Abstract
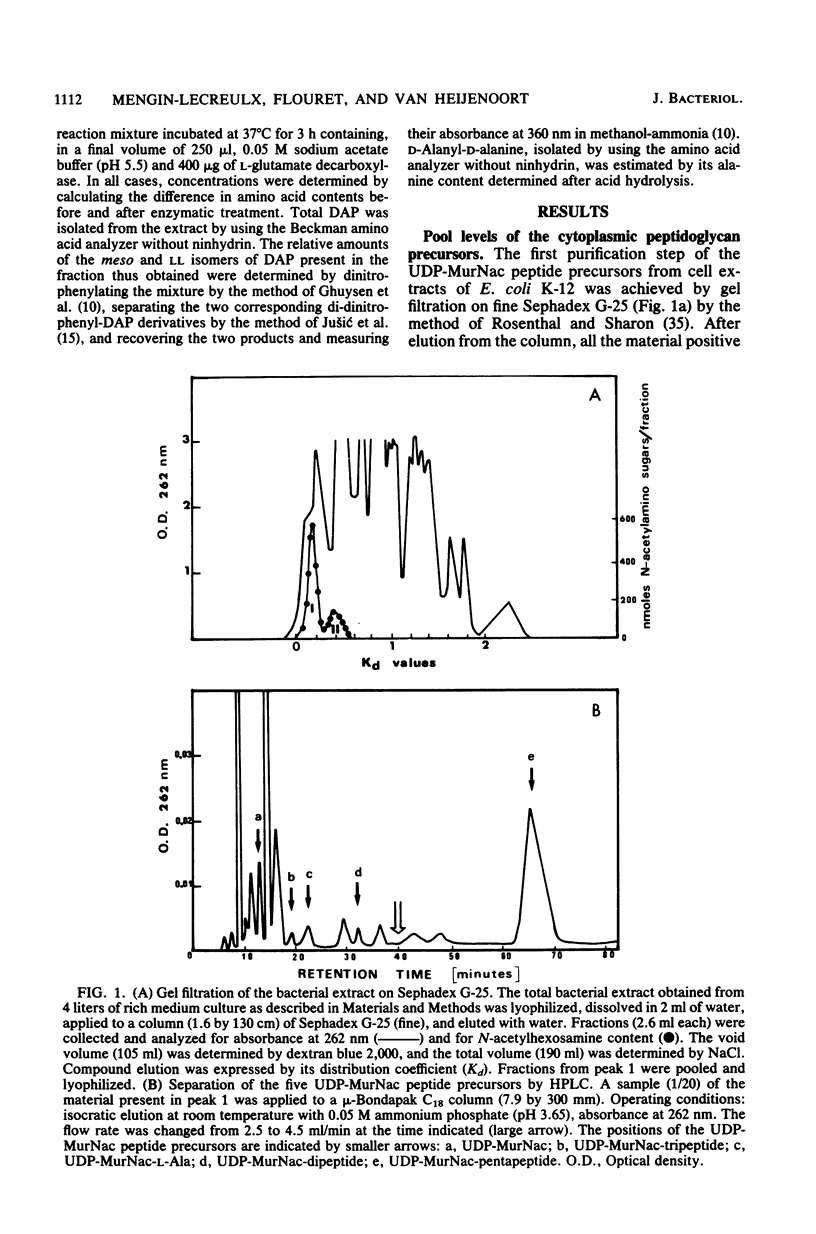
The cellular pool levels of most of the cytoplasmic precursors of peptidoglycan synthesis were determined for normally growing cells of Escherichia coli K-12. In particular, a convenient method for analyzing the uridine nucleotide precursor contents was developed by associating gel filtration and reverse-phase high-pressure liquid chromatography techniques. The enzymatic parameters of the four synthetases which catalyze the stepwise addition of L-alanine, D-glutamic acid, meso-diaminopimelic acid, and D-alanyl-D-alanine to uridine diphosphate-N-acetylmuramic acid were determined. It was noteworthy that the pool levels of L-alanine, D-glutamic acid, meso-diaminopimelic acid, and D-alanyl-D-alanine were much higher than the Km values determined for these substrates, whereas the molar concentrations of the uridine nucleotide precursors were lower than or about the same order of magnitude as the corresponding Km values. Taking into consideration the data obtained, an attempt was made to compare the in vitro activities of the D-glutamic acid, meso-diaminopimelic acid, and D-alanyl-D-alanine adding enzymes with their in vivo functioning, expressed by the amounts of peptidoglycan synthesized. The results also suggested that these adding activities were not in excess in the cell under normal growth conditions, but their amounts appeared adjusted to the requirements of peptidoglycan synthesis. Under the different in vitro conditions considered, only low levels of L-alanine adding activity were observed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar R. A., Vlaovic M. Purification of UDP-N-acetylenolpyruvoylglucosamine reductase from Escherichia coli by affinity chromatography, its subunit structure and the absence of flavin as the prosthetic group. Can J Biochem. 1979 Feb;57(2):188–196. doi: 10.1139/o79-023. [DOI] [PubMed] [Google Scholar]
- Atherton F. R., Hall M. J., Hassall C. H., Lambert R. W., Lloyd W. J., Ringrose P. S. Phosphonopeptides as antibacterial agents: mechanism of action of alaphosphin. Antimicrob Agents Chemother. 1979 May;15(5):696–705. doi: 10.1128/aac.15.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMB D. G., CHIN W., ROSEMAN S. Uridine nucleotides containing 2, 6-diaminopimelic acid from Escherichia coli. Biochim Biophys Acta. 1961 Jan 15;46:394–397. doi: 10.1016/0006-3002(61)90768-5. [DOI] [PubMed] [Google Scholar]
- Fletcher G., Irwin C. A., Henson J. M., Fillingim C., Malone M. M., Walker J. R. Identification of the Escherichia coli cell division gene sep and organization of the cell division-cell envelope genes in the sep-mur-ftsA-envA cluster as determined with specialized transducing lambda bacteriophages. J Bacteriol. 1978 Jan;133(1):91–100. doi: 10.1128/jb.133.1.91-100.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett A. J. The effect of magnesium ion deprivation on the synthesis of mucopeptide and its precursors in Bacillus subtilis. Biochem J. 1969 Nov;115(3):419–430. doi: 10.1042/bj1150419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondré B., Flouret B., van Heijenoort J. Release of D-alanyl-D-alanine from the precursor of the cell wall peptidoglycan by a peptidase of Escherichia coli K 12. Biochimie. 1973;55(6):685–691. doi: 10.1016/s0300-9084(73)80022-7. [DOI] [PubMed] [Google Scholar]
- ITO E., STROMINGER J. L. ENZYMATIC SYNTHESIS OF THE PEPTIDE IN BACTERIAL URIDINE NUCLEOTIDES. III. PURIFICATION AND PROPERTIES OF L-LYSIN-ADDING ENZYME. J Biol Chem. 1964 Jan;239:210–214. [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Involvement of the relA gene product and feedback inhibition in the regulation of DUP-N-acetylmuramyl-peptide synthesis in Escherichia coli. J Bacteriol. 1978 Sep;135(3):766–774. doi: 10.1128/jb.135.3.766-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E., Strominger J. L. Enzymatic synthesis of the peptide in bacterial uridine nucleotides. VII. Comparative biochemistry. J Biol Chem. 1973 May 10;248(9):3131–3136. [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLY M. D., CLARKE P. H., MEADOW P. M. THE ACCUMULATION OF NUCLEOTIDES BY ESCHERICHIA COLI STRAIN 26-26. J Gen Microbiol. 1963 Jul;32:103–116. doi: 10.1099/00221287-32-1-103. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambert M. P., Neuhaus F. C. Factors affecting the level of alanine racemase in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1156–1161. doi: 10.1128/jb.109.3.1156-1161.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M., van Heijenoort J. Autolysis of Escherichia coli. J Bacteriol. 1980 Apr;142(1):52–59. doi: 10.1128/jb.142.1.52-59.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., De Haas-Menger L., Ruyters W. H. Murein synthesis and identification of cell wall precursors of temperature-sensitive lysis mutants of Escherichia coli. J Bacteriol. 1972 Jan;109(1):326–335. doi: 10.1128/jb.109.1.326-335.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J. Studies on Escherichia coli enzymes involved in the synthesis of uridine diphosphate-N-acetyl-muramyl-pentapeptide. J Bacteriol. 1972 Apr;110(1):26–34. doi: 10.1128/jb.110.1.26-34.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., v Schijndel-van Dam A. Temperature-sensitive mutant of Escherichia coli K-12 with an impaired D-alanine:D-alanine ligase. J Bacteriol. 1973 Jan;113(1):96–104. doi: 10.1128/jb.113.1.96-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. The free amino acids in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):103–110. doi: 10.1042/bj0690103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J. M., Merrifield N. E., Jones W. M., Gotschlich E. C. Inhibition of bacterial growth by beta-chloro-D-alanine. Proc Natl Acad Sci U S A. 1974 Feb;71(2):417–421. doi: 10.1073/pnas.71.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y., Ito E. Purification and properties of uridine diphosphate N-acetylmuramyl-L-alanyl-D-glutamate:meso-2,6-diaminopimelate ligase. J Biol Chem. 1968 May 25;243(10):2665–2672. [PubMed] [Google Scholar]
- Mizuno Y., Yaegashi M., Ito E. Purification and properties of uridine diphosphate N-acetylmuramate: L-alanine ligase. J Biochem. 1973 Sep;74(3):525–538. doi: 10.1093/oxfordjournals.jbchem.a130273. [DOI] [PubMed] [Google Scholar]
- Moncany M. L., Kellenberger E. High magnesium content of Escherichia coli B. Experientia. 1981;37(8):846–847. doi: 10.1007/BF01985672. [DOI] [PubMed] [Google Scholar]
- NATHENSON S. G., STROMINGER J. L., ITO E. ENZYMATIC SYNTHESIS OF THE PEPTIDE IN BACTERIAL URIDINE NUCLEOTIDES. IV. PURIFICATION AND PROPERTIES OF D-GLUTAMIC ACID-ADDING ENZYME. J Biol Chem. 1964 Jun;239:1773–1776. [PubMed] [Google Scholar]
- NEUHAUS F. C., STRUVE W. G. ENZYMATIC SYNTHESIS OF ANALOGS OF THE CELL-WALL PRECURSOR. I. KINETICS AND SPECIFICITY OF URIDINE DIPHOSPHO-N-ACETYLMURAMYL-L-ALANYL-D-GLUTAMYL-L-LYSINE:D-ALANYL-D-ALANINE LIGASE (ADENOSINE DIPHOSPHATE) FROM STREPTOCOCCUS FAECALIS R. Biochemistry. 1965 Jan;4:120–131. doi: 10.1021/bi00877a020. [DOI] [PubMed] [Google Scholar]
- Pelzer H., Reuter W. Inhibition of peptidoglycan synthesis in ether-permeabilized Escherichia coli cells by structural analogs of D-alanyl-D-alanine. Antimicrob Agents Chemother. 1980 Dec;18(6):887–892. doi: 10.1128/aac.18.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENTHAL S., SHARON N. THE USE OF SEPHADEX G-25 FOR THE ISOLATION OF NUCLEOTIDE SUGAR DERIVATIVES FROM MICROCOCCUS LYSODEIKTICUS. Biochim Biophys Acta. 1964 Nov 1;83:378–380. doi: 10.1016/0926-6526(64)90025-4. [DOI] [PubMed] [Google Scholar]
- Raunio R., Rosenqvist H. Amino acid pool of Escherichia coli during the different phases of growth. Acta Chem Scand. 1970;24(8):2737–2744. doi: 10.3891/acta.chem.scand.24-2737. [DOI] [PubMed] [Google Scholar]
- Salmond G. P., Lutkenhaus J. F., Donachie W. D. Identification of new genes in a cell envelope-cell division gene cluster of Escherichia coli: cell envelope gene murG. J Bacteriol. 1980 Oct;144(1):438–440. doi: 10.1128/jb.144.1.438-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Venkateswaran P. S., Lugtenberg E. J., Wu H. C. Inhibition of phosphoenolpyruvate:uridine diphosphate N-acetylglucosamine enolpyruvyltransferase by uridine diphosphate N-acetylmuramyl peptides. Biochim Biophys Acta. 1973 Feb 15;293(2):570–574. doi: 10.1016/0005-2744(73)90367-7. [DOI] [PubMed] [Google Scholar]
- Wargel R. J., Shadur C. A., Neuhaus F. C. Mechanism of D-cycloserine action: transport systems for D-alanine, D-cycloserine, L-alanine, and glycine. J Bacteriol. 1970 Sep;103(3):778–788. doi: 10.1128/jb.103.3.778-788.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



