Abstract
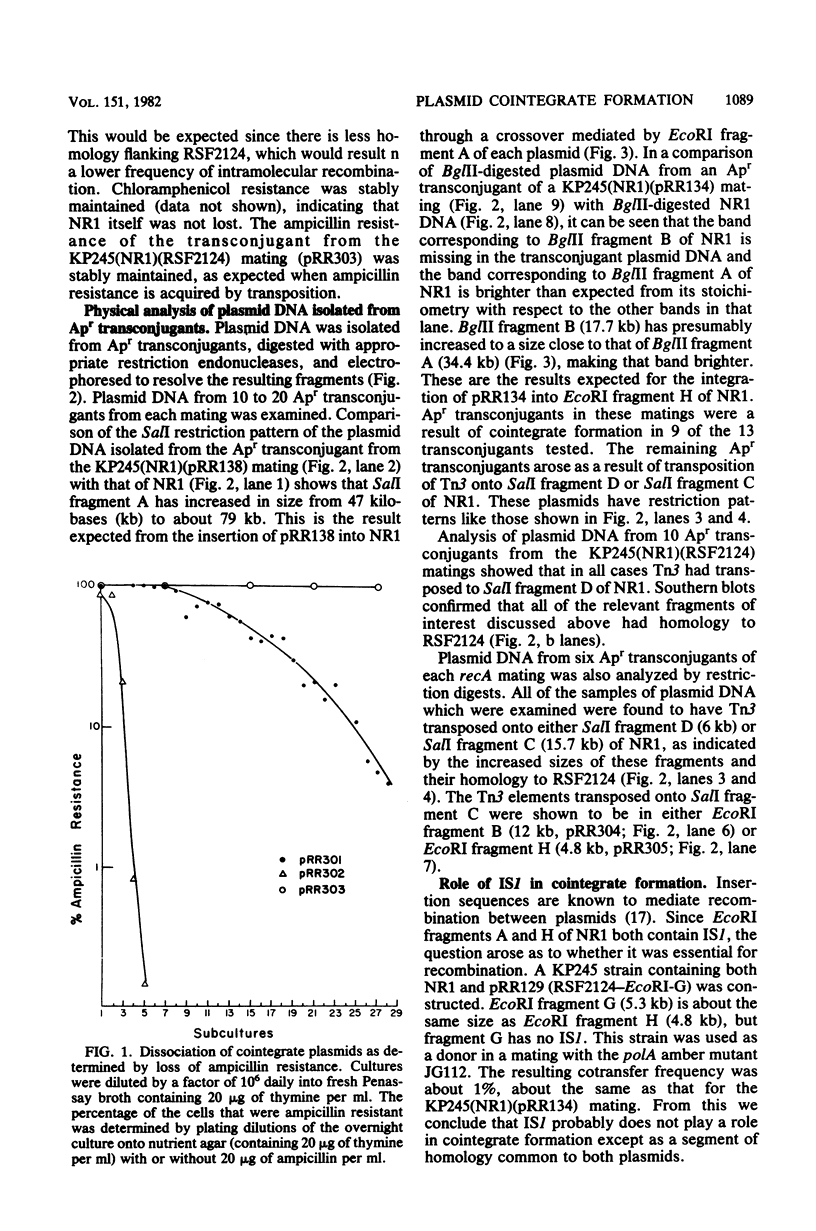
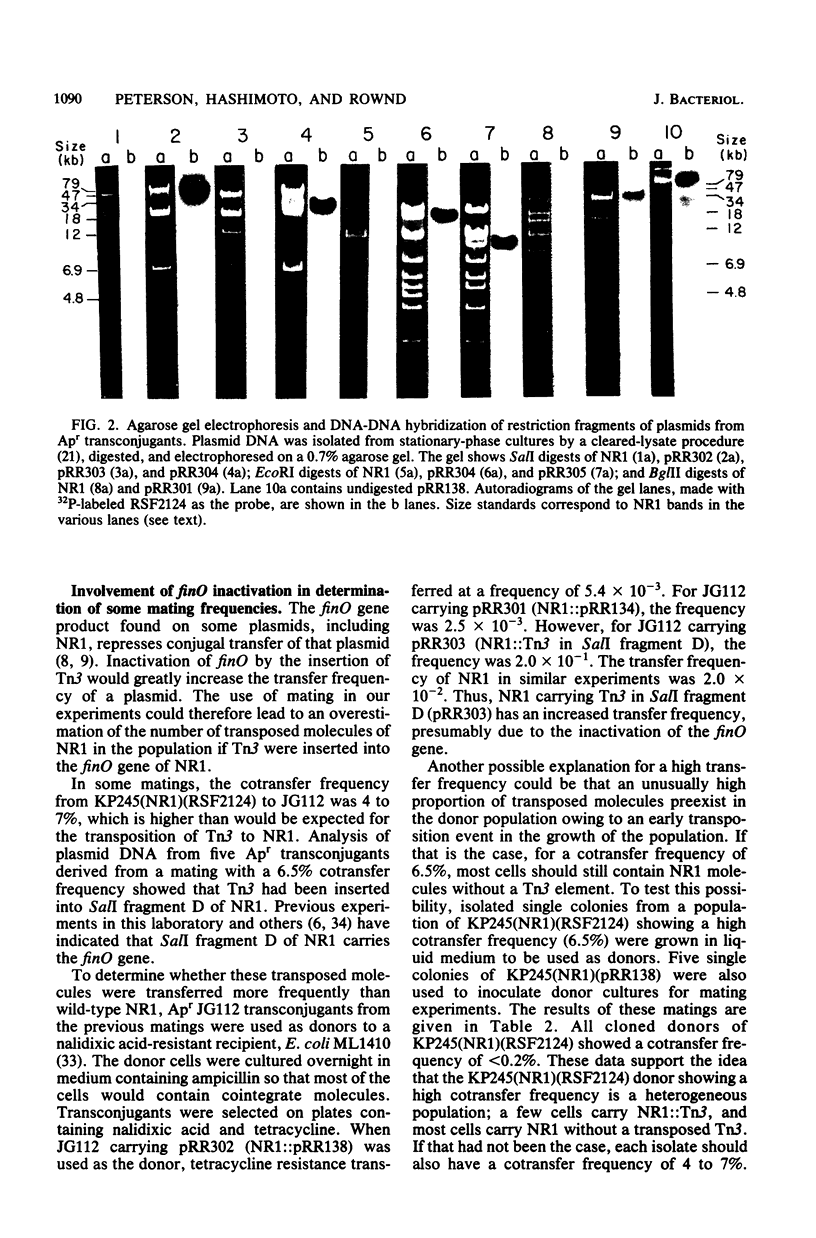
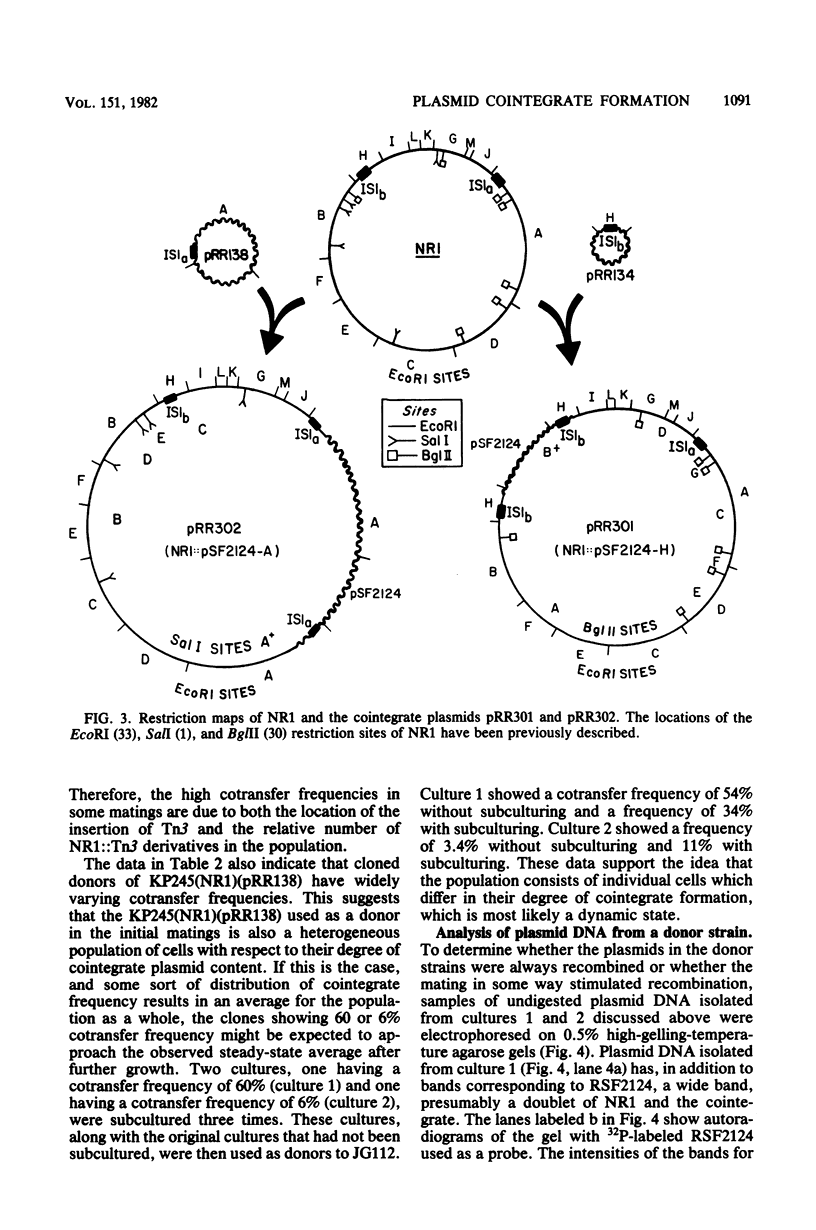
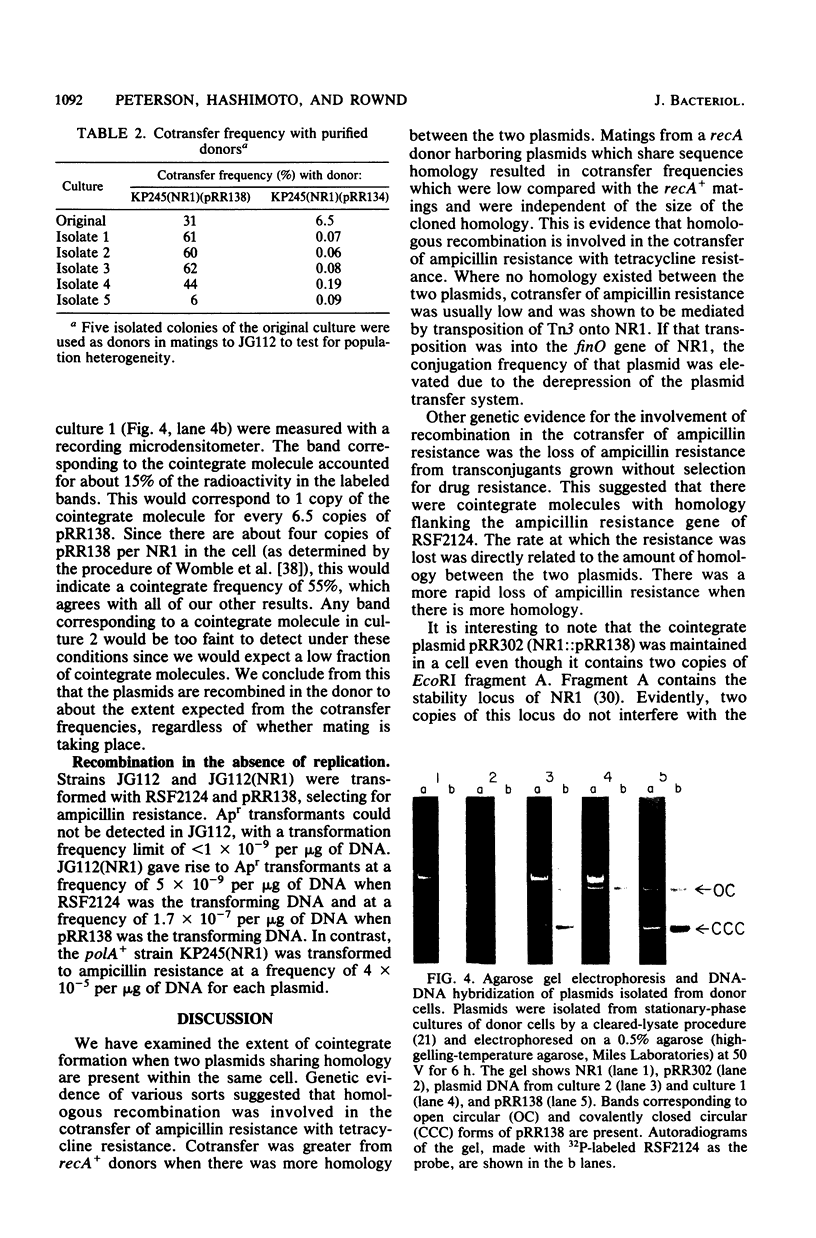
Conjugation experiments were performed in which the donor was Escherichia coli K-12 strain KP245 containing either R plasmid NR1 plus an ampicillin-resistant derivative of ColE1 (*ColE1::Tn3, called RSF2124) or NR1 plus RSF2124 carrying a cloned EcoRI fragment of NR1. The recipient was the polA amber mutant JG112, in which RSF2124 cannot replicate. Ampicillin-resistant transconjugants can arise only when the genes for ampicillin resistance are linked to NR1 or are transposed to the host chromosome. When EcoRI fragment A of NR1 (20.5 kilobases) was cloned to RSF2124, the frequency of cotransfer of ampicillin resistance with tetracycline resistance was 25 to 60%. Plasmid DNA from these ampicillin-resistant transconjugant cells was analyzed by gel electrophoresis and was shown to be a cointegrate of NR1 and the RSF2124 derivative. Analysis of plasmid DNA isolated from donor cultures showed that the cointegrates were present before conjugation, which indicates that the mating does not stimulate cointegrate formation. When the cloned fragment was EcoRI fragment H of NR1 (4.8 kilobases), the frequency of cotransfer of ampicillin resistance with tetracycline resistance was about 4%, and the majority of the ampicillin-resistant transconjugants were found to contain cointegrate plasmids. When the donor contained NR1 and RSF2124, the frequency of cotransfer of ampicillin resistance was less than 0.1%, and analysis of plasmid DNA from the ampicillin-resistant transconjugants showed that Tn3 had been transposed onto NR1. These data suggest that plasmids which share homology may exist in cointegrate form to a high degree within a host cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton C. R., Warren R. L., Jezo P., Easton A. M., Rownd R. H. Sa/I restriction endonuclease maps of FII incompatibility group R plasmids. Plasmid. 1979 Jan;2(1):150–154. doi: 10.1016/0147-619x(79)90013-1. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome-Smith J. RecA independent, site-specific recombination between ColE1 or ColK and a miniplasmid they complement for mobilization and relaxation: implications for the mechanism of DNA transfer during mobilization. Plasmid. 1980 Jul;4(1):51–63. doi: 10.1016/0147-619x(80)90082-7. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B., McIntire S. A. Lambda transducing phages derived from a FinO- R100::lambda cointegrate plasmid: proteins encoded by the R100 replication/incompatibility region and the antibiotic resistance determinant. Mol Gen Genet. 1979 Nov;176(3):319–334. doi: 10.1007/BF00333094. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., Willetts N. S. Two classes of Flac mutants insensitive to transfer inhibition by an F-like R factor. Mol Gen Genet. 1971;111(3):256–264. doi: 10.1007/BF00433110. [DOI] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The nature of the transfer inhibitor of several F-like plasmids. Mol Gen Genet. 1972;119(1):57–66. doi: 10.1007/BF00270444. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. M., Goldberg G. I., Chernin L. S., Gukova L. A., Avdienko I. D., Kuznetsova B. N., Kushner I. C. A protein produced by male strains of Escherichia coli K-12 which increases the yield of recombinants in conjugation: its nature and mode of action. Mol Gen Genet. 1973 Feb 2;120(3):211–226. doi: 10.1007/BF00267153. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. M., Gukova L. A., Chernin L. S., Avdienko I. D., Mnatsakanian G. G., Kushner I. C., Kuznetsova V. N., Strachova T. S. Rsf mutants of Escherichia coli HfrC defective in the production of the factor stimulating recombination in conjugation. Mol Gen Genet. 1974 Apr 3;129(4):295–310. doi: 10.1007/BF00265694. [DOI] [PubMed] [Google Scholar]
- Goto N., Terawaki Y., Nakaya R. Interactions between two heterogenic R plasmids: cointegrative suppression of the thermosensitive replication of Rts 1 by a nonconjugative derivative of NR1. Plasmid. 1978 Sep;1(4):589–593. doi: 10.1016/0147-619x(78)90017-3. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth R., Stephan R., Bulling E., van Leeuwen W. J., van Embden J. D., Guinée P. A., Portnoy D., Falkow S. R-factor cointegrate formation in Salmonella typhimurium bacteriophage type 201 strains. J Bacteriol. 1981 May;146(2):444–452. doi: 10.1128/jb.146.2.444-452.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M., Silva B., Genetello C., Engler G., van Vliet F., de Block M., Villarroel R., van Montagu M., Schell J. Spontaneous formation of cointegrates of the oncogenic Ti-plasmid and the wide-host-range P-plasmid RP4. Plasmid. 1978 Sep;1(4):456–467. doi: 10.1016/0147-619x(78)90004-5. [DOI] [PubMed] [Google Scholar]
- Hooykaas P. J., den Dulk-Ras H., Ooms G., Schilperoort R. A. Interactions between octopine and nopaline plasmids in Agrobacterium tumefaciens. J Bacteriol. 1980 Sep;143(3):1295–1306. doi: 10.1128/jb.143.3.1295-1306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S., Arber W. On the role of IS1 in the formation of hybrids between the bacteriophage P1 and the R plasmid NR1. Mol Gen Genet. 1980 Jan;177(2):261–270. doi: 10.1007/BF00267437. [DOI] [PubMed] [Google Scholar]
- Kilbane J. J., Malamy M. H. F factor mobilization of non-conjugative chimeric plasmids in Escherichia coli: general mechanisms and a role for site-specific recA-independent recombination at orV1. J Mol Biol. 1980 Oct 15;143(1):73–93. doi: 10.1016/0022-2836(80)90125-4. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli: requirement for deoxyribonucleic acid polymerase I in the replication of the plasmid ColE 1 . J Bacteriol. 1973 Jun;114(3):1116–1124. doi: 10.1128/jb.114.3.1116-1124.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Lovett M. A., Helinski D. R. Strand and site specificity of the relaxation event for the relaxation complex of the antibiotic resistance plasmid R6K. Biochemistry. 1974 Dec 31;13(27):5484–5490. doi: 10.1021/bi00724a005. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis J. J., Whitfield H. J. Physical characterization of a plasmid cointegrate containing an F'his gnd element and the Salmonella typhimurium LT2 cryptic plasmid. J Bacteriol. 1977 Mar;129(3):1601–1606. doi: 10.1128/jb.129.3.1601-1606.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Easton A. M., Rownd R. H. Mapping of the resistance genes of the R plasmid NR1. Mol Gen Genet. 1978 Jan 17;158(3):217–224. doi: 10.1007/BF00267192. [DOI] [PubMed] [Google Scholar]
- Miller J., Manis J., Kline B., Bishop A. Nonintegrated plasmid-folded chromosome complexes: genetic studies on formation and possible relationship to plasmid replication. Plasmid. 1978 Jun;1(3):273–283. doi: 10.1016/0147-619x(78)90045-8. [DOI] [PubMed] [Google Scholar]
- Nakaya R., Rownd R. Transduction of R factors by a Proteus mirabilis bacteriophage. J Bacteriol. 1971 Jun;106(3):773–783. doi: 10.1128/jb.106.3.773-783.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue-Geile K. L., DasSarma S., King S. R., Jaskunas S. R. Recombination between bacteriophage lambda and plasmid pBR322 in Escherichia coli. J Bacteriol. 1980 Jun;142(3):992–1003. doi: 10.1128/jb.142.3.992-1003.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Gill R., Falkow S. The generation of a ColE1-Apr cloning vehicle which allows detection of inserted DNA. Mol Gen Genet. 1975 Dec 30;142(3):239–249. doi: 10.1007/BF00425649. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanak N., Cramer J. H., Rownd R. H. EcoRI restriction endonuclease map of the composite R plasmid NR1. J Bacteriol. 1976 Jul;127(1):619–636. doi: 10.1128/jb.127.1.619-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Andrés I., Achtman M. Fertility repression of F-like conjugative plasmids: physical mapping of the R6--5 finO and finP cistrons and identification of the finO protein. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5836–5840. doi: 10.1073/pnas.75.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. J., Clark A. J. Sequence-specific recombination of plasmid ColE1. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6724–6728. doi: 10.1073/pnas.77.11.6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. F., Nester E. W. Hairy root: plasmid encodes virulence traits in Agrobacterium rhizogenes. J Bacteriol. 1980 Mar;141(3):1134–1141. doi: 10.1128/jb.141.3.1134-1141.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Taylor D. P., Rownd R. H. Method for obtaining more-accurate covalently closed circular plasmid-to-chromosome ratios from bacterial lysates by dye-buoyant density centrifugation. J Bacteriol. 1977 Apr;130(1):148–153. doi: 10.1128/jb.130.1.148-153.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Kanamaru Y., Mori R., Akiba T. Recombination between a thermosensitive kanamycin resistance factor and a nonthermosensitive multiple-drug resistant factor. J Bacteriol. 1969 Jun;98(3):863–873. doi: 10.1128/jb.98.3.863-873.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]