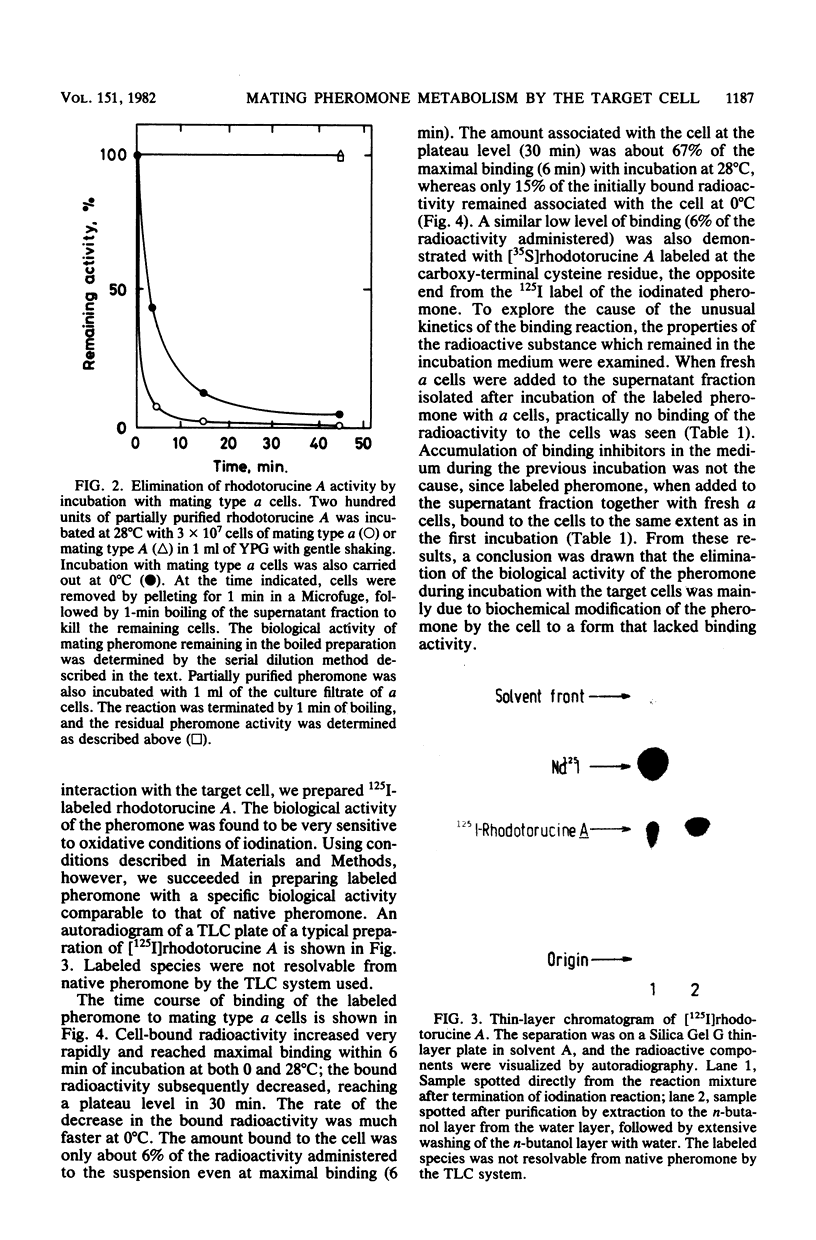
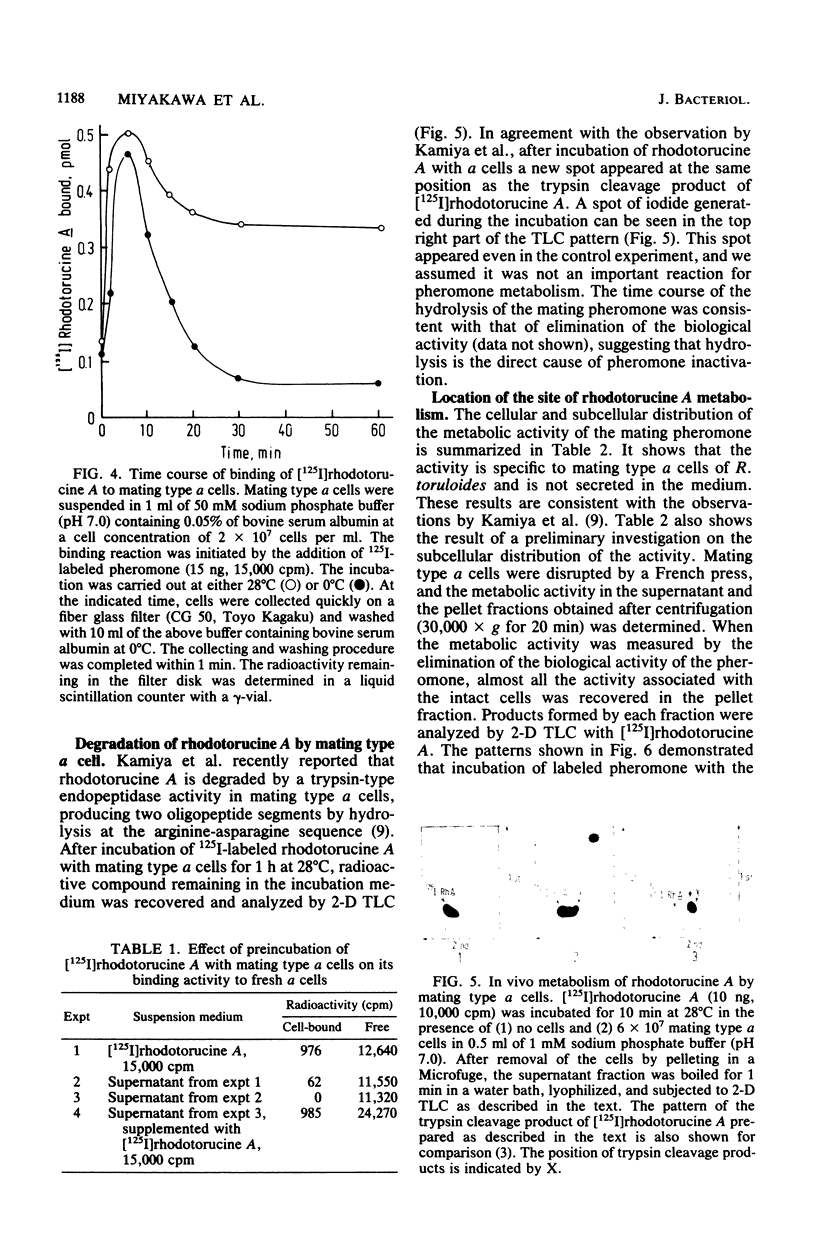
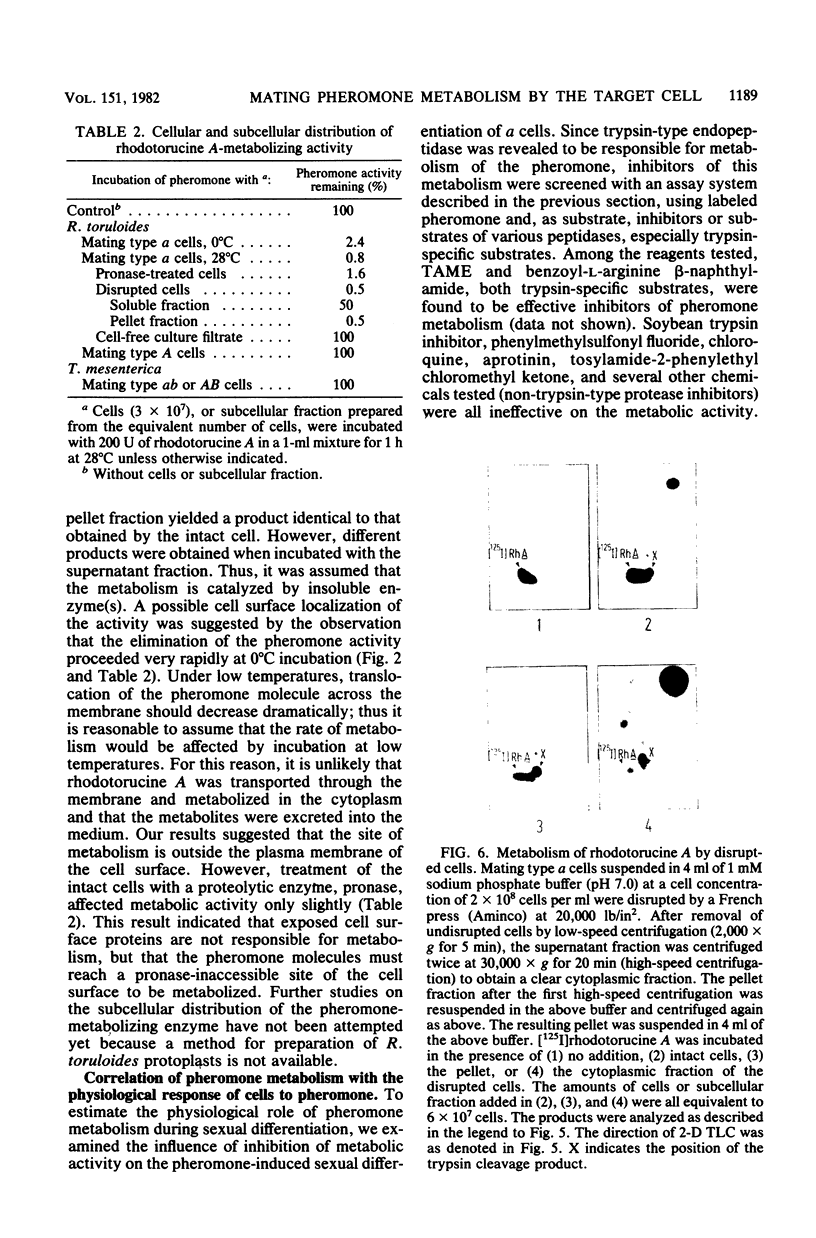
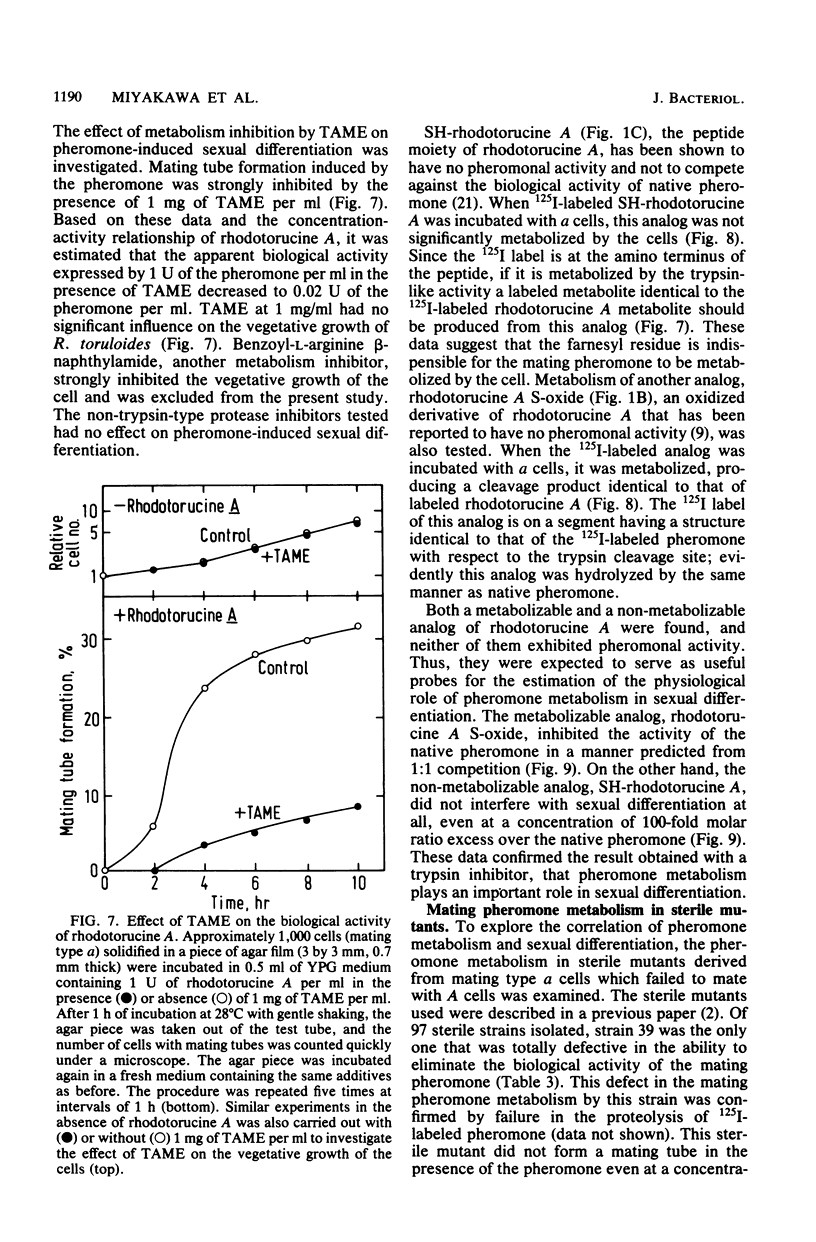
Abstract
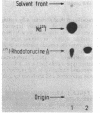
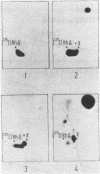
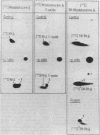
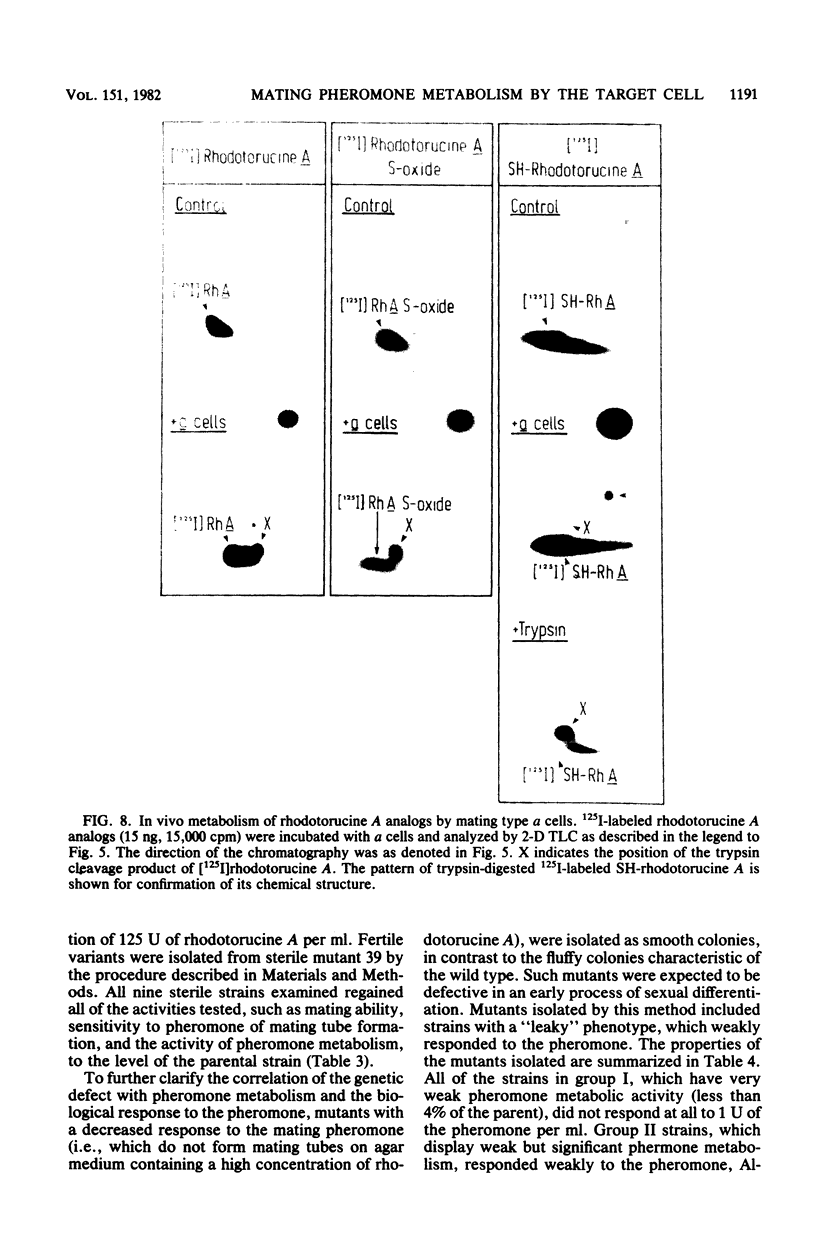
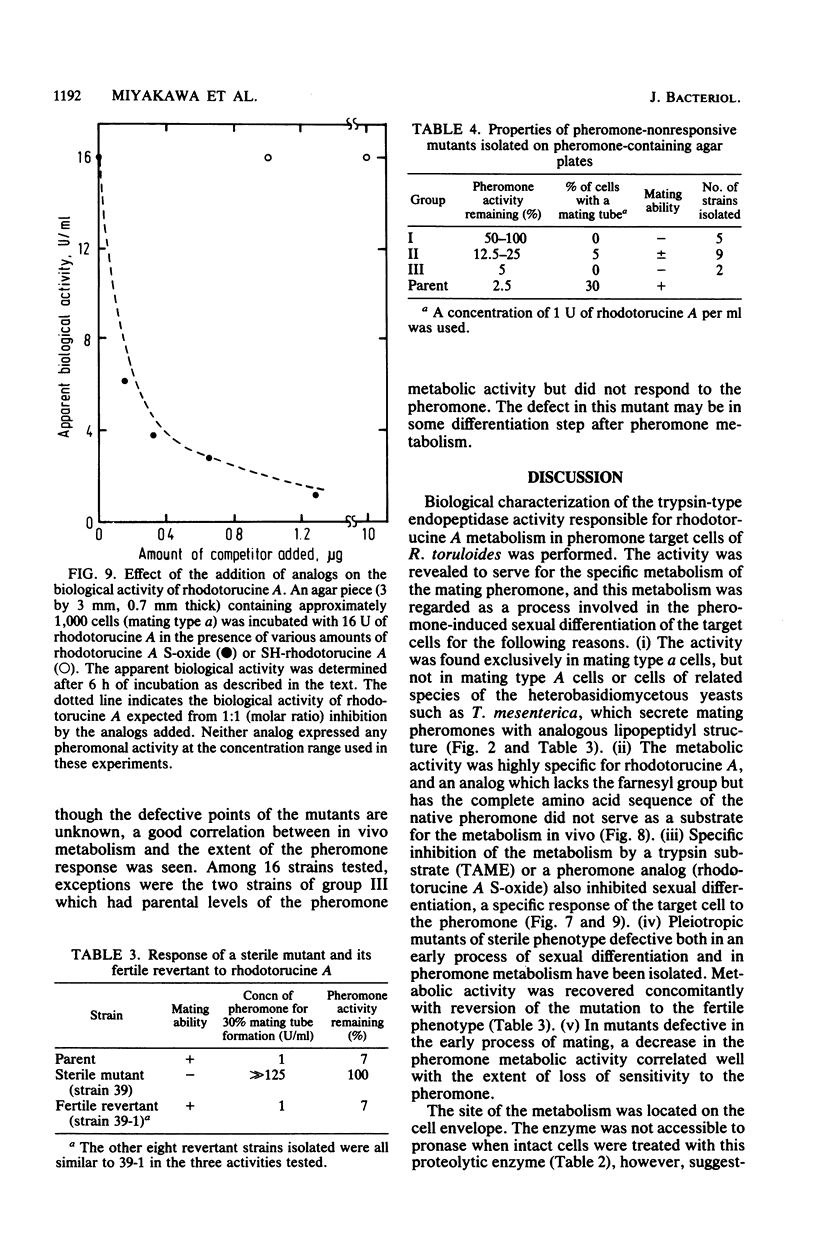
A trypsin-type endopeptidase (Kamiya et al., Biochem. Biophys. Res. Commun. 94:855-860, 1980) responsible for the metabolism of rhodotorucine A, the farnesyl undecapeptide mating pheromone secreted by mating type A cells of Rhodosporidium toruloides, was biologically characterized. Metabolic activity was found to be present exclusively on the cell surface of the pheromone target cell. The activity was highly specific to the pheromone, and a biologically inactive analog which has the complete amino acid sequence of rhodotorucine A but lacks the farnesyl residue was not metabolized by intact cells. Pheromone metabolism was inhibited by trypsin substrates such as tosyl-L-arginine methyl ester. The presence of tosyl-L-arginine methyl ester strongly inhibited the sexual differentiation induced by the pheromone at a concentration which did not affect the vegetative growth of R. toruloides. Pheromone-induced sexual differentiation was also strongly inhibited by a metabolizable analog, rhodotorucine A S-oxide, but not by a non-metabolizable one. In mutants defective in early processes of mating, the decrease in the pheromone metabolic activity correlated well with the extent of loss of sensitivity to the pheromone. Both the pheromone metabolism and the capacity for sexual differentiation of a sterile mutant were restored concomitantly with reversion from the sterile to the fertile phenotype. These results suggested that metabolism of the mating pheromone plays an essential role in the process of sexual differentiation in R. toruloides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Kusaka I., Fukui S. Morphological change in the early stages of the mating process of Rhodosporidium toruloides. J Bacteriol. 1975 May;122(2):710–718. doi: 10.1128/jb.122.2.710-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz R., Duntze W. Purification and partial characterization of a factor, a mating hormone produced by mating-type-a cells from Saccharomyces cerevisiae. Eur J Biochem. 1979 Apr;95(3):469–475. doi: 10.1111/j.1432-1033.1979.tb12986.x. [DOI] [PubMed] [Google Scholar]
- Ciejek E., Thorner J. Recovery of S. cerevisiae a cells from G1 arrest by alpha factor pheromone requires endopeptidase action. Cell. 1979 Nov;18(3):623–635. doi: 10.1016/0092-8674(79)90117-x. [DOI] [PubMed] [Google Scholar]
- Duntze W., MacKay V., Manney T. R. Saccharomyces cerevisiae: a diffusible sex factor. Science. 1970 Jun 19;168(3938):1472–1473. doi: 10.1126/science.168.3938.1472. [DOI] [PubMed] [Google Scholar]
- Finkelstein D. B., Strausberg S. Metabolism of alpha-factor by a mating type cells of Saccharomyces cerevisiae. J Biol Chem. 1979 Feb 10;254(3):796–803. [PubMed] [Google Scholar]
- Kamiya Y., Sakurai A., Takahashi N. Metabolites of mating pheromone, rhodotorucine A, by a cells of Rhodosporidium toruloides. Biochem Biophys Res Commun. 1980 Jun 16;94(3):855–860. doi: 10.1016/0006-291x(80)91313-3. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Edelman G. M. Inactivation and chemical alteration of mating factor alpha by cells and spheroplasts of yeast. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1304–1308. doi: 10.1073/pnas.75.3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y., Tanaka T., Chino N., Kita H., Sakakibara S. Amino acid substitution of mating factor of Saccharomyces cerevisiae structure-activity relationship. Biochem Biophys Res Commun. 1979 Feb 28;86(4):982–987. doi: 10.1016/0006-291x(79)90214-6. [DOI] [PubMed] [Google Scholar]
- Rasse-Messenguy F., Fink G. R. Temperature-sensitive nonsense suppressors in yeast. Genetics. 1973 Nov;75(3):459–464. doi: 10.1093/genetics/75.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics. 1980 Jul;95(3):561–577. doi: 10.1093/genetics/95.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya E., Fukui S. Binding of rhodotorucine A, a lipopeptidyl mating hormone, to a cells of Rhodosporidium toruloides for induction of sexual differentiation. Biochem Biophys Res Commun. 1978 Nov 14;85(1):473–479. doi: 10.1016/s0006-291x(78)80066-7. [DOI] [PubMed] [Google Scholar]
- Tsuchiya E., Fukui S., Kamiya Y., Sakagami Y., Fujino M. Requirements of chemical structure of hormonal activity of lipopeptidyl factors inducing sexual differentiation in vegetative cells of heterobasidiomycetous yeasts. Biochem Biophys Res Commun. 1978 Nov 14;85(1):459–463. doi: 10.1016/s0006-291x(78)80064-3. [DOI] [PubMed] [Google Scholar]