Abstract
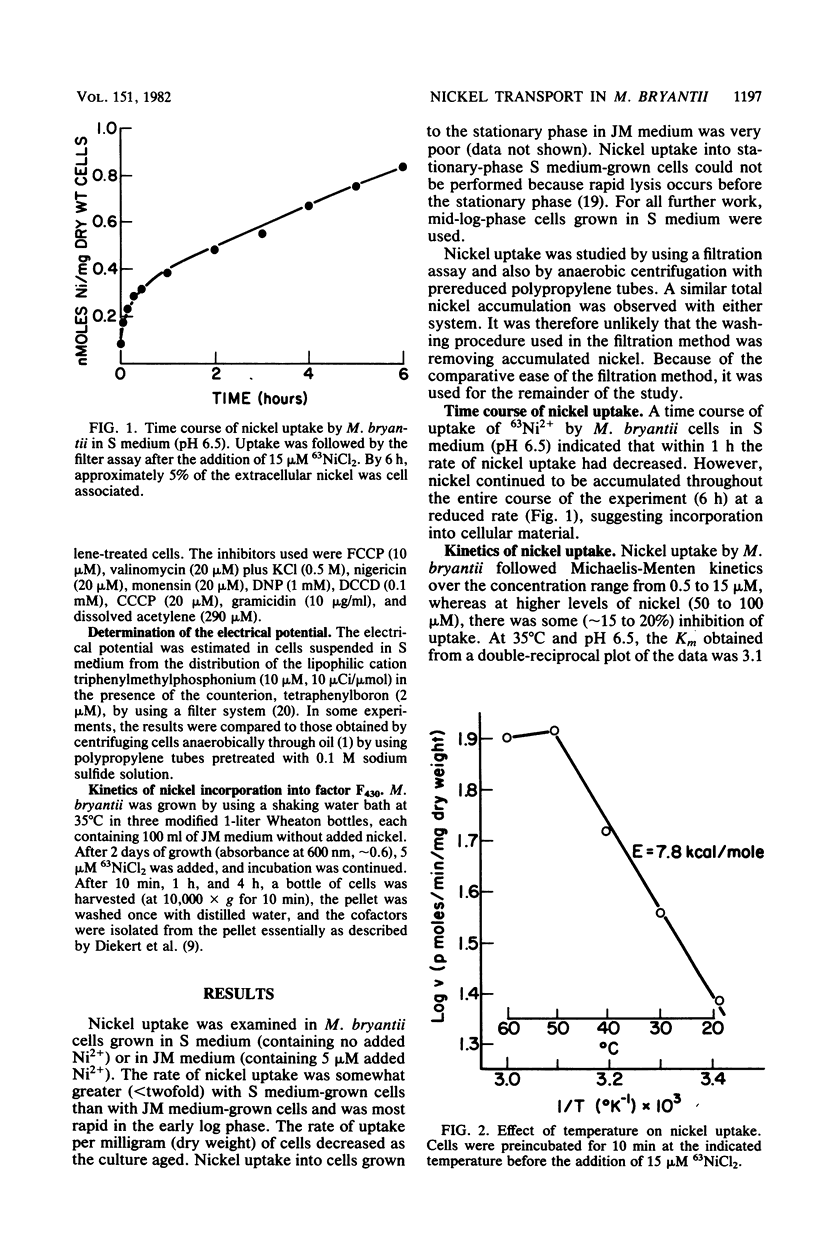
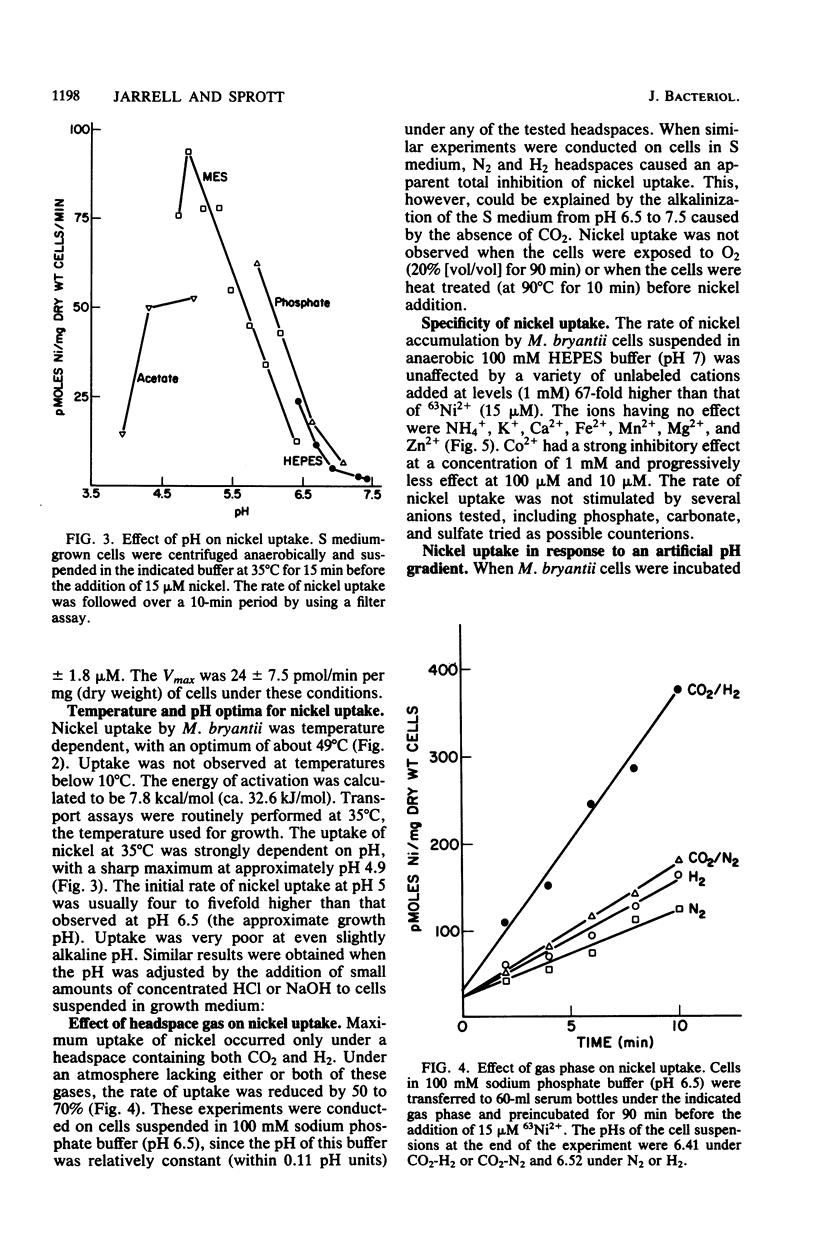
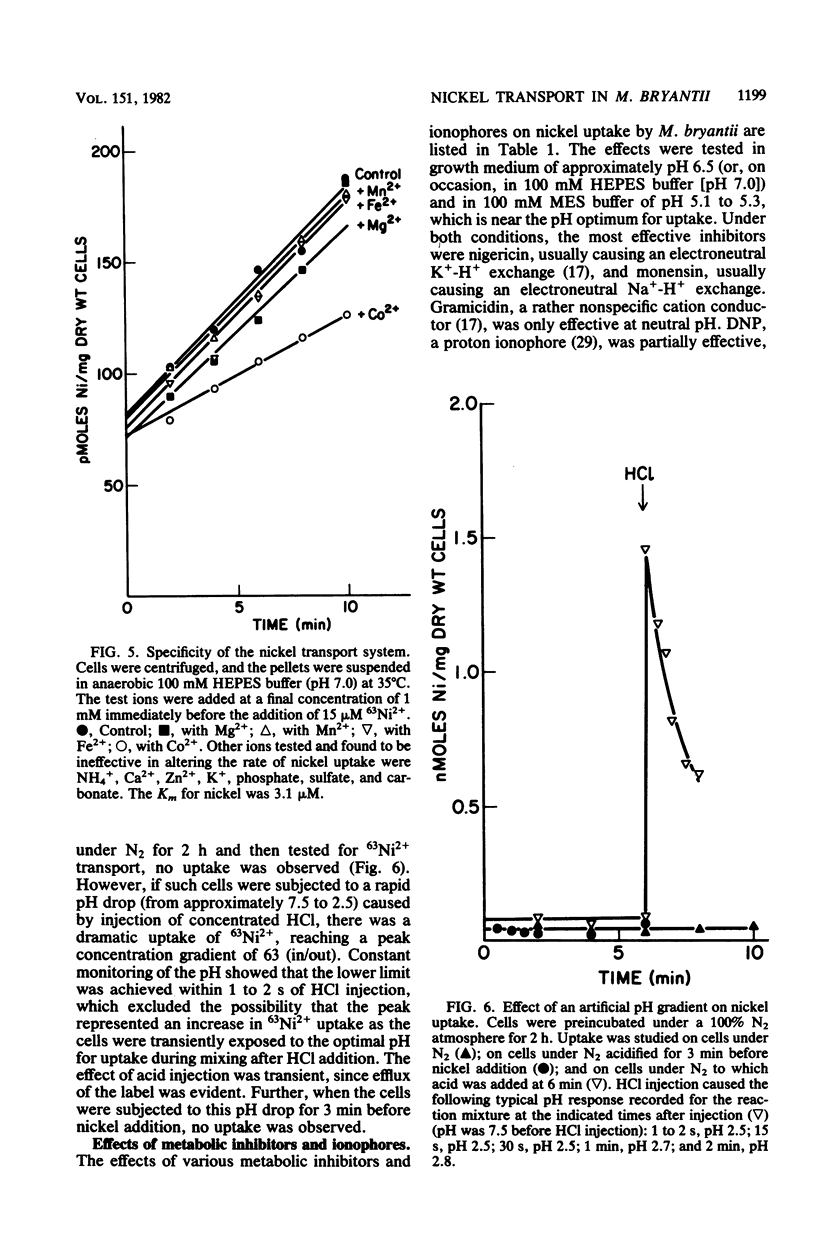
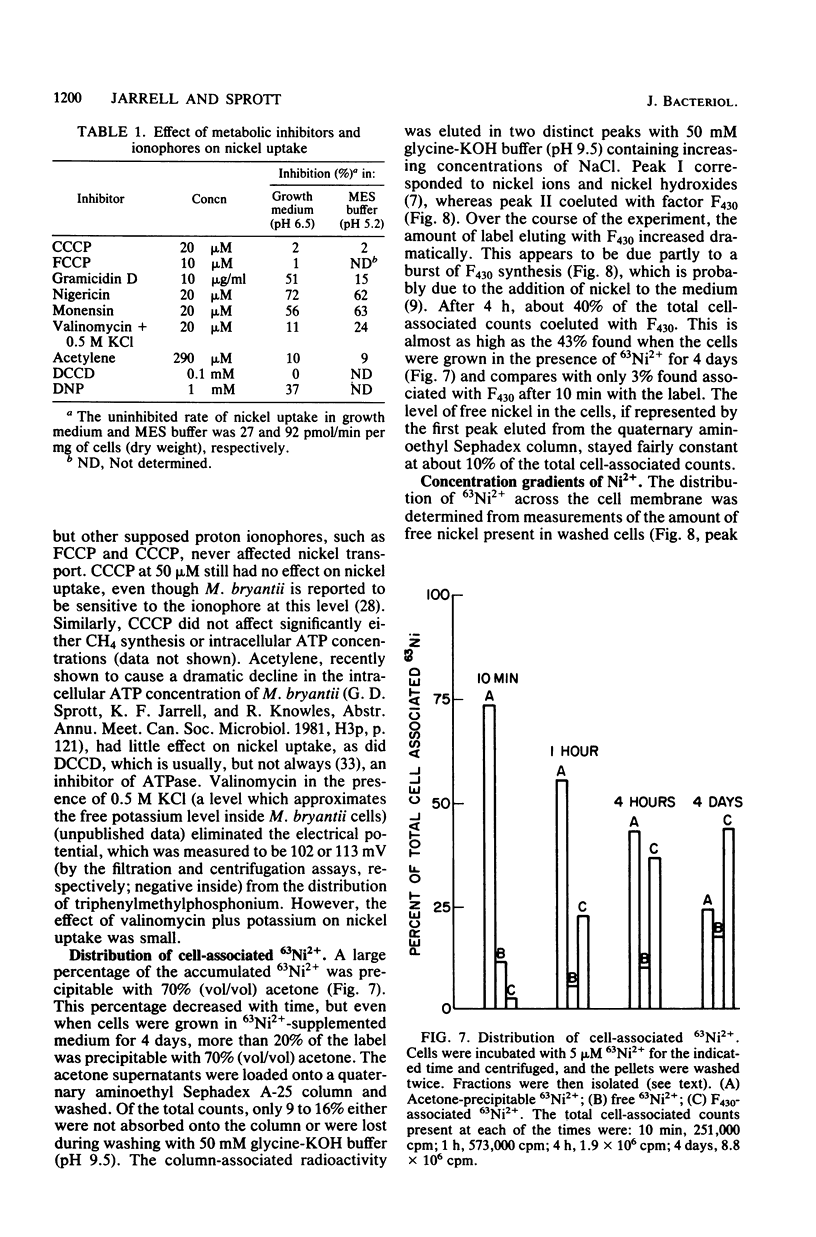
Methanobacterium bryantii, grown autotrophically on H2-CO2, transported nickel against a concentration gradient by a high-affinity system (Km = 3.1 microM). The system had a pH optimum of 4.9 and a temperature optimum of 49 degrees C with an energy of activation of 7.8 kcal/mol (ca. 32.6 kJ/mol). A headspace of H2-CO2 (4:1, vol/vol) was required for maximum rate of transport. The system was highly specific for nickel and was unaffected by high levels of all monovalent and divalent ions tested (including Mg2+) with the sole exception of Co2+. Kinetic experiments indicated that accumulated nickel became increasingly incorporated into cofactor F430 and protein. Nickel transport was inhibited by nigericin, monensin, and gramicidin but not by carbonyl cyanide-p-trifluoromethoxyphenyl hydrazone, carbonyl cyanide-m-chlorophenyl hydrazone, N,N'-dicyclohexylcarbodiimide, valinomycin plus potassium, or acetylene. The ineffectiveness of carbonyl cyanide-p-trifluoromethoxyphenyl hydrazone, carbonyl cyanide-m-chlorophenyl hydrazone, and N,N'-dicyclohexylcarbodiimide may be related to difficulties in the penetration of these compounds through the outer cell barriers. Nickel uptake was greatly stimulated by an artificially imposed pH gradient (inside alkaline). The data suggest that nickel transport is not dependent on the membrane potential or on intracellular ATP, but is coupled to proton movement.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTHA R., ORDAL E. J. NICKEL-DEPENDENT CHEMOLITHOTROPHIC GROWTH OF TWO HYDROGENOMONAS STRAINS. J Bacteriol. 1965 Apr;89:1015–1019. doi: 10.1128/jb.89.4.1015-1019.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Harold F. M. Energy coupling to potassium transport in Streptococcus faecalis. Interplay of ATP and the protonmotive force. J Biol Chem. 1980 Jan 25;255(2):433–440. [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. Transport of coenzyme M (2-mercaptoethanesulfonic acid) in Methanobacterium ruminantium. J Bacteriol. 1979 Jan;137(1):264–273. doi: 10.1128/jb.137.1.264-273.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuil C., Patel G. B. Composition of Methanospirillum hungatii GP1 during growth on different media. Can J Microbiol. 1980 May;26(5):577–582. doi: 10.1139/m80-102. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Diekert G., Klee B., Thauer R. K. Nickel, a component of factor F430 from Methanobacterium thermoautotrophicum. Arch Microbiol. 1980 Jan;124(1):103–106. doi: 10.1007/BF00407036. [DOI] [PubMed] [Google Scholar]
- Diekert G., Konheiser U., Piechulla K., Thauer R. K. Nickel requirement and factor F430 content of methanogenic bacteria. J Bacteriol. 1981 Nov;148(2):459–464. doi: 10.1128/jb.148.2.459-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doddema H. J. Anaerobic biochemical techniques applied in the purification of the hydrogenase of Methanobacterium thermoautotrophicum. Antonie Van Leeuwenhoek. 1980;46(1):107–107. doi: 10.1007/BF00422244. [DOI] [PubMed] [Google Scholar]
- Doddema H. J., Claesen C. A., Kell D. B., van der Drift C., Vogels G. D. An adenine nucleotide translocase in the procaryote Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1288–1293. doi: 10.1016/0006-291x(80)91613-7. [DOI] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Heine E., Finck A., Friedrich C. G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981 Mar;145(3):1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Altendorf K. H., Hirata H. Probing membrane transport mechanisms with inophores. Ann N Y Acad Sci. 1974 May 10;235(0):149–160. doi: 10.1111/j.1749-6632.1974.tb43264.x. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Colvin J. R., Sprott G. D. Spontaneous protoplast formation in Methanobacterium bryantii. J Bacteriol. 1982 Jan;149(1):346–353. doi: 10.1128/jb.149.1.346-353.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Sprott G. D. The transmembrane electrical potential and intracellular pH in methanogenic bacteria. Can J Microbiol. 1981 Jul;27(7):720–728. doi: 10.1139/m81-110. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Van Brunt J., Harold F. M. ATP-linked calcium transport in cells and membrane vesicles of Streptococcus faecalis. J Biol Chem. 1978 Apr 10;253(7):2085–2092. [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Sprott G. D., Smith I. C. Novel polar lipids from the methanogen Methanospirillum hungatei GP1. Biochim Biophys Acta. 1981 Apr 23;664(1):156–173. doi: 10.1016/0005-2760(81)90038-2. [DOI] [PubMed] [Google Scholar]
- Murray W. D., van den Berg L. Effects of nickel, cobalt, and molybdenum on performance of methanogenic fixed-film reactors. Appl Environ Microbiol. 1981 Sep;42(3):502–505. doi: 10.1128/aem.42.3.502-505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G. B., Agnew B. J. A simple apparatus for measuring the Eh of anaerobic media. Can J Microbiol. 1981 Aug;27(8):853–855. doi: 10.1139/m81-134. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Wolfe R. S. Adenosine triphosphate pools in Methanobacterium. J Bacteriol. 1970 Apr;102(1):43–51. doi: 10.1128/jb.102.1.43-51.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F. D., Erfle J. D., Mahadevan S. Evidence for an internal electrochemical proton gradient in Methanobacterium thermoautotrophicum. J Biol Chem. 1981 Oct 10;256(19):9843–9848. [PubMed] [Google Scholar]
- Sauer F. D., Erfle J. D., Mahadevan S. Methane synthesis without the addition of adenosine triphosphate by cell membranes isolated from Methanobacterium ruminantium. Biochem J. 1979 Jan 15;178(1):165–172. doi: 10.1042/bj1780165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönheit P., Moll J., Thauer R. K. Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch Microbiol. 1979 Oct;123(1):105–107. doi: 10.1007/BF00403508. [DOI] [PubMed] [Google Scholar]
- Sedgwick E. G., MacLeod R. A. Energy coupling to K+ transport in a marine bacterium. Can J Biochem. 1980 Oct;58(10):1206–1214. doi: 10.1139/o80-161. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Jarrell K. F. K+, Na+, and Mg2+ content and permeability of Methanospirillum hungatei and Methanobacterium thermoautotrophicum. Can J Microbiol. 1981 Apr;27(4):444–451. doi: 10.1139/m81-067. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Langworthy T. A. Diphytanyl and dibiphytanyl glycerol ether lipids of methanogenic archaebacteria. Science. 1979 Jan 5;203(4375):51–53. doi: 10.1126/science.758677. [DOI] [PubMed] [Google Scholar]
- Webb M. Interrelationships between the utilization of magnesium and the uptake of other bivalent cations by bacteria. Biochim Biophys Acta. 1970 Nov 24;222(2):428–439. doi: 10.1016/0304-4165(70)90133-9. [DOI] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Presence of nickel in factor F430 from Methanobacterium bryantii. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1196–1201. doi: 10.1016/0006-291x(80)90413-1. [DOI] [PubMed] [Google Scholar]



