Abstract
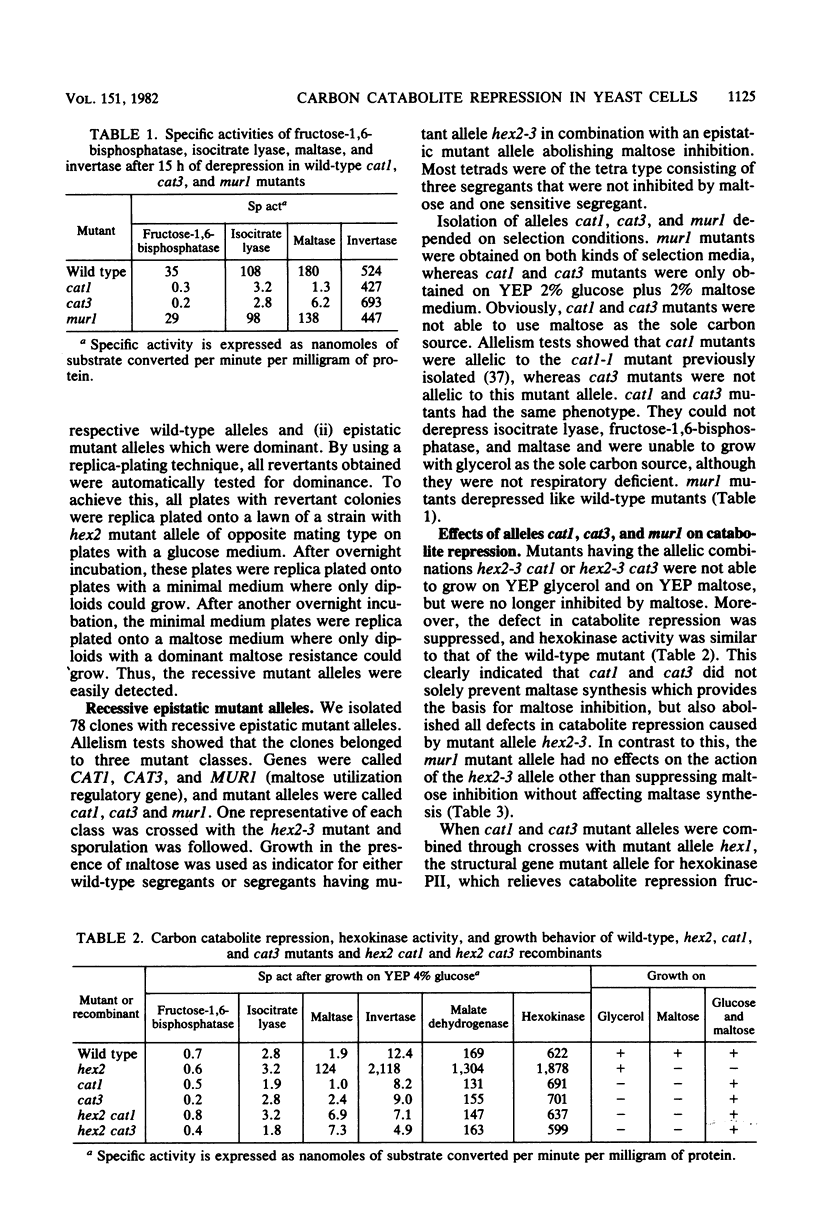
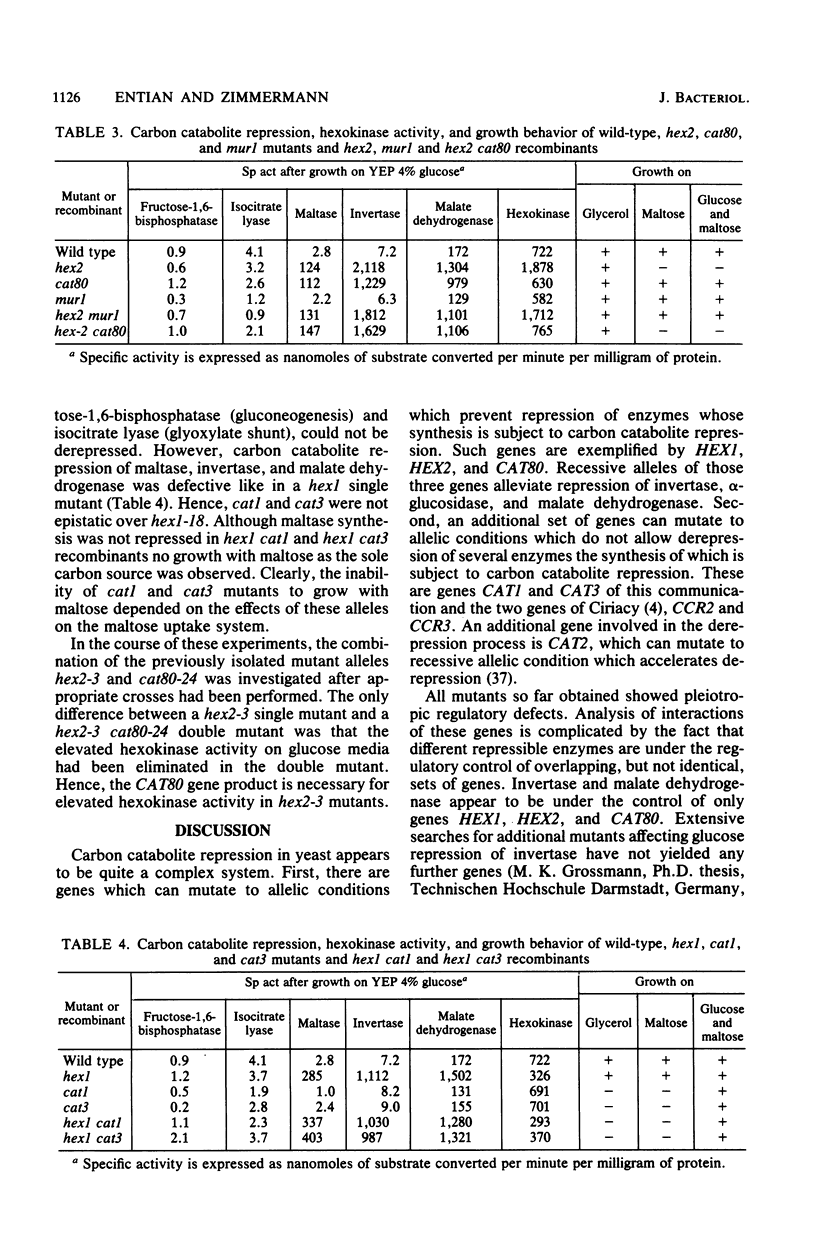
A mutation causing resistance to carbon catabolite repression in gene HEX2, mutant allele hex2-3, causes an extreme sensitivity to maltose when in combination with the genes necessary for maltose metabolism. This provided a convenient system for the selective isolation of mutations in genes specifically required for maltose metabolism and other genes involved in general carbon catabolite repression. In addition to reversion of the hex2-3 allele, mutations in three other genes were detected. These genes were called CAT1, CAT3, and MUR1 and in a mutated form abolished maltose inhibition caused by mutant allele hex2-3. Mutant alleles cat1 and cat3 also restored normal repression in the presence of the hex2-3 allele. Segregants having only mutant alleles cat1 or cat3 were obtained by tetrad analysis. These segregants could not grow on nonfermentable carbon sources. Mutant alleles of gene CAT1 were allelic to a mutant allele cat1-1 previously isolated (Zimmermann et al., Mol. Gen. Genet. 151:95-103). Such mutants prevented derepression not only of the maltose catabolizing system, the selected property, but also of glyoxylate shunt and gluconeogenic enzymes. However, respiratory activities and invertase formation were not affected under derepressing conditions. cat3 mutants had the same phenotypic properties as cat1 mutants. This showed that carbon metabolism in yeast cells is under a very complex and ramified control of repressing and derepressing genes, which are interdependent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron J. R., Loh E. Y., Davis R. W. Evidence for transposition of dispersed repetitive DNA families in yeast. Cell. 1979 Apr;16(4):739–751. doi: 10.1016/0092-8674(79)90090-4. [DOI] [PubMed] [Google Scholar]
- Ciriacy M. Isolation and characterization of yeast mutants defective in intermediary carbon metabolism and in carbon catabolite derepression. Mol Gen Genet. 1977 Jul 20;154(2):213–220. doi: 10.1007/BF00330840. [DOI] [PubMed] [Google Scholar]
- Ciriacy M., Williamson V. M. Analysis of mutations affecting Ty-mediated gene expression in Saccharomyces cerevisiae. Mol Gen Genet. 1981;182(1):159–163. doi: 10.1007/BF00422784. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entian K. D. A carbon catabolite repression mutant of Saccharomyces cerevisiae with elevated hexokinase activity: evidence for regulatory control of hexokinase PII synthesis. Mol Gen Genet. 1981;184(2):278–282. doi: 10.1007/BF00272917. [DOI] [PubMed] [Google Scholar]
- Entian K. D. A defect in carbon catabolite repression associated with uncontrollable and excessive maltose uptake. Mol Gen Genet. 1980;179(1):169–175. doi: 10.1007/BF00268460. [DOI] [PubMed] [Google Scholar]
- Entian K. D. Genetic and biochemical evidence for hexokinase PII as a key enzyme involved in carbon catabolite repression in yeast. Mol Gen Genet. 1980;178(3):633–637. doi: 10.1007/BF00337871. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K., Scheel I. A partial defect in carbon catabolite repression in mutants of Saccharomyces cerevisiae with reduced hexose phosphyorylation. Mol Gen Genet. 1977 Nov 4;156(1):99–105. doi: 10.1007/BF00272258. [DOI] [PubMed] [Google Scholar]
- Gancedo C., Schwerzmann K. Inactivation by glucose of phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae. Arch Microbiol. 1976 Sep 1;109(3):221–225. doi: 10.1007/BF00446632. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Mikrobiol. 1971;76(2):132–138. doi: 10.1007/BF00411787. [DOI] [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- Grossmann M. K., Zimmermann F. K. The structural genes of internal invertases in Saccharomyces cerevisiae. Mol Gen Genet. 1979 Sep;175(2):223–229. doi: 10.1007/BF00425540. [DOI] [PubMed] [Google Scholar]
- Lobo Z., Maitra P. K. Genetics of yeast hexokinase. Genetics. 1977 Aug;86(4):727–744. doi: 10.1093/genetics/86.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. Genetic control of catabolite repression of the lac operon in Escherichia coli. Biochem Biophys Res Commun. 1965 Jul 12;20(2):230–234. doi: 10.1016/0006-291x(65)90351-7. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Michels C. A., Romanowski A. Pleiotropic glucose repression-resistant mutation in Saccharomyces carlesbergensis. J Bacteriol. 1980 Aug;143(2):674–679. doi: 10.1128/jb.143.2.674-679.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenecourt B. S., Kuo S. C., Lampen J. O. Saccharomyces mutants with invertase formation resistant to repression by hexoses. J Bacteriol. 1973 Apr;114(1):233–238. doi: 10.1128/jb.114.1.233-238.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Application of two new methods for cleavage of polysaccharides into specific oligosaccharide fragments. Structure of the capsular and extracellular polysaccharides of Rhizobium japonicum that bind soybean lectin. J Biol Chem. 1982 Feb 25;257(4):1870–1875. [PubMed] [Google Scholar]
- Neeff J., Mecke D. In vivo and in vitro studies on the glucose dependent inactivation of yeast cytoplasmic malate dehydrogenase. Arch Microbiol. 1977 Oct 24;115(1):55–60. doi: 10.1007/BF00427845. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the activities of respiratory enzymes during the aerobic growth of yeast on different carbon sources. Biochem J. 1965 Oct;97(1):298–302. doi: 10.1042/bj0970298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamhart D. H., Ten Berge A. M., Van De Poll K. W. Isolation of a catabolite repression mutant of yeast as a revertant of a strain that is maltose negative in the respiratory-deficient state. J Bacteriol. 1975 Mar;121(3):747–752. doi: 10.1128/jb.121.3.747-752.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Wishnow R., Loomis W. F., Jr, Magasanik B. Catabolite repression gene of Escherichia coli. J Bacteriol. 1969 Nov;100(2):809–816. doi: 10.1128/jb.100.2.809-816.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijk R., Ouwehand J., van den Bos T., Koningsberger V. V. Induction and catabolite repression of alpha-glucosidase synthesis in protoplasts of Saccharomyces carlsbergensis. Biochim Biophys Acta. 1969 Jul 22;186(1):178–191. doi: 10.1016/0005-2787(69)90501-2. [DOI] [PubMed] [Google Scholar]
- Witt I., Kronau R., Holzer H. Repression von Alkoholdehydrogenase, Malatdehydrogenase, Isocitratlyase und Malatsynthase in Hefe durch Glucose. Biochim Biophys Acta. 1966 Jun 15;118(3):522–537. [PubMed] [Google Scholar]
- Zimmermann F. K., Eaton N. R. Genetics of induction and catabolite repression of Maltese synthesis in Saccharomyces cerevisiae. Mol Gen Genet. 1974;134(3):261–272. doi: 10.1007/BF00267720. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Kaufmann I., Rasenberger H., Haubetamann P. Genetics of carbon catabolite repression in Saccharomycess cerevisiae: genes involved in the derepression process. Mol Gen Genet. 1977 Feb 28;151(1):95–103. doi: 10.1007/BF00446918. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Scheel I. Mutants of Saccharomyces cerevisiae resistant to carbon catabolite repression. Mol Gen Genet. 1977 Jul 7;154(1):75–82. doi: 10.1007/BF00265579. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Varmus H. E., Perlman R. L., Pastan I. H. Stimulation of lac mRNA synthesis by cyclic AMP in cell free extracts of Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 12;38(5):894–901. doi: 10.1016/0006-291x(70)90805-3. [DOI] [PubMed] [Google Scholar]



