Abstract
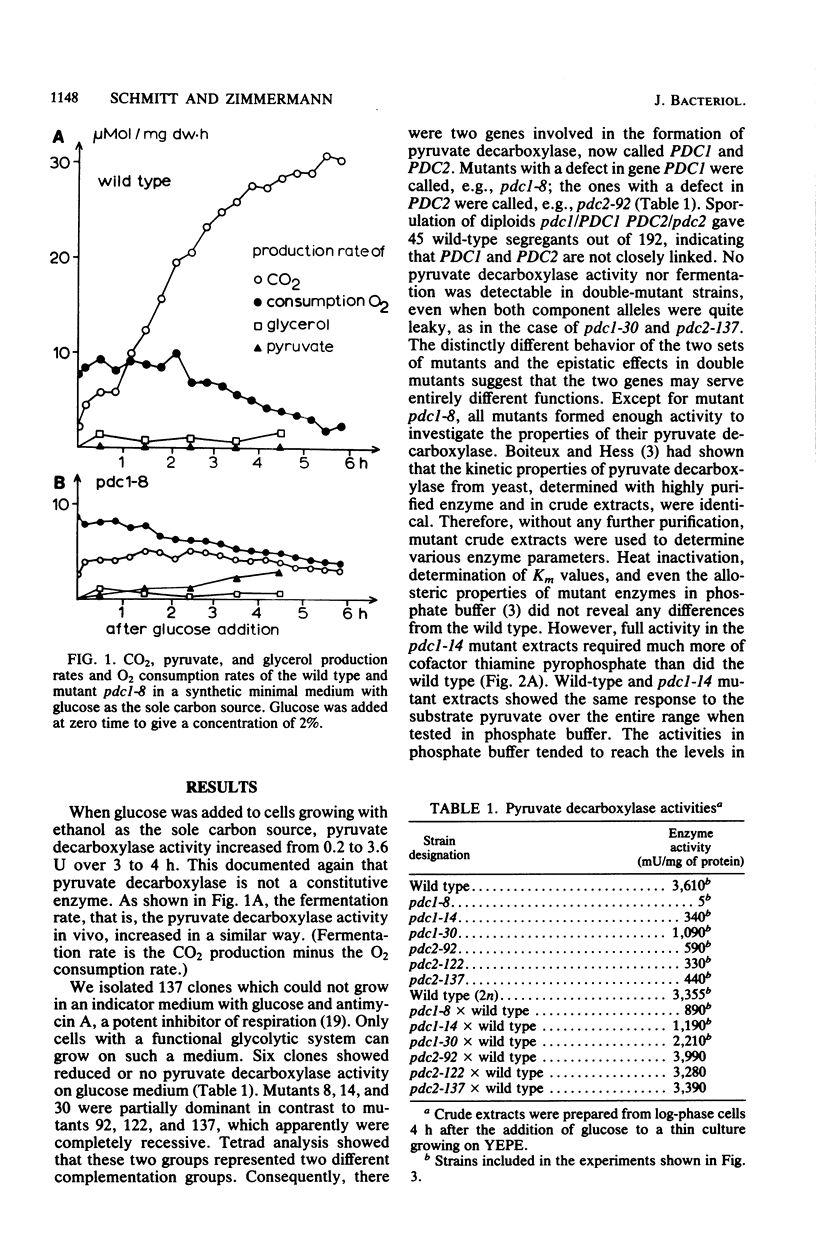
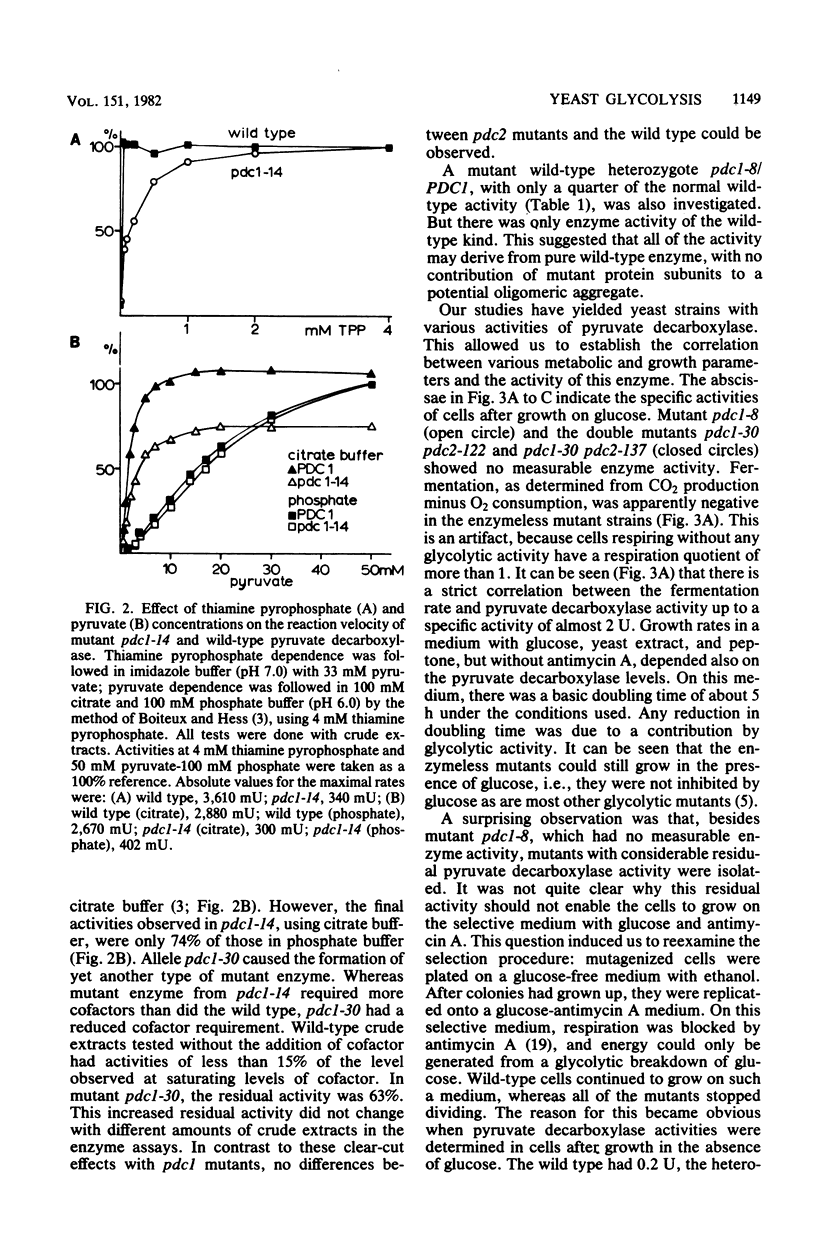
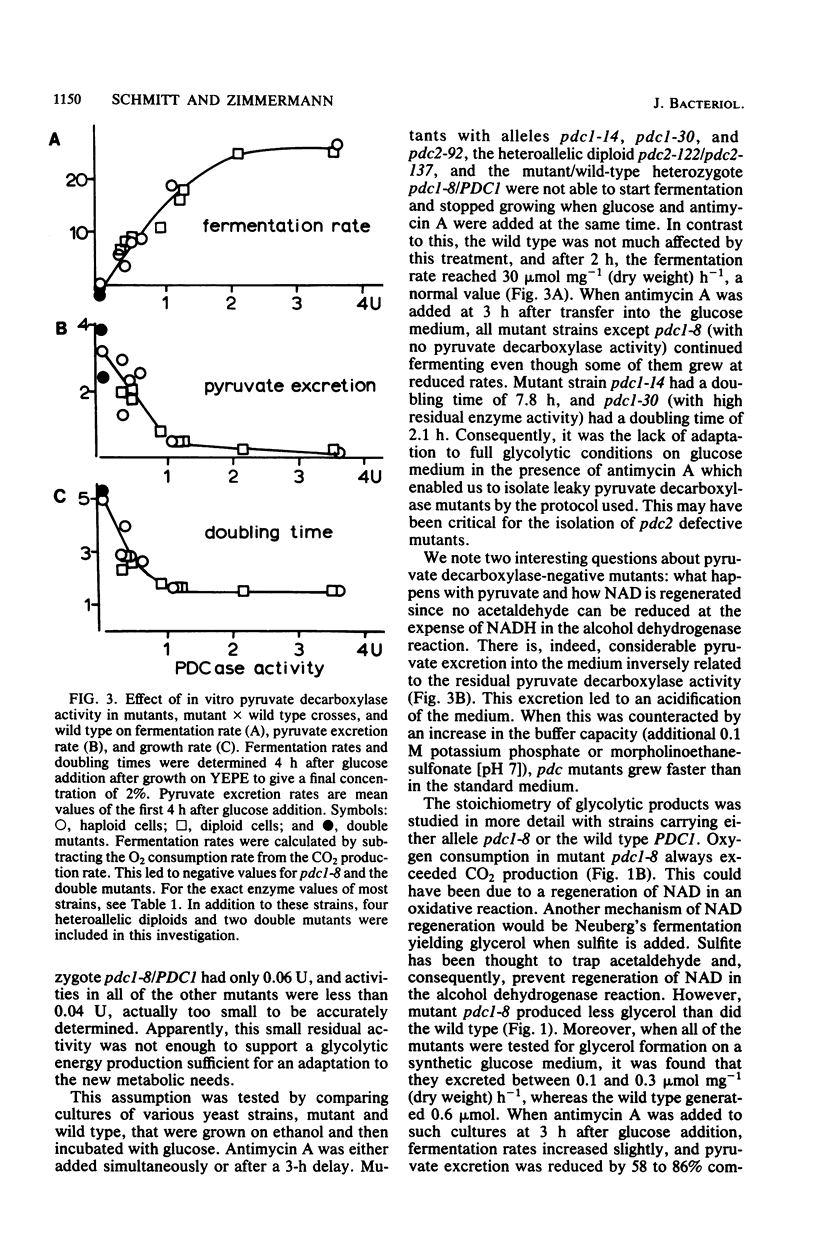
Six different pyruvate decarboxylase mutants of Saccharomyces cerevisiae were isolated. They belong to two unlinked complementation groups. Evidence is presented that one group is affected in a structural gene. The fact that five of the six mutants had residual pyruvate decarboxylase activity provided the opportunity for an intensive physiological characterization. It was shown that the loss of enzyme activity in vitro is reflected in a lower fermentation rate, an increased pyruvate secretion, and slower growth on a 2% glucose medium. The different effects of antimycin A on leaky mutants grown on ethanol versus the same mutants grown on glucose support the view that glucose induces some of the glycolytic enzymes, especially pyruvate decarboxylase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bañuelos M., Gancedo C., Gancedo J. M. Activation by phosphate of yeast phosphofructokinase. J Biol Chem. 1977 Sep 25;252(18):6394–6398. [PubMed] [Google Scholar]
- Boiteux A., Hess B. Allosteric properties of yeast pyruvate decarboxylase. FEBS Lett. 1970 Aug 31;9(5):293–296. doi: 10.1016/0014-5793(70)80381-7. [DOI] [PubMed] [Google Scholar]
- Ciriacy M., Breitenbach I. Physiological effects of seven different blocks in glycolysis in Saccharomyces cerevisiae. J Bacteriol. 1979 Jul;139(1):152–160. doi: 10.1128/jb.139.1.152-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriacy M. Isolation and characterization of yeast mutants defective in intermediary carbon metabolism and in carbon catabolite derepression. Mol Gen Genet. 1977 Jul 20;154(2):213–220. doi: 10.1007/BF00330840. [DOI] [PubMed] [Google Scholar]
- Clifton D., Weinstock S. B., Fraenkel D. G. Glycolysis mutants in Saccharomyces cerevisiae. Genetics. 1978 Jan;88(1):1–11. doi: 10.1093/genetics/88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K. Glycolytic enzymes and intermediates in carbon catabolite repression mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1980 Jan;177(2):345–350. doi: 10.1007/BF00267449. [DOI] [PubMed] [Google Scholar]
- Gounaris A. D., Turkenkopf I., Buckwald S., Young A. Pyruvate decarboxylase. I. Protein dissociation into subunits under conditions in which thiamine pyrophosphate is released. J Biol Chem. 1971 Mar 10;246(5):1302–1309. [PubMed] [Google Scholar]
- Gounaris A. D., Turkenkopf I., Civerchia L. L., Greenlie J. Pyruvate decarboxylase III. Specificity restrictions for thiamine pyrophosphate in the protein association step, sub-unit structure. Biochim Biophys Acta. 1975 Oct 20;405(2):492–499. [PubMed] [Google Scholar]
- HOLZER H. Regulation of carbohydrate metabolism by enzyme competition. Cold Spring Harb Symp Quant Biol. 1961;26:277–288. doi: 10.1101/sqb.1961.026.01.034. [DOI] [PubMed] [Google Scholar]
- Hopmann R. F. Hydroxyl-ion-induced subunit dissociation of east cytoplasmic pyruvate decarboxylase. A circular dichroism study. Eur J Biochem. 1980 Sep;110(1):311–318. doi: 10.1111/j.1432-1033.1980.tb04869.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam K. B., Marmur J. Isolation and characterization of Saccharomyces cerevisiae glycolytic pathway mutants. J Bacteriol. 1977 May;130(2):746–749. doi: 10.1128/jb.130.2.746-749.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancashire W. E., Payton M. A., Webber M. J., Hartley B. S. Petite-negative mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1981;181(3):409–410. doi: 10.1007/BF00425622. [DOI] [PubMed] [Google Scholar]
- Maitra P. K., Lobo Z. A kinetic study of glycolytic enzyme synthesis in yeast. J Biol Chem. 1971 Jan 25;246(2):475–488. [PubMed] [Google Scholar]
- Maitra P. K., Lobo Z. Genetic studies with a phosphoglucose isomerase mutant of Saccharomyces cerevisiae. Mol Gen Genet. 1977 Nov 4;156(1):55–60. doi: 10.1007/BF00272252. [DOI] [PubMed] [Google Scholar]
- Maitra P. K., Lobo Z. Yeast pyruvate kinase: a mutant from catalytically insensitive to fructose 1,6-bisphosphate. Eur J Biochem. 1977 Sep;78(2):353–360. doi: 10.1111/j.1432-1033.1977.tb11747.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 1975 Aug 1;56(1):1–6. doi: 10.1016/0014-5793(75)80098-6. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae. Microbiol Rev. 1980 Dec;44(4):519–571. doi: 10.1128/mr.44.4.519-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamhart D. H., Ten Berge A. M., Van De Poll K. W. Isolation of a catabolite repression mutant of yeast as a revertant of a strain that is maltose negative in the respiratory-deficient state. J Bacteriol. 1975 Mar;121(3):747–752. doi: 10.1128/jb.121.3.747-752.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. An enrichment method for auxotrophic yeast mutants using the antibiotic 'nystatin'. Nature. 1966 Jul 9;211(5045):206–207. doi: 10.1038/211206a0. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Jr Isolation and characterization of a Saccharomyces cerevisiae mutant deficient in pyruvate kinase activity. J Bacteriol. 1977 Apr;130(1):232–241. doi: 10.1128/jb.130.1.232-241.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich J., Kempfle M. Subunit size of cytoplasmic yeast pyruvate decarboxylase. FEBS Lett. 1969 Aug;4(4):273–274. doi: 10.1016/0014-5793(69)80253-x. [DOI] [PubMed] [Google Scholar]
- Wills C., Phelps J. A technique for the isolation of yeast alcohol dehydrogenase mutants with altered substrate specificity. Arch Biochem Biophys. 1975 Apr;167(2):627–637. doi: 10.1016/0003-9861(75)90506-8. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Eaton N. R. Genetics of induction and catabolite repression of Maltese synthesis in Saccharomyces cerevisiae. Mol Gen Genet. 1974;134(3):261–272. doi: 10.1007/BF00267720. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Kaufmann I., Rasenberger H., Haubetamann P. Genetics of carbon catabolite repression in Saccharomycess cerevisiae: genes involved in the derepression process. Mol Gen Genet. 1977 Feb 28;151(1):95–103. doi: 10.1007/BF00446918. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Scheel I. Mutants of Saccharomyces cerevisiae resistant to carbon catabolite repression. Mol Gen Genet. 1977 Jul 7;154(1):75–82. doi: 10.1007/BF00265579. [DOI] [PubMed] [Google Scholar]
- Zimmermann F. K., Schmiedt I., ten Berge A. M. Dominance and recessiveness at the protein level in mutant x wildtype crosses in Sacchaomyces cerevisiae. Mol Gen Genet. 1969 Aug 15;104(4):321–330. doi: 10.1007/BF00334231. [DOI] [PubMed] [Google Scholar]



