protoplasts. This effect appeared to involve small (0.1- to 0.3-micrometer diameter) poorly sedimented liposomes. It was most effective (more than 100-fold stimulation) with positively charged liposome supernatants and high (about 50% [wt/vol]) PEG concentrations. Stimulation of transfection was also observed with cloning ligation mixtures containing phi C31 DNA as the vector. Transformation by plasmids (but not by chromosomal DNA fragments) was also significantly more efficient in these conditions than in conventional protoplast transformation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham A. D., Standish M. M., Watkins J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965 Aug;13(1):238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Bibb M., Schottel J. L., Cohen S. N. A DNA cloning system for interspecies gene transfer in antibiotic-producing Streptomyces. Nature. 1980 Apr 10;284(5756):526–531. doi: 10.1038/284526a0. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J., Springer W., Suarez J. E. Dispensable sequences and packaging constraints of DNA from the Streptomyces temperate phage phi C31. Gene. 1981 Nov;15(2-3):249–256. doi: 10.1016/0378-1119(81)90134-7. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J., Suarez J. E. Restriction mapping of the DNA of the Streptomyces temperate phage phi C31 and its derivatives. Gene. 1981 Aug;14(3):183–194. doi: 10.1016/0378-1119(81)90114-1. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Wilde L. C. Restriction of a bacteriophage of Streptomyces albus G involving endonuclease SalI. J Bacteriol. 1976 Nov;128(2):644–650. doi: 10.1128/jb.128.2.644-650.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Fraley R. T., Fornari C. S., Kaplan S. Entrapment of a bacterial plasmid in phospholipid vesicles: potential for gene transfer. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3348–3352. doi: 10.1073/pnas.76.7.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley R., Straubinger R. M., Rule G., Springer E. L., Papahadjopoulos D. Liposome-mediated delivery of deoxyribonucleic acid to cells: enhanced efficiency of delivery related to lipid composition and incubation conditions. Biochemistry. 1981 Nov 24;20(24):6978–6987. doi: 10.1021/bi00527a031. [DOI] [PubMed] [Google Scholar]
- Fraley R., Subramani S., Berg P., Papahadjopoulos D. Introduction of liposome-encapsulated SV40 DNA into cells. J Biol Chem. 1980 Nov 10;255(21):10431–10435. [PubMed] [Google Scholar]
- Hopwood D. A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967 Dec;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic studies with bacterial protoplasts. Annu Rev Microbiol. 1981;35:237–272. doi: 10.1146/annurev.mi.35.100181.001321. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. Bacterial protoplast fusion: recombination in fused protoplasts of Streptomyces coelicolor. Mol Gen Genet. 1978 Jul 4;162(3):307–317. doi: 10.1007/BF00268856. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M. Factors affecting recombinant frequency in protoplast fusions of Streptomyces coelicolor. J Gen Microbiol. 1979 Mar;111(1):137–143. doi: 10.1099/00221287-111-1-137. [DOI] [PubMed] [Google Scholar]
- Krügel H., Fiedler G., Noack D. Transfection of protoplasts from Streptomyces lividans 66 with actinophage SH10 DNA. Mol Gen Genet. 1980 Jan;177(2):297–300. doi: 10.1007/BF00267442. [DOI] [PubMed] [Google Scholar]
- Liposomes and their uses in biology and medicine. Ann N Y Acad Sci. 1978;308:1–462. [PubMed] [Google Scholar]
- Lomovskaya N. D., Mkrtumian N. M., Gostimskaya N. L., Danilenko V. N. Characterization of temperate actinophage phi C31 isolated from Streptomyces coelicolor A3(2). J Virol. 1972 Feb;9(2):258–262. doi: 10.1128/jvi.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurquin P. F. Entrapment of plasmid DNA by liposomes and their interactions with plant protoplasts. Nucleic Acids Res. 1979 Aug 24;6(12):3773–3784. doi: 10.1093/nar/6.12.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makins J. F., Holt G. Liposome-mediated transformation of streptomycetes by chromosomal DNA. Nature. 1981 Oct 22;293(5834):671–673. doi: 10.1038/293671a0. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Orloff S., Butler J. D., Triche T., Lalley P., Schulman J. D. Entrapment of metaphase chromosomes into phospholipid vesicles (lipochromosomes): carrier potential in gene transfer. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1361–1365. doi: 10.1073/pnas.75.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez J. E., Chater K. F. DNA cloning in Streptomyces: a bifunctional replicon comprising pBR322 inserted into a Streptomyces phage. Nature. 1980 Jul 31;286(5772):527–529. doi: 10.1038/286527a0. [DOI] [PubMed] [Google Scholar]
- Suarez J. E., Chater K. F. Polyethylene glycol-assisted transfection of Streptomyces protoplasts. J Bacteriol. 1980 Apr;142(1):8–14. doi: 10.1128/jb.142.1.8-14.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. DNA cloning in Streptomyces: resistance genes from antibiotic-producing species. Nature. 1980 Jul 31;286(5772):525–527. doi: 10.1038/286525a0. [DOI] [PubMed] [Google Scholar]



