Abstract
We have previously shown that a decrease in GABA tone and a subsequent increase in glutamatergic tone occur in association with the pubertal increase in LHRH release in primates. To further determine the causal relationship between developmental changes in GABA and glutamate levels and the pubertal increase in LHRH release, we examined monkeys with precocious puberty induced by lesions in the posterior hypothalamus (PH). Six prepubertal female rhesus monkeys (17.5±0.1 months of age) received lesions in the PH, 3 prepubertal females (17.5±0.1 months) received sham lesions, and 2 females received no treatments. LHRH, GABA, and glutamate levels in the stalk-median eminence before and after lesions were assessed over two 6-h periods (6:00–12:00 and 18:00–24:00) using push-pull perfusion. Monkeys with PH lesions exhibited external signs of precocious puberty including significantly earlier menarche in PH lesion animals (18.9±0.2 months) than in sham/controls (25.5±0.6 months, p<0.001). Moreover, PH lesion animals had elevated LHRH levels and higher evening glutamate levels after lesions, whereas LHRH changes did not occur in sham/controls until later. Changes in GABA release were not discernible, as evening GABA levels already deceased at 18–20 months of age in both groups and morning levels remain at the prepubertal levels. The age of first ovulation in both groups did not differ. Collectively, PH lesions may not be a good tool to investigate the mechanism of puberty and taking into account the recent findings on the role of kisspeptins, the mechanism of the puberty onset in primates is more complex than we initially anticipated.
Keywords: timing of puberty, LHRH release, lesions in the hypothalamus, primates
Introduction
The concept that an increase in pulsatile LHRH release triggers the onset of puberty has been well established (26, 44, 45). However, the mechanism of the pubertal increase in LHRH release remains unclear (39). It has been reported that children with tumors or hamartomas in the hypothalamus exhibit precocious puberty (37). A previous study from our laboratory showed that bilateral lesions made in the posterior hypothalamus of female rhesus monkeys during the prepubertal stage result in an early rise in LH release followed by precocious puberty (42). Moreover, similar lesions in ovariectomized prepubertal females result in early pubertal LH increases and accelerated timing of the estrogen positive feedback effect on the LH surge when compared to those in ovariectomized sham control females (35), indicating that the hypothalamic lesion-induced LH increase in prepubertal monkeys is independent of ovarian steroid hormones and is suggestive that lesions cause changes in the control mechanism of the LHRH neurosecretory system.
A series of studies in our laboratory indicate that an increase in LHRH release is associated with a decrease in γ-aminobutyric acid (GABA) followed by an increase in glutamate in the stalk-median eminence (S-ME), when female rhesus monkeys undergo normal puberty (21, 41). The importance of the decrease in GABA tone at the onset of puberty is further suggested by our observation that the GABAA receptor blocker, bicuculline, results in precocious puberty (15). However, it is still unclear whether the pubertal increase in LHRH release is causally related to the decrease in GABA release followed by an increase in glutamate release. Although producing lesions in the hypothalamus may not be the best approach, this is only the known method for induction of precocious puberty in non-human primates.
Therefore, the objective of this study is 1) to determine if precocious puberty induced by posterior hypothalamic lesions is due to an increase in LHRH release in the S-ME and 2) to examine whether hypothalamic lesion-induced LHRH increases occur in association with a decrease in GABA release and an increase in glutamate release.
Material and Methods
Animals
Eleven female rhesus monkeys (Macaca mulatta), born at the Wisconsin National Primate Research Center, at 13–14 months of age were used in this study. The animals were housed in pairs (172 × 86 × 86 cm) under controlled lighting conditions (lights on 6:00–18:00 and lights off 18:00–6:00) with room temperature maintained at 22 C. The monkeys were fed Purina Monkey Chow once every morning, supplemented with fresh fruit every afternoon. Tap water was available ad libitum. The protocol for this study was reviewed and approved by the Animal Care and Use Committee, University of Wisconsin, and all experiments were conducted under the guidelines established by the NIH and USDA.
Six of the 11 monkeys were assigned to the lesion group, whereas 3 of the 11 monkeys were assigned to the sham control, and the other 2 monkeys remained intact. Body weights were recorded weekly and daily observations on development of sex skin color and menstruation were made throughout the entire experimental period, as described previously (9). Blood samples (3ml) were collected once a week for measurement of circulating hormones.
Experimental Designs
Experiments were designed to observe acute and chronic effects of hypothalamic lesions on precocious puberty. Lesions were made between 17.0–17.9 months of age. Acute effects of posterior hypothalamic lesions on release of LHRH, GABA, and glutamate were to occur in the first 3 months post operatively (before 21 months of age), whereas the chronic effects of posterior hypothalamic lesions on LHRH release would occur throughout the pubertal process.
At approximately 14 months of age the monkeys were anesthetized with isoflurane and cranial pedestals were implanted on their skulls using a stereotaxic apparatus. The center of the cranial pedestal was positioned above the third ventricle recess using x-ray ventriculograms, with which the third ventricle was visualized (11). The cranial pedestals allowed us to collect in vivo perfusates in the S-ME from monkeys at various ages during their development, as described below. To obtain control data in all animals push-pull perfusion experiments were conducted 6 to 9 weeks after the cranial pedestal implantation (between 15.5–17.0 months of age), as described below. Between 17.0–17.9 months of age bilateral posterior hypothalamic lesions were made through cranial pedestals using x-ray ventriculography. The lesions were made by passing a radiofrequency current through a thermister electrode with a tip diameter of 0.7 mm, as described previously (35, 42). Briefly, the radiofrequency current was raised slowly over 30 sec to bring the tip temperature to 73 °C, which was maintained for 1 min, followed by a gradual current reduction during the 30 sec period. In sham controls, the identical procedure was applied, except for no radiofrequency current was passed through the electrode. Intact controls did not receive any experimental procedure. Subsequently, in all animals, except for one animal in the lesion group (see below), a push-pull perfusion experiment was conducted between 18 and 20 months of age. In one lesion animal only one push-pull experiment was conducted after lesions. To examine the chronic effect of lesions, push-pull perfusion experiments were conducted at 3 to 5 month intervals until the animals ovulated for the first time.
Push-pull perfusion
To assess the changes in release of LHRH, GABA, and glutamate in the S-ME, the push-pull perfusion method was used. Prior to initiation of the push-pull perfusion experiments the monkeys were adapted to the researchers and the experimental environments, as described previously. Three days prior to the perfusion experiment the animals were anesthetized with ketamine and xylazine and placed in a stereotaxic apparatus. An outer cannula (20 ga.) with an inner stylet (28 ga.) was inserted into the median eminence-stalk using a hydraulic microdrive unit (model MO95-B, Narshige Instrument, Tokyo, Japan), which allows three-dimensional micro-adjustments for accurate placement. Cannula placement was visualized with x-ray ventriculograms, and compared with the ventriculograms obtained during pedestal implantation. After implantation the animals were placed in a primate chair and allowed three days to recover before the experiment started. On the day of the experiment, the stylet was replaced with inner cannula (28 ga.) and artificial CSF was infused through the inner (push) cannula, while the perfusate samples were collected from the outer (pull) cannula at 10-min intervals. Perfusate samples were collected for 6 hours in the morning (6:00–12:00) and 6 hours in the evening (18:00–24:00) using two peristaltic pumps with identical flow speeds (~22 μl/min). The order of morning and evening sampling sessions was randomized. Perfusates were aliquoted to 150 μl for LHRH assay and the remaining (~70 μl) for GABA and glutamate assay and stored in μ70 °C.
Assays
The LHRH levels in all perfusates were measured using RIA antiserum R1245, kindly provided by Dr. Nett (Colorado State University, Fort Collins, CO, USA). The method for LHRH assay has been reported previously (11). Synthetic LHRH (Richelieu Biotechnologies Inc., Montreal, Quebec, Canada) was used for the radiolabeled antigen and reference standard. The antigen-antibody complex was precipitated with a goat anti-rabbit gamma globulin. Sensitivity of the assay was 0.1 pg/tube. Intra and inter assay coefficient of variations were 8.1% and 11.3%, respectively.
The GABA and glutamate levels in one set of perfusate samples before and after lesions (or sham surgery in sham controls or at an equivalent age in intact controls) were measured by HPLC with electrochemical detection, as described previously (21, 41). Two aliquots from the same perfusate samples used for LHRH measurements, at 20 μl each (one for GABA and the other for glutamate) were derivatized, with 45 μl of a mixture containing o-phthaldialdehyde reagent and 2-methyl-2-propanethiol (Sigma, St. Louis, MO) and separated by centrifugation. Samples were injected at 50 μl onto a C18 column with a mobile phase consisting of 2.5 mM sodium phosphate dibasic, 0.5 mM EDTA, 40% methanol, and 10% acetonitrile at pH 7.1 for GABA assay, or 5 mM sodium phosphate dibasic, 0.5 mM EDTA, 40% methanol, and 5% acetonitrile at pH 6.9 for glutamate assay. Minimum detectability of GABA and glutamate were 0.2 and 2.0 pg per 20 μl sample, respectively.
For evidence of first and second ovulations, progesterone in serum samples was measured by RIA (40). The ages of first and second ovulations were assessed by the pattern of the sex-skin color break-down followed by progesterone levels above 1 ng/ml.
Histological Examinations
After their second ovulation the lesion and sham animals were anesthetized and perfused with 4% buffered paraformaldehyde solution. The brains were removed and placed in the cryoprotectant sucrose PBS (30g/100ml). Serial sections were cut by cryostat in the frontal plane at 50 μm, and every 10th slide was stained with cresyl violet to visualize the region of the lesions using an atlas by Bleier for reference (2). To identify the damage of the LHRH neuronal system by lesions, remaining serial sections were systematically immunostained with a cocktail of LR1 and GF-6 as described previously (30, 33) and tissue sections were further analyzed.
Statistical Analysis
The data from the three-sham lesion controls and two intact animals were pooled together as the control group (sham/controls, n=5), as they were very similar. Because one of the 6 lesion animals had to be discontinued 4 weeks after menarche at 20.5 months of age, the lesion group became n=5 after menarche. In one of the 6 lesion animals GABA and glutamate were not measured. Acute lesion effects on LHRH, GABA, and, glutamate levels were examined by analysis of variance repeated measures followed by post hoc Tukey test using hourly mean from individual animals. Lesion effects on the pulsatility of LHRH release were assessed with the PULSAR computer algorithm (20). The key parameters for PULSAR were similar to those previously reported (49), i.e., the cut-off criteria for G1, G2, G3, G4 and G5 were 3.8, 2.6, 1.9, 1.5 and 1.2, respectively, and the intra-assay coefficients of variation for LHRH were described by the formula Y=3.38X+3.14/100. Chronic effects of posterior hypothalamic lesions on LHRH release were examined from the data obtained periodically after 21 months of age through the confirmation of first ovulation: developmental changes in LHRH release at the age of 21–24 months, 25–29 months, 30–34 months and over 35 months in lesion and sham/control groups were compared using hourly mean from individual animals. Again, if multiple push-pull perfusion experiments were conducted in an animal during an age category, the results from only one experiment were used for the calculation. Chronic effects on GABA, and glutamate levels were not assessed, because of the large total sample number and the amount of labor required for GABA and glutamate analysis with HPLC in each sample. The effects of the treatments and ages on LHRH levels were examined by the analysis of variance repeated measures followed by post hoc Tukey test using hourly mean from individual animals. The effects of lesions on the age of menarche, first and second ovulations, and body weights at these events, were examined with Student t-test. Significance was attained at p<0.05.
Results
Posterior Hypothalamic Lesions on Timing of Puberty
Posterior hypothalamic lesions resulted in precocious menarche (Table 1). The age (18.8 ± 0.2 months) at menarche in the lesion group was significantly (p< 0.001) earlier than in the sham/control group (25.5 ± 0.9 months). However, the ages of first and second ovulations in the lesion group (40.1 ± 3.4 months and 44.9 ± 4.1 months) were not significantly different from those in the sham group (41.3 ± 1.3 months and 42.9 ± 1.3 months).
Table 1.
Effects of lesions in the posterior hypothalamic area on the timing of puberty: The ages of menarche, first ovulation (Ov), and second ovulation (Ov) and body weights (BW) at the corresponding ages are shown.
| Animals | N | Surgery (months) | Menarche (months) | BW (kg) | 1st Ov (months) | BW (kg) | 2nd Ov (months | BW (kg) |
|---|---|---|---|---|---|---|---|---|
| Lesions | 6† | 17.4±0.1# | 18.8±0.2*** | 2.6±0.1** | 40.1±3.4 | 5.3±0.5 | 44.9±4. 1 | 5.5±0.4 |
| Sham/Controls | 5†† | 17.5±0.1 | 25.5±0.9 | 3.2±0.1 | 41.3±1.3 | 5.2±0.2 | 42.9±1.3 | 5.2±0.2 |
the number of animals reduced to 5 after menarche,
three received sham surgery,
Mean±SEM,
p<0.001 vs. sham control,
p<0.01 vs. sham/control
The body weight (2.6 ± 0.1 kg) at menarche in the lesion group was also significantly (P <0.01) smaller than that (3.2 ± 0.1 kg) in the sham/control group (Table 1). However body weights at first and second ovulations (5.3 ± 0.5 kg and 5.5 ± 0.4 kg, respectively) were not different from those (5.2 ± 0.2 kg and 5.2 ± 0.2 kg) in the sham/control group.
Acute effects of posterior hypothalamic lesions on LHRH release
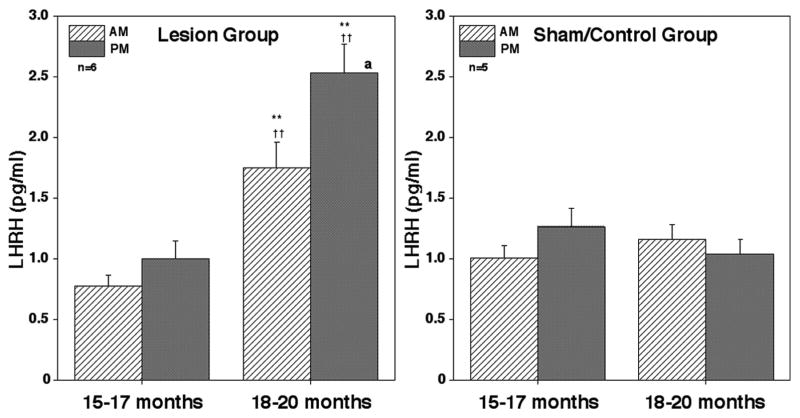
The morning and evening mean LHRH levels before the lesion surgery in the lesion group and those in sham/controls at the equivalent age were low and similar between the two groups (Figs. 1 and 2). After posterior hypothalamic lesions the morning and evening LHRH levels increased as shown in the example in Fig. 1. Similarly, mean LHRH levels (± SEM) after lesions in the lesion group were significantly (p<0.01) higher than those prior to lesions (Fig. 2). In contrast, the morning and evening LHRH levels within 3 months in the sham/control group did not significantly change. The mean LHRH levels after the surgery in the lesion group were also higher (p<0.01) than those in the sham/control group at the corresponding age (Fig. 2).
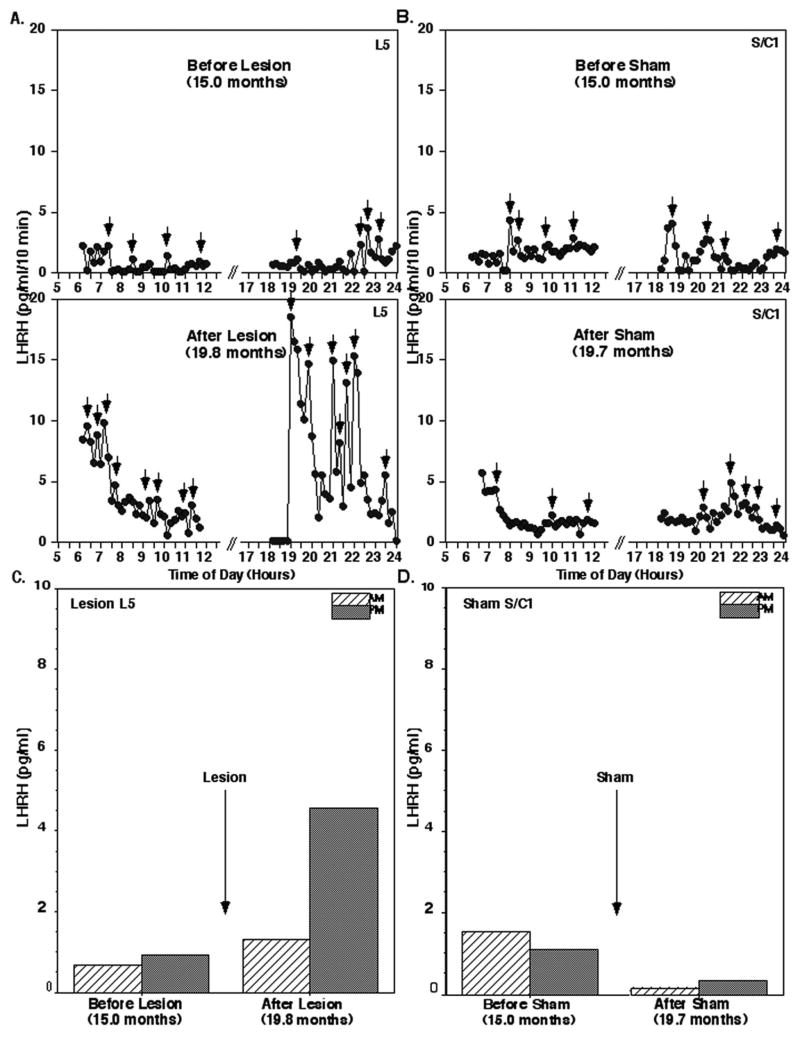
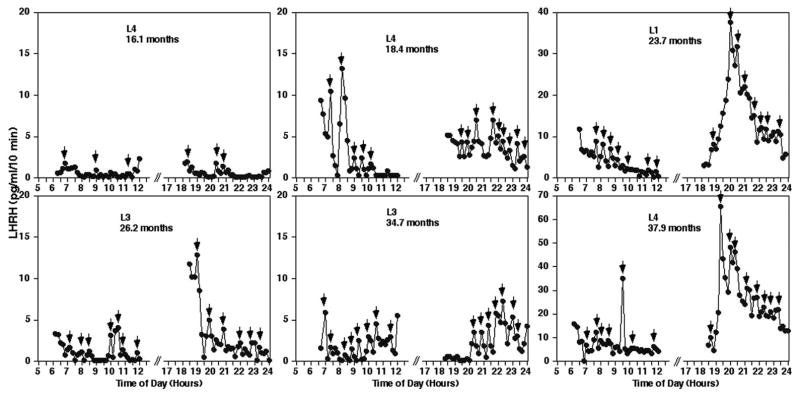
Figure 1.
Acute effects of posterior hypothalamic lesions on LHRH release in the stalk-median eminence. Representative examples from prepubertal monkeys before and after hypothalamic lesions (L5, A) or sham lesions (S/C1, B) are shown. Mean LHRH levels during the 6 hours in the morning and 6 hours in the evening of the same lesion animal (L5, C) and sham control (S/C1, D) are also shown. Note that in monkey L5 which received lesions, LHRH levels, especially the evening levels, increased dramatically, whereas LHRH levels in monkey S/C1 were not significantly affected by the sham surgery. Small arrows indicate the LHRH peaks identified by PULSAR analysis.
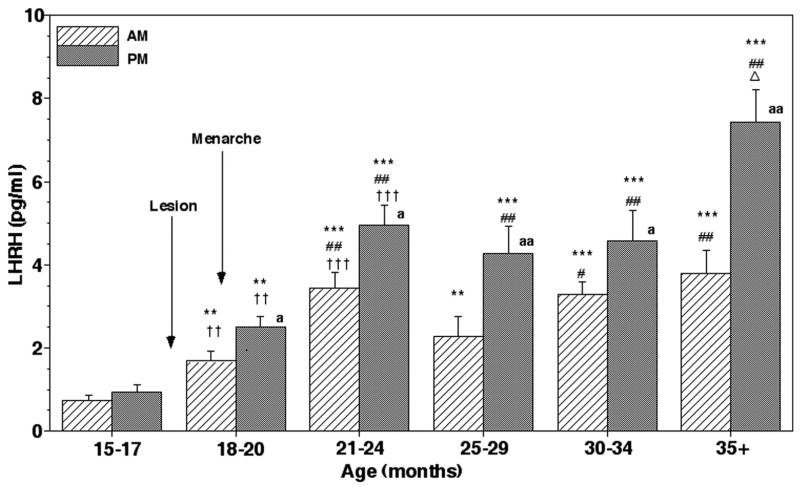
Figure 2.
Acute effects of posterior hypothalamic lesions on LHRH release in the stalk-median eminence. Group means (± SEM) are shown. Number of monkeys in the lesion group and sham/control group were 6 and 5, respectively. Note that lesion resulted in a significant increase in LHRH levels in both morning and evening when compared to the value prior to lesions (within group) as well as to those in the sham/control group at the corresponding age (between groups). In the sham/control group LHRH levels did not change. **: p<0.01 vs. 15–17 months (before lesions, within group); ††: p<0.01 vs. control/sham at the corresponding age (between groups); a: p<0.05 vs. morning values.
The results from PULSAR analysis indicated that the inter-pulse intervals in the morning and evening at 18–20 months of age were significantly reduced after lesions when compared to those before lesions at 15–17 months of age (P<0.01 for morning; p<0.001 for evening, Table 2) as well as those in sham/controls at the corresponding 18–20 months age (p<0.01). Similarly, the pulse amplitudes in both morning and evening were significantly (p<0.05) increased after lesions. Both inter-pulse intervals and pulse amplitude in sham/controls were not different between 15–17 and 18–20 months of age (Table 2).
Table 2.
Acute effects of posterior hypothalamic lesions on the inter-pulse intervals (IPI) and pulse amplitude of LHRH release
| Groups | AM IPI (min) | PM IPI (min) | AM Amplitude (pg/ml) | PM Amplitude (pg/ml) |
|---|---|---|---|---|
| Lesions (N = 6) | ||||
| 15–17 months | 137.7±35.1# | 114.7±11.5 | 1.4±0.2 | 1.3±0.2 |
| 18–20 months | 47.7±5.1**,a | 51.2±1.6***,aa | 3.4±1.0*,a | 5.0±1.6*,a |
| 21–24 months | 55.0±5.2***,a | 50.2±2.6*** | 5.1±1.3*,a | 6.6±1.4**,a |
| 25–29 months | 49.6±2.9*** | 54.2±3.1*** | 8.6±3.1* | 10.6±3.2* |
| 30–3å4 months | 56.4±2.1*** | 54.3±3.6*** | 7.4±2.6* | 8.7±1.5** |
| > 35 months | 44.5±3.3*** | 48.4±1.3*** | 11.6±5.0* | 14.2±5.0* |
|
| ||||
| Sham/controls (N=5) | ||||
| 15–17 months | 135.7±16.8 | 99.2±15.6 | 1.3±0.3 | 1.2±0.1 |
| 18–20 months | 132.0±30.9 | 122.0±23.1 | 1.5±0.3 | 1.8±0.8 |
| 21–24 months | 115.2±19.4 | 73.2±9.1 | 2.0±0.3 | 1.8±0.8 |
| 25–29 months | 71.8±8.6** | 58.0±3.6* | 2.1±0.4 | 4.8±1.4* |
| 30–34 months | 54.3±3.6*** | 51.1±5.1** | 9.3±3.5* | 15.7±3.5** |
| > 35 months | 57.3±5.2*** | 47.5±3.6** | 7.4±2.6* | 17.1±4.5** |
Mean±SEM
p<0.05 vs. 15–17 months;
p<0.01 vs. 15–17 months;
p<0.001 vs. 15–17 months (within group)
p<0.05 vs. sham/controls at corresponding age;
p<0.01 vs. sham/controls at corresponding age (between groups)
Acute effects of posterior hypothalamic lesions on GABA and glutamate levels
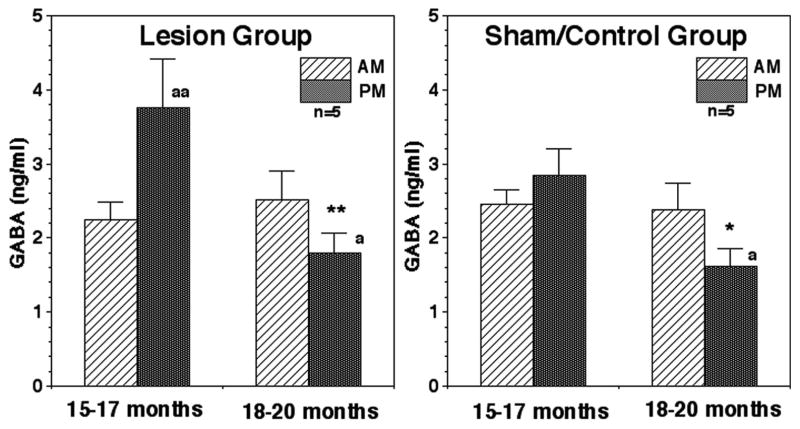
Prior to hypothalamic lesions, the evening GABA levels in juvenile animals at 15–17 months of age in lesion group were significantly higher (p<0.01) than morning levels. After lesions, the evening GABA levels became significantly lower than those before lesions (p<0.01, Fig. 3). The morning GABA levels were not affected by lesions. At 18–20 months of age evening GABA levels in lesion animals became lower than those in the morning (p<0.05). In the sham/control group at 15–17 months of age the nocturnal increase was not significant. However, to our surprise, evening GABA levels in the sham/control group at 18–20 months of age were significantly lower than those at 15–17 months of age as well as those in the morning at the same age (for both p<0.05, Fig. 3).
Figure 3.
Acute effects of posterior hypothalamic lesions on GABA release in the stalk-median eminence. Number of monkeys in the lesion group and sham/control group were 5 and 5, respectively. Note that evening GABA levels at 18–20 months were lower than those at 15–17 months of age in the lesion as well as sham/control groups (within group). **: p<0.01 vs. 15–17 months; *: p<0.05 vs. 15–17 months; aa: p<0.01 vs. morning values; a: p<0.05 vs. morning values.
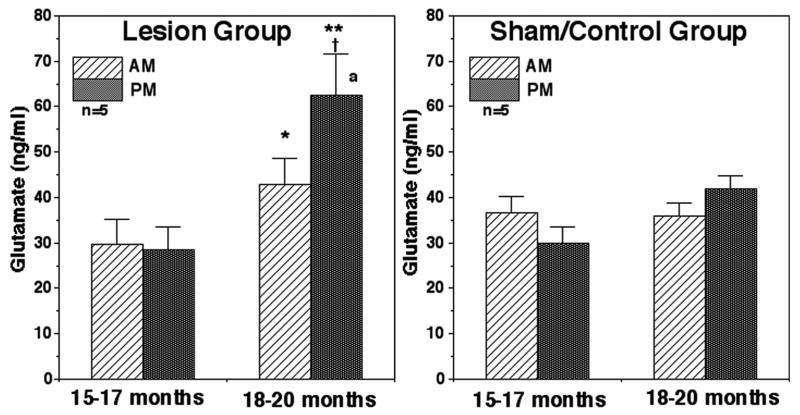
Prior to the treatment, there was no difference in morning and evening glutamate levels. After lesions, both morning and evening levels became significantly higher (p<0.05 for AM and p<0.01 for PM) than those prior to lesions (Fig. 4) The evening glutamate levels were also higher than those in sham/control at the corresponding age (p<0.05, Fig. 4). At 18–20 months of age evening glutamate levels in the lesion group became higher than morning levels (p<0.05). In contrast, neither evening nor morning glutamate levels in the sham/control group at the 15–17 months of age were different from those at 18–20 months of age. Similarly, no circadian changes in glutamate levels in the sham/control group were observed at either age (Fig. 4).
Figure 4.
Acute effects of posterior hypothalamic lesions on glutamate release in the stalk-median eminence. The number of monkeys in the lesion group and sham/control group was 5 for both. Note that glutamate levels after lesions at 18–20 months of age were significantly higher than those at 15–17 months of age prior to lesions (within group) as well as evening values in the sham/control group at the corresponding age (between groups). At 18–20 months of age evening glutamate levels were also higher than those in the morning. In the sham/control group glutamate levels did not change between two developmental ages. **: p<0.01 vs. 15–17 months; *: p<0.05 vs. 15–17 months; †: p<0.05 vs. sham/control group at the corresponding age (between groups); a: p<0.05 vs. morning values.
Chronic Effects of Posterior Hypothalamic Lesions on LHRH release
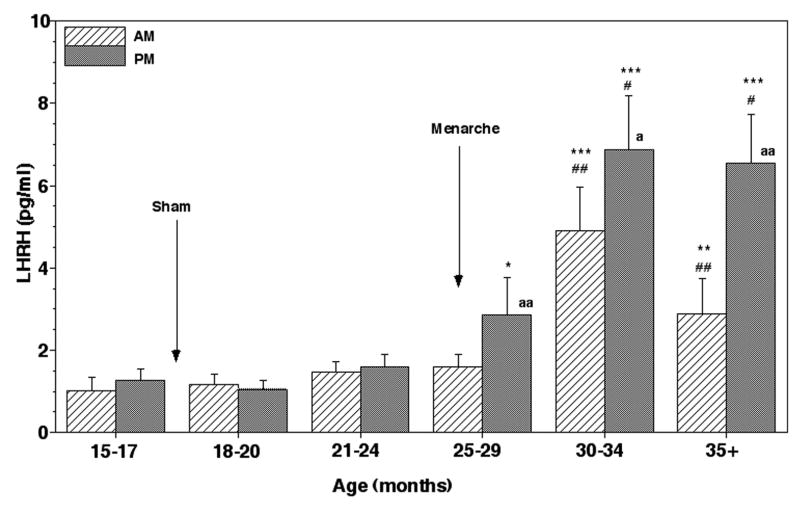
After menarche both morning and evening LHRH levels in the lesion group further increased at 21–24 months of age, and stayed at an elevated level until 30–34 months of age (p<0.001, Figs. 5 and 6). At the age prior to first ovulation (35+ months), the evening LHRH levels in the lesion group further elevated reaching the plateau value (Figs. 5 and 6). In contrast, both morning and evening LHRH levels in the sham/control group remained low through 24 months of age, and evening LHRH levels at 25–29 months finally became significantly higher (p<0.05) than those at the juvenile age coinciding with menarche (Figs. 7 and 8). After 30 months both morning and evening LHRH levels in the sham/control group further increased achieving the highest values, which were also highly significant (p<0.001) compared to those at 15–17 months of age. LHRH levels in the lesion group before 25 months of age were also significantly higher than in the sham/control group (p<0.001–0.01), but LHRH levels after 25 months of age did not differ from those during the corresponding age in the sham/control group (Fig. 6). Evening LHRH levels in the lesion group were significantly higher than morning levels during all periods examined after lesions, whereas the evening LHRH levels were only significant after 25 months of age in sham/control animals (Figs. 6 and 8).
Figure 5.
Chronic effects of posterior hypothalamic lesions on LHRH release in the stalk-median eminence. Representative examples in monkeys before lesion (L4, 16.1 months) and after hypothalamic lesions during the course of sexual development (L4 at 18.4 months, L1 at 23.7 months, L3 at 26.2 months, L3 at 34.7 months and L4 at 37.9 months) are shown. Note that the scale of y-axis differs in each case. Lesion resulted in elevated levels of LHRH release throughout pubertal development starting at ~18 months of age. Small arrows indicate LHRH peaks identified by PULSAR analysis.
Figure 6.
Chronic effects of posterior hypothalamic lesions on LHRH release in the stalk-median eminence. Group means (± SEM) are shown. The number of lesion monkeys in each developmental period was 5–6. Approximate age of lesion and menarche are indicated by arrows. After lesions, both morning (AM) and evening (PM) LHRH levels were significantly higher than the pre-lesion values (within group) as well as the values in sham/lesion animals at the corresponding age (between groups). Note that LHRH levels further increased at 21–24 months of age, and stayed the same through 34 months of age. The evening levels after 35 months of age further increased over the levels at 21–24 months of age. The evening values were higher than the morning values in all examined periods after posterior hypothalamic lesions. ***: p<0.001 vs. 15–17 months (before lesion); **: p<0.01 vs. 15–17 months (before lesion); ## p<0.01 vs. 18–20 months; # p<0.05 vs. 18–20 months; Δ: p<0.05 vs. 21–24 months, 25–29 months, and 30–34 months; †††: p<0.001 vs. values of sham/controls at the corresponding age; ††: p<0.01 vs. values of sham/controls at the corresponding age; aa: p<0.01 vs. morning levels at each age; a: p<0.05 vs. morning levels at each age.
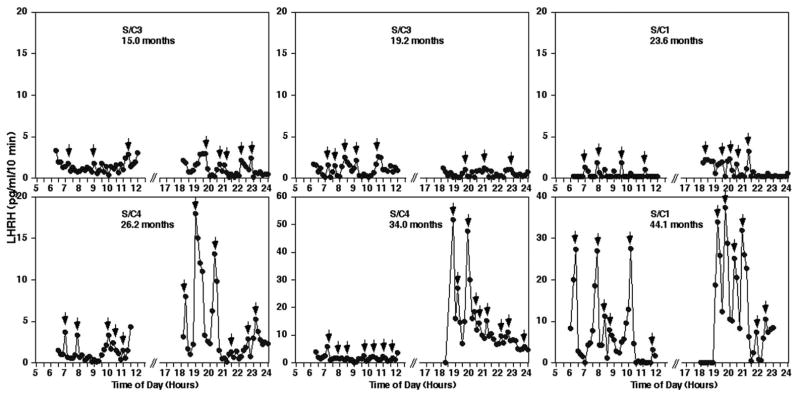
Figure 7.
Changes in LHRH release in the stalk-median eminence during pubertal development in sham/control animals. Representative examples in monkeys during the course of pubertal development (S/C3 at 15.0 months, S/C3 at 19.2 months, S/C1 at 23.6 months, S/C4 at 26.2 months, S/C34 at 34.0 months and S/C1 at 44.1 months) are shown. Note that the scale of the y-axis differs in each case. In sham/control animals a significant increase in LHRH release did not occur until around 25 months of age. Small arrows indicate LHRH peaks identified by PULSAR analysis.
Figure 8.
Changes in LHRH release in the stalk-median eminence during pubertal development in sham/control animals. Group means (± SEM) are shown. The number of sham/control monkeys in each developmental period was 4–5. Approximate age of sham lesion (in 3 animals) and menarche are indicated by arrows. Note that the first elevated LHRH levels were observed at 25–29 months of age. LHRH levels further increased after 30 months of age. A significant increase in the evening and morning LHRH levels started to occur at 25–29 months, and 30–34 months of age, respectively, and the increase continued until the end of the examination. ***: p<0.001 vs. 15–17 months; **: p<0.01 vs. 15–17 months; *: p<0.05 vs. 15–17 months; ##: p<0.01 vs. 25–29 months; #: p<0.05 vs. 25–29 months; aa: p<0.01 vs. morning levels at each age; a: p<0.05 vs. morning levels at each age.
The results from PULSAR analysis indicated that in the lesion group the inter-pulse intervals of LHRH release in both morning and evening after 21–24 months of age were continuously shorter than those of the pre-lesion controls at 15–17 months throughout the experiment (Table 2). Similarly, the pulse amplitudes in both morning and evening after 21–24 months of age were also significantly higher than the pre-lesion controls at 15–17 months throughout the experiment. However, comparison between the lesion and sham/control groups suggested that the morning inter-pulse intervals and both morning and evening pulse amplitudes in the lesion group were significantly different only at 21–24 months of age, but not older, because in sham/control animals the inter-pulse interval and pulse amplitude both underwent pubertal changes (Table 2).
Histological Observations
The lesioned areas of the hypothalamus in this study were similar (Fig. 9), but slightly more ventral to those observed in our previous study (42). The lesion scars were found bilaterally crossing the midline of the hypothalamus. The maximum extent of lesions was 3.1 ± 0.2 mm rostrocaudally. The areas included in all lesion animals were the premammillary area, a posterior portion of the infundibular (arcuate) nucleus, ventral portion of the ventromedial nucleus, the ventral portion of the dorsomedial nucleus and anterior portion of the mammillary body. While in 1 of the 6 monkeys the median eminence was slightly damaged, the remaining 5 monkeys the median eminence was completely spared. In 4 of the 6 monkeys only the caudal portion of the infundibular nucleus was lesioned, in the 2 of the 6 monkeys approximately 40% the infundibular nucleus was lesioned. Immunocytochemical staining indicated that LHRH fibers and perikarya in the stalk region were abundant in the lesion animals, but there were no LHRH fibers and perikarya in the lesioned area.
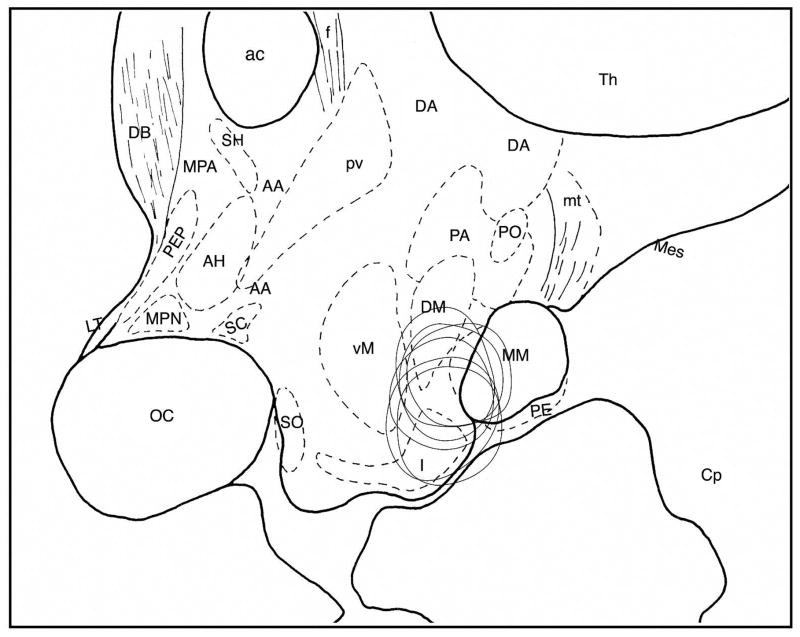
Figure 9.
Reconstruction of lesion area in the posterior hypothalamus of 6 monkeys shown in the sagittal plane. Schema was drawn based on Bleier’s atlas (2) with some modifications. Abbreviations: AA, Anterior hypothalamic area; ac, anterior commissura; AH, anterior hypothalamic nucleus; Cp, cerebral peduncle; DA, dorsal hypothalamic nucleus; DB, nucleus of diagonal bundle of Broca; DM, dorsomedial nucleus; f, fornix; I, infundibular nucleus; LT, lamina terminalis; Mes, mesencephalic tegmentum; MM, medial mammillary nucleus; MPA, medial preoptic area; MPN, medial preoptic nucleus; oc, optic chiasm; PA, posterior hypothalamic area; PE, perimammillary nucleus; PEP, periventricular preoptic nucleus; PM, paramammillary nucleus; PO, posterior nucleus; PV, paraventricular nucleus; SC, suprachiasmatic nucleus; SH, septohypothalamic nucleus; SO, supraoptic nucleus; Th, thalamus; VM, ventromedial nucleus.
Discussions
The results of the present study indicate that an increase in LHRH release occurs when the age of puberty was advanced by lesions in the posterior hypothalamus. Although it has been clearly shown that pulsatile LHRH release increases at the onset of spontaneous puberty in monkeys and rats (38, 44), to date an elevated release of LHRH in association with precocious puberty has only been shown indirectly by measurement of LH. Clinical studies have demonstrated that pulsatile LH release increases in association with precocious puberty in humans and that injection of an LHRH super agonist arrests precocious puberty, presumably due to pituitary down-regulation (see 3, 34). In rhesus monkeys an increase in LH release occurs when precocious puberty is induced by posterior hypothalamic lesions (42), infusion of the GABAA receptor antagonist, bicuculline into the basal hypothalamus (15), systemic injection of IGF1 or leptin (47, 48), or systemic injection of NMDA (28). Thus, the findings of this study are significantly important to support the concept that an increase in LHRH release is accompanied with an alteration of the timing of puberty.
The posterior hypothalamic lesion-induced increase in LHRH release was followed by precocious menarche. Although in our previous study posterior hypothalamic lesions advanced the timing of both menarche and first ovulation (42), in the present study the lesion did not result in early first ovulation. This difference does not appear to be due to the difference in the size and location of lesions within the hypothalamus, as they were comparable, but it may rather be attributed to the trend in our colony during the last 2 decades that menarche and first ovulation occur at much younger ages (Terasawa, unpublished observation). The cause of changes in the normal puberty age in our female rhesus colony remains to be investigated. Nonetheless, the absence of the advanced timing of first ovulation after precocious menarche with hypothalamic lesions is quite different from the precocious puberty induced by the infusion of the GABAA receptor antagonist, bicuculline, into the basal hypothalamus (15). While in that study, first ovulation occurred at the menarcheal age of control animals, in the present study first ovulation did not occur until the same age in sham/control animals, despite the fact that mean LHRH levels, pulse frequency, and pulse amplitude in lesion animals all increased after lesions.
The control mechanisms involved in the timing of first ovulation and menarche appear to differ, even though both require an increase in pulsatile LHRH release. Previously, we proposed a hypothesis that a larger amount of LHRH output from the hypothalamus is required for the positive-feedback effects of estrogen in the pubertal rhesus monkey (15). Consistent with the hypothesis, in the present study we observed that mean LHRH levels dramatically increased after 35 months of age in both lesion and sham/control animals (Figs. 6 and 8). Previous studies also suggest that after an increase in pulsatile LHRH release (pulse frequency, pulse amplitude, and basal release) occurs at the onset of puberty, total output of LHRH release (pulse amplitude and basal release together) further increases between the early and midpubertal stages in female rhesus monkeys (7, 44). Moreover, escape from estrogen suppression or a decrease in hyper sensitivity to estrogen in ovariectomized female monkeys occurred at the age of first ovulation, but not at the age of menarche (32, 46). Therefore, while lesions in the posterior hypothalamus can stimulate pulsatile LHRH release resulting precocious menarche, this procedure does not modify the mechanism responsible for the tempo of events leading to the entire process of puberty in primates.
While in the previous study with ovariectomized monkeys posterior hypothalamic lesions advanced the age at which the positive feedback effect of estrogen on LH release occurs (15), in the present study in ovarian intact monkeys posterior hypothalamic lesions did not modify the age of first ovulation. This difference in ovariectomized and ovarian intact monkeys can be interpreted to mean that a small amount of circulating estrogen from the ovary after menarche in ovarian intact monkeys causes the restraint in the positive feedback mechanism on LH and/or LHRH release. The suppression of LH by a small elevation of estrogen from the ovary in leptin treated female monkeys has been shown in a study by Wilson and colleagues (48). Again, these observations support the concept that the control mechanisms involved in the timing of first ovulation in normal puberty in primates differs from the mechanism initiating puberty.
A nocturnal elevation of GABA levels was seen in the lesion group at 15–17 months of age. Previously, because we only examined GABA levels during the morning hours (8:00 to 11:00; 21), we did not notice a daily GABA rhythm. A circadian variation in GABA release in the preoptic area of adult female rats (22) and a daily fluctuation of GABA content in the suprachiasmatic nucleus or hypothalamus in adult male rats (1, 5, 6) have been reported. Nonetheless, we need to conduct an additional study to establish a nocturnal elevation of GABA levels at 15–17 months of age, as changes were not significant in sham/control animals.
The results of the present study also indicate that evening GABA levels in both lesion and sham/control groups at 18–20 months were significantly lower than those at 15–17 months. These results were quite surprising to us based on our previous observations (21, 41), as we expected that a high level of GABA would continue until very close to the early pubertal age. Although there is a possibility that sham lesion surgery with insertion of a lesion electrode into the posterior hypothalamus may have caused similar effects as lesions, further comparison of the results from two studies suggest that this difference may be due to developmental changes between 15–17 months and 18–20 months of age. In the previous studies (21) GABA levels of prepubertal monkeys were measured at an average of 15.8±1.1 months of age (21), which is comparable to the pre-lesion age in the present study. Thus, while the developmental decrease in morning GABA levels does not occur until later age, it is possible that the developmental decrease in nocturnal GABA release occurs several months prior to the early pubertal stage, at 18–20 months of age. If this assumption is correct, a difference in nocturnal GABA levels between the lesion and sham/control groups may have been seen, if the lesion effects were examined shortly after lesions, rather than a couple of months after lesions. The question of whether posterior hypothalamic lesions decrease GABA release in the stalk-median eminence leading to an increase in LHRH release remains to be resolved.
In contrast to the changes in evening GABA release, the lesion-induced LHRH increase appears to be associated with an increase in evening levels of glutamate. Evening glutamate levels at 18–20 months of age in the lesion group were higher than those at 15–17 months, whereas evening glutamate levels in the sham/lesion group at 18–20 months of age did not differ from those at 15–17 months. Corresponding to these glutamate changes, LHRH levels at 18–20 months in the lesion group were higher than those at 15–17 months of age, whereas this was not seen in sham/control group. This observation underscores the significance of a pubertal increase in glutamate release and supports the hypothesis that glutamate in the hypothalamus plays a role in the mechanism of puberty (4, 28, 43).
A series of studies by Ojeda and colleagues have shown that growth factors, such as transforming growth factor alpha (TGFα), insulin-like growth factor-1 (IGF-1), and fibroblast growth factor-1 (FGF-1) from astroglia are important for the mechanism of the onset of puberty (see 24, 25). Hypothalamic expression of TGFα̣in it receptor, erbB1, genes increases prior to puberty not only in rats but also in monkeys (16, 18, 19). While transplantation of genetically engineered cells containing TGFα̣causes precocious puberty (17, 31), the interference of TGFα̣synthesis results in delayed puberty (19). Importantly, in female rats lesions in the anterior hypothalamus resulting in precocious puberty induces extensive astrogliosis, from which TGFα̣is released (13). Astrogila also contain erbB1 receptors through which TGFα̣in astroglia stimulate prostaglandin E2 resulting in LHRH release (14). Moreover, TGFα̣mRNA expression in astroglia is observed in hypothalamic hamartomas removed from human patients exhibiting precocious puberty (12). Thus, in the present study it is likely that posterior hypothalamic lesions induced astrogliosis, which might have released TGFα leading to LHRH release triggering the initial phase of puberty, including changes in sex-skin swelling (presumably due to a small increase in circulating estrogen) and menarche. However, posterior hypothalamic lesions are not sufficient to result in a cascade of events necessary to advance the phase of puberty, as lesions did not alter the age of first ovulation. These results further suggest that modifying the timing of puberty by the placement of lesions in the hypothalamus is not a good tool for studying the mechanism of puberty in primates.
Stimulation of the glutamatergic neuronal system, and more recently stimulation of the kisspeptin/GPR54 neuronal system induce puberty in sexually immature rats and mice and pubertal changes in gonadotropin release in prepubertal male monkeys (4, 23, 28, 29, 36, 43). Similarly, electrical stimulation of the basal hypothalamus in female prepubertal monkeys, where LHRH neuroterminals are concentrated, results in an elevation of LHRH release (8). However, stimulation of the LHRH neuronal system directly or indirectly through excitation of upstream regulatory systems may not provide answers to the precise mechanism of the onset of puberty in primates. Unlike in rodents, the reduction of central GABAergic and/or neuropeptide Y inhibition is a prerequisite for the initiation and progression of spontaneous puberty in non-human primates (10, 26, 39). In the present study the reduction in evening GABA levels had occurred at 18–20 months of age, well before the early pubertal stage, although a causal relationship is not clearly established. Rather, a precocious increase in glutamate occurred in association with LHRH increase after hypothalamic lesions. Therefore, several critical questions remain to be answered. Does excitatory input, such as kisspeptin, give a signal for a reduction in the tonic GABA inhibition in the evening? Alternatively, does the developmental reduction in evening GABA inhibition precede an increase in other stimulatory neuromodulators, including kisspeptin? The investigation of the sequence of events in the basal hypothalamus will be very important for understanding the mechanism of the onset of puberty.
Acknowledgments
The authors express their sincere appreciation to Laure Lee Luchansky for her HPLC analysis.
Grant Supports: This work is supported by the NIH grant R01HD11355. This publication was made possible in part by Grant Number P51 RR000167 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), to the Wisconsin National Primate Research Center, University of Wisconsin-Madison.
References
- 1.Aguilar-Roblero R, Verduzco-Carbajal L, Rodriguez C, Mendez-Franco J, Moran J, de la Mora MP. Circadian rhythmicity in the GABAergic system in the suprachiasmatic nuclei of the rat. Neurosci Lett. 1993;157:199–202. doi: 10.1016/0304-3940(93)90736-5. [DOI] [PubMed] [Google Scholar]
- 2.Bleier R. The hypothalamus of the rhesus monkey: A cytoarchitechtonic atlas. Madison, WI: University of Wisconsin Press; 1984. [Google Scholar]
- 3.Boepple PA, Mansfield MJ, Wierman ME, Rudlin CR, Bode HH, Crigler JF, Jr, Crawford JD, Crowley WF., Jr Use of a potent, long acting agonist of gonadotropin-releasing hormone in the treatment of precocious puberty. Endocr Rev. 1986;7:24–33. doi: 10.1210/edrv-7-1-24. [DOI] [PubMed] [Google Scholar]
- 4.Bourguignon JP, Gerard A, Mathieu J, Mathieu A, Franchimont P. Maturation of the hypothalamic control of pulsatile gonadotropin-releasing hormone secretion at onset of puberty. I. Increased activation of N-methyl-D aspartate receptors. Endocrinology. 1990;127:873–881. doi: 10.1210/endo-127-2-873. [DOI] [PubMed] [Google Scholar]
- 5.Cardinali DP, Golombek DA. The rhythmic GABAergic system. Neurochem Res. 1998;23:607–614. doi: 10.1023/a:1022426519297. [DOI] [PubMed] [Google Scholar]
- 6.Cattabeni F, Maggi A, Monduzzi M, De Angelis L, Racagni G. GABA: circadian fluctuations in rat hypothalamus. J Neurochem. 1978;31:565–567. doi: 10.1111/j.1471-4159.1978.tb02676.x. [DOI] [PubMed] [Google Scholar]
- 7.Chongthammakun S, Claypool LE, Terasawa E, Chongthammakun S, Claypool LE, Terasawa E. Ovariectomy increases in vivo luteinizing hormone-releasing hormone release in pubertal, but not prepubertal, female rhesus monkeys. J Neuroendocrinol. 1993;5:41–50. doi: 10.1111/j.1365-2826.1993.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 8.Claypool LE, Watanabe G, Terasawa E. Effects of electrical stimulation of medial basal hypothalamus on the in vivo release of luteinizing hormone-releasing hormone in the prepubertal and peripubertal monkey. Endocrinology. 1990;127:3014–3022. doi: 10.1210/endo-127-6-3014. [DOI] [PubMed] [Google Scholar]
- 9.Czaja JA, Robinson JA, Eisele SG, Scheffler G, Goy RW. Relationship between sexual skin colour of female rhesus monkeys and midcycle plasma levels of oestradiol and progesterone. J Reprod Fertil. 1977;49:147–150. doi: 10.1530/jrf.0.0490147. [DOI] [PubMed] [Google Scholar]
- 10.El Majdoubi M, Sahu A, Ramaswamy S, Plant TM. Neuropeptide Y: A hypothalamic brake restraining the onset of puberty in primates. Proc Natl Acad Sci USA. 2000;97:6179–6184. doi: 10.1073/pnas.090099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gearing M, Terasawa E. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull. 1988;21:117–121. doi: 10.1016/0361-9230(88)90126-8. [DOI] [PubMed] [Google Scholar]
- 12.Jung H, Carmel P, Schwartz MS, Witkin JW, Bentele KH, Westphal M, Piatt JH, Costa ME, Cornea A, Ma YJ, Ojeda SR. Some hypothalamic hamartomas contain transforming growth factor alpha, a puberty-inducing growth factor but not luteinizing hormone-releasing hormone neurons. J Clin Endocrinol Metab. 1999;84:4695–4701. doi: 10.1210/jcem.84.12.6185. [DOI] [PubMed] [Google Scholar]
- 13.Junier MP, Hill DF, Costa ME, Felder S, Ojeda SR. Hypothalamic lesions that induce female precocious puberty activate glial expression of the epidermal growth factor receptor gene: differential regulation of alternatively spliced transcripts. J Neurosci. 1993;13:703–713. doi: 10.1523/JNEUROSCI.13-02-00703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junier MP, Ma YJ, Costa ME, Hoffman G, Hill DF, Ojeda SR. Transforming growth factor alpha contributes to the mechanism by which hypothalamic injury induces precocious puberty. Proc Natl Acad Sci USA. 1991;88:9743–9747. doi: 10.1073/pnas.88.21.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E. Effects of pulsatile infusion of the GABAA receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology. 1999;140:5257–5266. doi: 10.1210/endo.140.11.7139. [DOI] [PubMed] [Google Scholar]
- 16.Ma YJ, Costa ME, Ojeda SR. Developmental expression of the genes encoding transforming growth factor alpha and its receptor in the hypothalamus of female rhesus macaques. Neuroendocrinology. 1994;60:346–359. doi: 10.1159/000126769. [DOI] [PubMed] [Google Scholar]
- 17.Ma YJ, Dissen GA, Merlino G, Coquelin A, Ojeda SR. Overexpression of a human transforming growth factor-alpha (TGF alpha) transgene reveals a dual antagonistic role of TGF alpha in female sexual development. Endocrinology. 1994;135:1392–1400. doi: 10.1210/endo.135.4.7925101. [DOI] [PubMed] [Google Scholar]
- 18.Ma YJ, Hill DF, Junier MP, Costa ME, Felder SE, Ojeda SR. Expression of epidermal growth factor receptor changes in the hypothalamus during the onset of female puberty. Mol Cell Neurosci. 1994;5:246–262. doi: 10.1006/mcne.1994.1029. [DOI] [PubMed] [Google Scholar]
- 19.Ma YJ, Junier MP, Costa ME, Ojeda SR. Transforming growth factor-alpha gene expression in the hypothalamus is developmentally regulated and linked to sexual maturation. Neuron. 1992;9:657–670. doi: 10.1016/0896-6273(92)90029-d. [DOI] [PubMed] [Google Scholar]
- 20.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- 21.Mitsushima D, Hei DL, Terasawa E. GABA is an inhibitory neurotransmitter restricting the release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci USA. 1994;91:395–399. doi: 10.1073/pnas.91.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsushima D, Shwe TT, Funabashi T, Shinohara K, Kimura F. GABA release in the medial preoptic area of cyclic female rats. Neuroscience. 2002;113:109–114. doi: 10.1016/s0306-4522(02)00160-4. [DOI] [PubMed] [Google Scholar]
- 23.Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ojeda SR, Ma YJ. Glial-neuronal interactions in the neuroendocrine control of mammalian puberty: facilitatory effects of gonadal steroids. J Neurobiol. 1999;40:528–540. doi: 10.1002/(sici)1097-4695(19990915)40:4<528::aid-neu9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 25.Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE. Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–1174. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- 26.Plant TM. Puberty in primates. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 453–485. [Google Scholar]
- 27.Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Hum Reprod Update. 2004;10:67–77. doi: 10.1093/humupd/dmh001. [DOI] [PubMed] [Google Scholar]
- 28.Plant TM, Gay VL, Marshall GR, Arslan M. Puberty in monkeys is triggered by chemical stimulation of the hypothalamus. Proc Natl Acad Sci USA. 1989;86:2506–2510. doi: 10.1073/pnas.86.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 30.Quanbeck C, Sherwood NM, Millar RP, Terasawa E. Two populations of luteinizing hormone-releasing hormone neurons in the forebrain of the rhesus macaque during embryonic development. J Comp Neurol. 1997;380:293–309. [PubMed] [Google Scholar]
- 31.Rage F, Hill DF, Sena-Esteves M, Breakefield XO, Coffey RJ, Costa ME, McCann SM, Ojeda SR. Targeting transforming growth factor alpha expression to discrete loci of the neuroendocrine brain induces female sexual precocity. Proc Natl Acad Sci USA. 1997;94:2735–2740. doi: 10.1073/pnas.94.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rapisarda JJ, Bergman KS, Steiner RA, Foster DL. Response to estradiol inhibition of tonic luteinizing hormone secretion decreases during the final stage of puberty in the rhesus monkey. Endocrinology. 1983;112:1172–1179. doi: 10.1210/endo-112-4-1172. [DOI] [PubMed] [Google Scholar]
- 33.Richter TA, Keen KL, Terasawa E. Synchronization of Ca(2+) oscillations among primate LHRH neurons and nonneuronal cells in vitro. J Neurophysiol. 2002;88:1559–1567. doi: 10.1152/jn.2002.88.3.1559. [DOI] [PubMed] [Google Scholar]
- 34.Rosenfield RL. Puberty and its disorders in girls. Endocrinol Metab Clin North Am. 1991;20:15–42. [PubMed] [Google Scholar]
- 35.Schultz NJ, Terasawa E. Posterior hypothalamic lesions advance the time of the pubertal changes in luteinizing hormone release in ovariectomized female rhesus monkeys. Endocrinology. 1988;123:445–455. doi: 10.1210/endo-123-1-445. [DOI] [PubMed] [Google Scholar]
- 36.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegel-Witchel S. CNS lesions, neurologic disorders, and puberty in man. In: Plant TM, Lee PA, editors. The Neurobiology of Puberty. Bristol: Journal of Endocrinology Ltd; 1995. pp. 229–239. [Google Scholar]
- 38.Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology. 2001;142:2929–2936. doi: 10.1210/endo.142.7.8239. [DOI] [PubMed] [Google Scholar]
- 39.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 40.Terasawa E, Bridson WE, Nass TE, Noonan JJ, Dierschke DJ. Developmental changes in the LH secretory pattern in peripubertal female rhesus monkeys: comparisons between gonadally intact and ovariectomized animals. Endocrinology. 1984;115:2233–2240. doi: 10.1210/endo-115-6-2233. [DOI] [PubMed] [Google Scholar]
- 41.Terasawa E, Luchansky LL, Kasuya E, Nyberg CL. An increase in glutamate release follows a decrease in gamma aminobutyric acid and the pubertal increase in luteinizing hormone releasing hormone release in female rhesus monkeys. J Neuroendocrinol. 1999;11:275–282. doi: 10.1046/j.1365-2826.1999.00325.x. [DOI] [PubMed] [Google Scholar]
- 42.Terasawa E, Noonan JJ, Nass TE, Loose MD. Posterior hypothalamic lesions advance the onset of puberty in the female rhesus monkey. Endocrinology. 1984;115:2241–2250. doi: 10.1210/endo-115-6-2241. [DOI] [PubMed] [Google Scholar]
- 43.Urbanski HF, Ojeda SR. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990;126:1774–1776. doi: 10.1210/endo-126-3-1774. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe G, Terasawa E. In vivo release of luteinizing hormone-releasing hormone (LHRH) increases with puberty in the female rhesus monkey. Endocrinology. 1989;125:92–99. doi: 10.1210/endo-125-1-92. [DOI] [PubMed] [Google Scholar]
- 45.Wildt L, Marshall G, Knobil E. Experimental induction of puberty in the infantile female rhesus monkey. Science. 1980;207:1373–1375. doi: 10.1126/science.6986658. [DOI] [PubMed] [Google Scholar]
- 46.Wilson ME. IGF-I administration advances the decrease in hypersensitivity to oestradiol negative feedback inhibition of serum LH in adolescent female rhesus monkeys. J Endocrinol. 1995;145:121–130. doi: 10.1677/joe.0.1450121. [DOI] [PubMed] [Google Scholar]
- 47.Wilson ME. Premature elevation in serum insulin-like growth factor-I advances first ovulation in rhesus monkeys. J Endocrinol. 1998;58:247–257. doi: 10.1677/joe.0.1580247. [DOI] [PubMed] [Google Scholar]
- 48.Wilson ME, Fisher J, Chikazawa K, Yoda R, Legendre A, Mook D, Gould KG. Leptin administration increases nocturnal concentrations of luteinizing hormone and growth hormone in juvenile female rhesus monkeys. J Clin Endocrinol Metab. 2003;88:4874–4883. doi: 10.1210/jc.2003-030782. [DOI] [PubMed] [Google Scholar]
- 49.Woller MJ, McDonald JK, Reboussin DM, Terasawa E. Neuropeptide Y is a neuromodulator of pulsatile luteinizing hormone-releasing hormone release in the gonadectomized rhesus monkey. Endocrinology. 1992;130:2333–2342. doi: 10.1210/endo.130.4.1547745. [DOI] [PubMed] [Google Scholar]